The Role of Apoptin in Chicken Anemia Virus Replication
Abstract
1. Chicken Anemia Virus
2. Apoptin Structure and Regulation
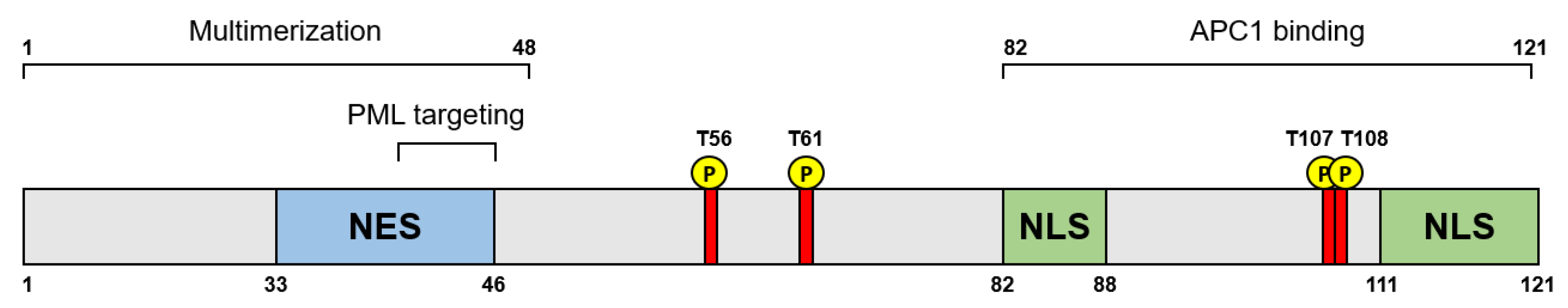
2.1. Structure of Apoptin
2.2. Localization and Regulation of Apoptin
3. Role of Apoptin in CAV Infection
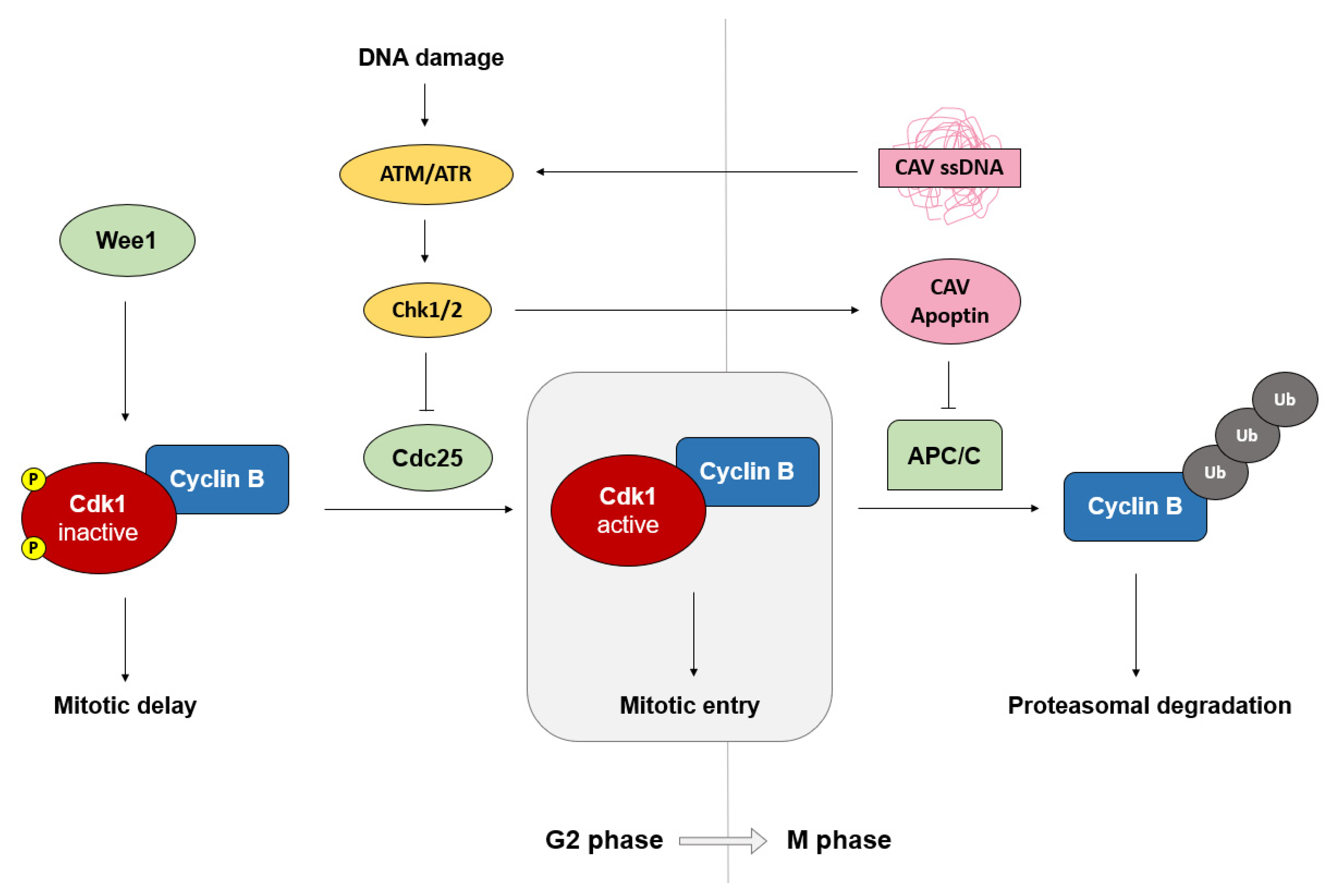
3.1. Apoptin and the APC/C
3.2. Apoptin and G2/M Cell Cycle Arrest
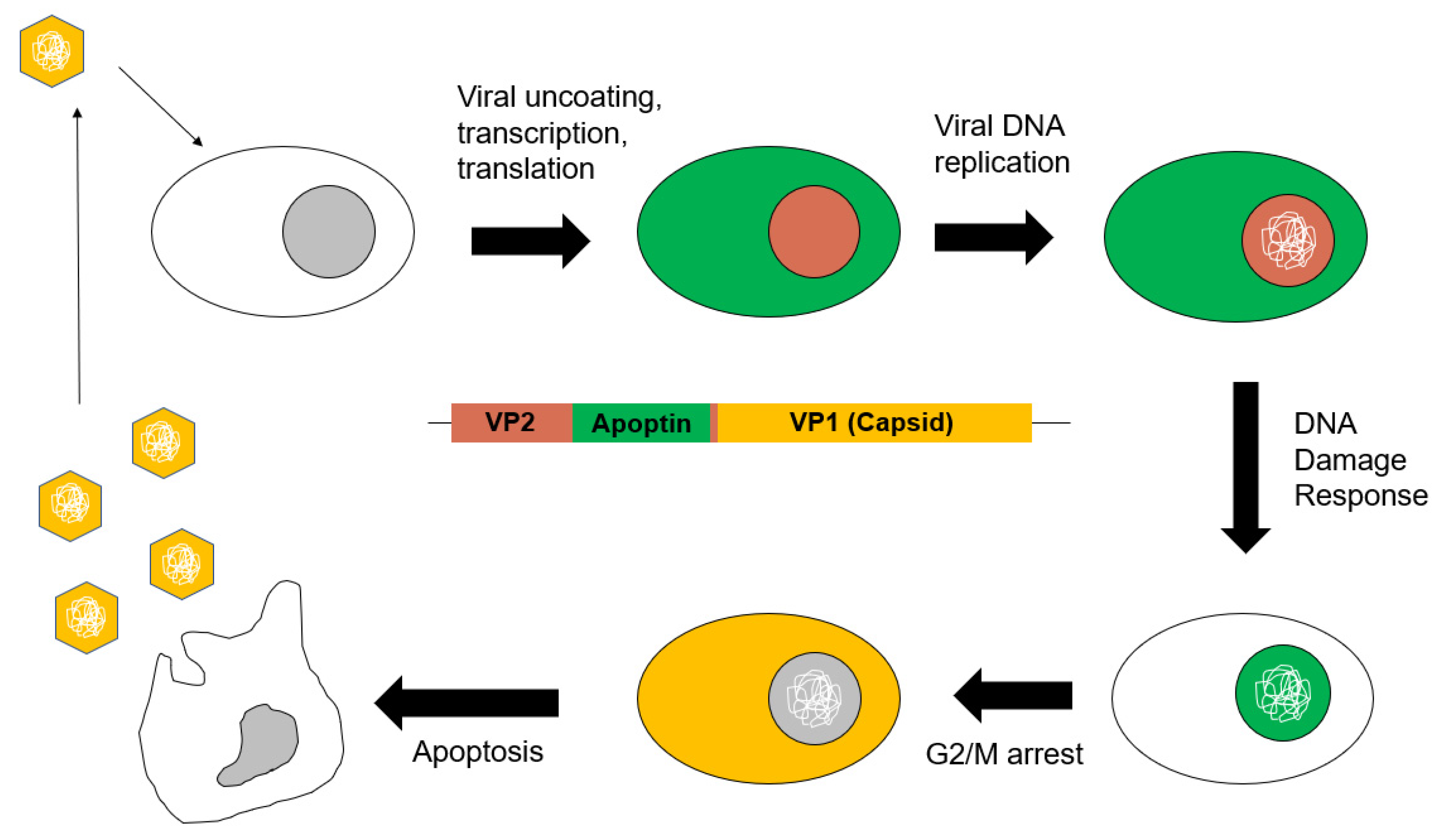
3.3. Apoptin in the Viral Life Cycle
4. Apoptin-Like Proteins in Other Single-stranded DNA Viruses
4.1. Comparing Apoptin to Similar Viral Proteins
4.2. Anelloviruses
4.3. Circoviruses
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rosenberger, J.K.; Cloud, S.S. Chicken anemia virus. Poult. Sci. 1998, 77, 1190–1192. [Google Scholar] [CrossRef] [PubMed]
- Jeurissen, S.H.; Pol, J.M.; de Boer, G.F. Transient depletion of cortical thymocytes induced by chicken anaemia agent. Thymus 1989, 14, 115–123. [Google Scholar] [PubMed]
- Yuasa, N.; Taniguchi, T.; Yoshida, I. Isolation and some characteristics of an agent inducing anemia in chicks. Avian Dis. 1979, 23, 366–385. [Google Scholar] [CrossRef]
- Gelderblom, H.; Kling, S.; Lurz, R.; Tischer, I.; Bülow, V. Morphological characterization of chicken anaemia agent (CAA). Arch. Virol. 1989, 109, 115–120. [Google Scholar] [CrossRef]
- Adams, M.J.; Lefkowitz, E.J.; King, A.M.Q.; Harrach, B.; Harrison, R.L.; Knowles, N.J.; Kropinski, A.M.; Krupovic, M.; Kuhn, J.H.; Mushegian, A.R. Ratification vote on taxonomic proposals to the International Committee on Taxonomy of Viruses (2016). Arch. Virol. 2016, 161, 2921–2949. [Google Scholar] [CrossRef]
- Noteborn, M.H.; Todd, D.; Verschueren, C.A.; De Gauw, H.W.; Curran, W.L.; Veldkamp, S.; Douglas, A.J.; McNulty, M.S.; Koch, G. A single chicken anemia virus protein induces apoptosis. J. Virol. 1994, 68, 346–351. [Google Scholar] [CrossRef]
- Noteborn, M.H.; Kranenburg, O.; Zantema, A.; Koch, G.; de Boer, G.F.; van der Eb, A.J. Transcription of the chicken anemia virus (CAV) genome and synthesis of its 52-kDa protein. Gene 1992, 118, 267–271. [Google Scholar] [CrossRef]
- Noteborn, M.H.; Verschueren, C.A.; Koch, G.; Van der Eb, A.J. Simultaneous expression of recombinant baculovirus-encoded chicken anaemia virus (CAV) proteins VP1 and VP2 is required for formation of the CAV-specific neutralizing epitope. J. Gen. Virol. 1998, 79, 3073–3077. [Google Scholar] [CrossRef][Green Version]
- Peters, M.A.; Jackson, D.C.; Crabb, B.S.; Browning, G.F. Chicken anemia virus VP2 is a novel dual specificity protein phosphatase. J. Biol. Chem. 2002, 277, 39566–39573. [Google Scholar] [CrossRef]
- Grand, R.J.; Ibrahim, A.P.; Taylor, A.M.R.; Milner, A.E.; Gregory, C.D.; Gallimore, P.H.; Turnell, A.S. Human cells arrest in S phase in response to adenovirus 12 E1A. Virology 1998, 244, 330–342. [Google Scholar] [CrossRef]
- Münger, K.; Basile, J.R.; Duensing, S.; Eichten, A.; Gonzalez, S.L.; Grace, M.; Zacny, V.L. Biological activities and molecular targets of the human papillomavirus E7 oncoprotein. Oncogene 2001, 20, 7888. [Google Scholar] [CrossRef] [PubMed]
- Teodoro, J.G.; Heilman, D.W.; Parker, A.E.; Green, M.R. The viral protein Apoptin associates with the anaphase-promoting complex to induce G2/M arrest and apoptosis in the absence of p53. Genes Dev. 2004, 18, 1952–1957. [Google Scholar] [CrossRef] [PubMed]
- Jeurissen, S.H.; De Boer, G.F. Chicken anaemia virus influences the pathogenesis of Marek’s disease in experimental infections, depending on the dose of Marek’s disease virus. Vet. Q. 1993, 15, 81–84. [Google Scholar] [CrossRef][Green Version]
- Zhuang, S.-M.; Shvarts, A.; van Ormondt, H.; Jochemsen, A.G.; van der Eb, A.J.; Noteborn, M.H. Apoptin, a protein derived from chicken anemia virus, induces p53-independent apoptosis in human osteosarcoma cells. Cancer Res. 1995, 55, 486–489. [Google Scholar] [PubMed]
- Haridy, M.; Goryo, M.; Sasaki, J.; Okada, K. Pathological and immunohistochemical study of chickens with co-infection of Marek’s disease virus and chicken anaemia virus. Avian Pathol. 2009, 38, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Danen-Van Oorschot, A.A.; Fischer, D.F.; Grimbergen, J.M.; Klein, B.; Zhuang, S.-M.; Falkenburg, J.H.; Backendorf, C.; Quax, P.H.; Van der Eb, A.J.; Noteborn, M.H. Apoptin induces apoptosis in human transformed and malignant cells but not in normal cells. PNAS 1997, 94, 5843–5847. [Google Scholar] [CrossRef]
- Los, M.; Panigrahi, S.; Rashedi, I.; Mandal, S.; Stetefeld, J.; Essmann, F.; Schulze-Osthoff, K. Apoptin, a tumor-selective killer. Biochim. Biophys. Acta 2009, 1793, 1335–1342. [Google Scholar] [CrossRef]
- Backendorf, C.; Visser, A.E.; De Boer, A.; Zimmerman, R.; Visser, M.; Voskamp, P.; Zhang, Y.-H.; Noteborn, M. Apoptin: Therapeutic potential of an early sensor of carcinogenic transformation. Annu. Rev. Pharmacol. Toxicol. 2008, 48, 143–169. [Google Scholar] [CrossRef]
- Pietersen, A.M.; Van der Eb, M.M.; Rademaker, H.J.; Van den Wollenberg, D.J.M.; Rabelink, M.J.W.E.; Kuppen, P.J.K.; Van Dierendonck, J.H.; Van Ormondt, H.; Masman, D.; Van de Velde, C.J.H. Specific tumor-cell killing with adenovirus vectors containing the apoptin gene. Gene Ther. 1999, 6, 882. [Google Scholar] [CrossRef][Green Version]
- Zhang, M.; Wang, J.; Li, C.; Hu, N.; Wang, K.; Ji, H.; He, D.; Quan, C.; Li, X.; Jin, N. Potent growth-inhibitory effect of a dual cancer-specific oncolytic adenovirus expressing apoptin on prostate carcinoma. Int. J. Oncol. 2013, 42, 1052–1060. [Google Scholar] [CrossRef]
- Ruiz-Martínez, S.; Castro, J.; Vilanova, M.; Bruix, M.; Laurents, D.V.; Ribó, M.; Benito, A. A truncated apoptin protein variant selectively kills cancer cells. Invest. New Drugs 2017, 35, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, J.; Müller, M.M.; Tavassoli, M. Cancer Treatment Goes Viral: Using Viral Proteins to Induce Tumour-Specific Cell Death. Cancers 2019, 11, 1975. [Google Scholar] [CrossRef]
- Heilman, D.W.; Teodoro, J.G.; Green, M.R. Apoptin nucleocytoplasmic shuttling is required for cell type-specific localization, apoptosis, and recruitment of the anaphase-promoting complex/cyclosome to PML bodies. J. Virol. 2006, 80, 7535–7545. [Google Scholar] [CrossRef] [PubMed]
- Pyronnet, S.; Dostie, J.; Sonenberg, N. Suppression of cap-dependent translation in mitosis. Genes Dev. 2001, 15, 2083–2093. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.; Mathews, M.B.; Mohr, I. Tinkering with translation: Protein synthesis in virus-infected cells. Cold Spring Harb. Perspect. Biol. 2013, 5, a012351. [Google Scholar] [CrossRef]
- Mo, M.; Shahar, S.; Fleming, S.B.; Mercer, A.A. How viruses affect the cell cycle through manipulation of the APC/C. Trends Microbiol. 2012, 20, 440–448. [Google Scholar] [CrossRef]
- Jeurissen, S.H.; Wagenaar, F.; Pol, J.M.; Van der Eb, A.J.; Noteborn, M.H. Chicken anemia virus causes apoptosis of thymocytes after in vivo infection and of cell lines after in vitro infection. J. Virol. 1992, 66, 7383–7388. [Google Scholar] [CrossRef]
- Danen-van Oorschot, A.A.; Zhang, Y.-H.; Leliveld, S.R.; Rohn, J.L.; Seelen, M.C.; Bolk, M.W.; van Zon, A.; Erkeland, S.J.; Abrahams, J.-P.; Mumberg, D. Importance of nuclear localization of apoptin for tumor-specific induction of apoptosis. J. Biol. Chem. 2003, 278, 27729–27736. [Google Scholar] [CrossRef]
- Leliveld, S.R.; Zhang, Y.-H.; Rohn, J.L.; Noteborn, M.H.; Abrahams, J.P. Apoptin induces tumor-specific apoptosis as a globular multimer. J. Biol. Chem. 2003, 278, 9042–9051. [Google Scholar] [CrossRef]
- Janssen, K.; Hofmann, T.G.; Jans, D.A.; Hay, R.T.; Schulze-Osthoff, K.; Fischer, U. Apoptin is modified by SUMO conjugation and targeted to promyelocytic leukemia protein nuclear bodies. Oncogene 2007, 26, 1557. [Google Scholar] [CrossRef][Green Version]
- Seeler, J.-S.; Dejean, A. Nuclear and unclear functions of SUMO. Nat. Rev. Mol. Cell Biol. 2003, 4, 690. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, R.; Pandolfi, P.P. Role of PML and the PML-nuclear body in the control of programmed cell death. Oncogene 2003, 22, 9048. [Google Scholar] [CrossRef] [PubMed]
- Dellaire, G.; Bazett-Jones, D.P. PML nuclear bodies: Dynamic sensors of DNA damage and cellular stress. Bioessays 2004, 26, 963–977. [Google Scholar] [CrossRef] [PubMed]
- Everett, R.D. DNA viruses and viral proteins that interact with PML nuclear bodies. Oncogene 2001, 20, 7266. [Google Scholar] [CrossRef] [PubMed]
- Dieckhoff, P.; Bolte, M.; Sancak, Y.; Braus, G.H.; Irniger, S. Smt3/SUMO and Ubc9 are required for efficient APC/C-mediated proteolysis in budding yeast. Mol. Microbiol. 2004, 51, 1375–1387. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Cheng, C.-M.; Chang, Y.-F.; Wang, T.-Y.; Yuo, C.-Y. Apoptin T108 phosphorylation is not required for its tumor-specific nuclear localization but partially affects its apoptotic activity. Biochem. Biophys. Res. Commun. 2007, 354, 391–395. [Google Scholar] [CrossRef]
- Rohn, J.L.; Zhang, Y.-H.; Aalbers, R.I.; Otto, N.; den Hertog, J.; Henriquez, N.V.; van de Velde, C.J.; Kuppen, P.J.; Mumberg, D.; Donner, P. A tumor-specific kinase activity regulates the viral death protein Apoptin. J. Biol. Chem. 2002, 277, 50820–50827. [Google Scholar] [CrossRef]
- Kucharski, T.J.; Ng, T.F.; Sharon, D.M.; Navid-Azarbaijani, P.; Tavassoli, M.; Teodoro, J.G. Activation of the Chicken Anemia Virus Apoptin protein by Chk1/2 phosphorylation is required for apoptotic activity and efficient viral replication. J. Virol. 2016, 90, 9433–9445. [Google Scholar] [CrossRef]
- Rohn, J.L.; Zhang, Y.-H.; Leliveld, S.R.; Danen-van Oorschot, A.A.; Henriquez, N.V.; Abrahams, J.P.; Noteborn, M.H. Relevance of apoptin’s integrity for its functional behavior. J. Virol. 2005, 79, 1337–1338. [Google Scholar] [CrossRef]
- Lanz, H.L.; Florea, B.I.; Noteborn, M.H.; Backendorf, C. Development and application of an in vitro apoptin kinase assay. Anal. Biochem. 2012, 421, 68–74. [Google Scholar] [CrossRef]
- Maddika, S.; Panigrahi, S.; Wiechec, E.; Wesselborg, S.; Fischer, U.; Schulze-Osthoff, K.; Los, M. Unscheduled Akt-triggered activation of cyclin-dependent kinase 2 as a key effector mechanism of apoptin’s anticancer toxicity. Mol. Cell. Biol. 2009, 29, 1235–1248. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Han, S.-X.; Ma, J.-L.; Ying, X.; Liu, P.; Li, J.; Wang, L.; Zhang, Y.; Ma, J.; Zhang, L. The role of CDK1 in apoptin-induced apoptosis in hepatocellular carcinoma cells. Oncol. Rep. 2013, 30, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Cole, D.; Westwood, N.; Macpherson, L.; Farzaneh, F.; Mufti, G.; Tavassoli, M.; Gäken, J. Crucial roles for protein kinase C isoforms in tumor-specific killing by apoptin. Cancer Res. 2010, 70, 7242–7252. [Google Scholar] [CrossRef] [PubMed]
- Guelen, L.; Paterson, H.; Gäken, J.; Meyers, M.; Farzaneh, F.; Tavassoli, M. TAT-apoptin is efficiently delivered and induces apoptosis in cancer cells. Oncogene 2004, 23, 1153. [Google Scholar] [CrossRef]
- Kucharski, T.J.; Gamache, I.; Gjoerup, O.; Teodoro, J.G. DNA damage response signaling triggers nuclear localization of the chicken anemia virus protein Apoptin. J. Virol. 2011, 85, 12638–12649. [Google Scholar] [CrossRef]
- Hills, S.A.; Diffley, J.F. DNA replication and oncogene-induced replicative stress. Curr. Biol. 2014, 24, R435–R444. [Google Scholar] [CrossRef]
- Lai, G.-H.; Lien, Y.-Y.; Lin, M.-K.; Cheng, J.-H.; Tzen, J.T.; Sun, F.-C.; Lee, M.-S.; Chen, H.-J.; Lee, M.-S. VP2 of chicken Anaemia virus interacts with Apoptin for Down-regulation of apoptosis through De-phosphorylated threonine 108 on Apoptin. Sci. Rep. 2017, 7, 14799. [Google Scholar] [CrossRef]
- Chang, L.; Zhang, Z.; Yang, J.; McLaughlin, S.H.; Barford, D. Molecular architecture and mechanism of the anaphase-promoting complex. Nature 2014, 513, 388. [Google Scholar] [CrossRef]
- Castro, A.; Bernis, C.; Vigneron, S.; Labbe, J.-C.; Lorca, T. The anaphase-promoting complex: A key factor in the regulation of cell cycle. Oncogene 2005, 24, 314. [Google Scholar] [CrossRef]
- Peters, J.-M. The anaphase promoting complex/cyclosome: A machine designed to destroy. Nat. Rev. Mol. Cell Biol. 2006, 7, 644. [Google Scholar] [CrossRef]
- Primorac, I.; Musacchio, A. Panta rhei: The APC/C at steady state. J. Cell Biol. 2013, 201, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Zhang, Z.; Yang, J.; McLaughlin, S.H.; Barford, D. Atomic structure of the APC/C and its mechanism of protein ubiquitination. Nature 2015, 522, 450. [Google Scholar] [CrossRef]
- Sivakumar, S.; Gorbsky, G.J. Spatiotemporal regulation of the anaphase-promoting complex in mitosis. Nat. Rev. Mol. Cell Biol. 2015, 16, 82–94. [Google Scholar] [CrossRef]
- Hochegger, H.; Takeda, S.; Hunt, T. Cyclin-dependent kinases and cell-cycle transitions: Does one fit all? Nat. Rev. Mol. Cell Biol. 2008, 9, 910. [Google Scholar] [CrossRef]
- Kousholt, A.N.; Menzel, T.; Sørensen, C.S. Pathways for genome integrity in G2 phase of the cell cycle. Biomolecules 2012, 2, 579–607. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.Y.; Elder, R.T. Viral infections and cell cycle G2/M regulation. Cell Res. 2005, 15, 143. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, M.; Gomez, N.; Lenicov, F.R.; Echeverría, E.; Shayo, C.; Moglioni, A.; Fernández, N.; Davio, C. G2/M cell cycle arrest and tumor selective apoptosis of acute leukemia cells by a promising benzophenone thiosemicarbazone compound. PLoS ONE 2015, 10, e0136878. [Google Scholar] [CrossRef]
- Hu, A.; Huang, J.-J.; Zhang, J.-F.; Dai, W.-J.; Li, R.-L.; Lu, Z.-Y.; Duan, J.-L.; Li, J.-P.; Chen, X.-P.; Fan, J.-P. Curcumin induces G2/M cell cycle arrest and apoptosis of head and neck squamous cell carcinoma in vitro and in vivo through ATM/Chk2/p53-dependent pathway. Oncotarget 2017, 8, 50747. [Google Scholar] [CrossRef]
- Shangguan, W.-J.; Li, H.; Zhang, Y.-H. Induction of G2/M phase cell cycle arrest and apoptosis by ginsenoside Rf in human osteosarcoma MG-63 cells through the mitochondrial pathway. Oncol. Rep. 2014, 31, 305–313. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, T.; Sun, W.; Wang, Z.; Zuo, D.; Zhou, Z.; Li, S.; Xu, J.; Yin, F.; Hua, Y. Erianin induces G2/M-phase arrest, apoptosis, and autophagy via the ROS/JNK signaling pathway in human osteosarcoma cells in vitro and in vivo. Cell Death Dis. 2016, 7, e2247. [Google Scholar] [CrossRef]
- Xia, W.; Spector, S.; Hardy, L.; Zhao, S.; Saluk, A.; Alemane, L.; Spector, N.L. Tumor selective G2/M cell cycle arrest and apoptosis of epithelial and hematological malignancies by BBL22, a benzazepine. PNAS 2000, 97, 7494–7499. [Google Scholar] [CrossRef] [PubMed]
- Sachs, A.B. Cell cycle–dependent translation initiation: IRES elements prevail. Cell 2000, 101, 243–245. [Google Scholar] [CrossRef]
- Goh, W.C.; Rogel, M.E.; Kinsey, C.M.; Michael, S.F.; Fultz, P.N.; Nowak, M.A.; Hahn, B.H.; Emerman, M. HIV-1 Vpr increases viral expression by manipulation of the cell cycle: A mechanism for selection of Vpr in vivo. Nat. Med. 1998, 4, 65. [Google Scholar] [CrossRef]
- Kashanchi, F.; Agbottah, E.T.; Pise-Masison, C.A.; Mahieux, R.; Duvall, J.; Kumar, A.; Brady, J.N. Cell cycle-regulated transcription by the human immunodeficiency virus type 1 Tat transactivator. J. Virol. 2000, 74, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Dove, B.; Brooks, G.; Bicknell, K.; Wurm, T.; Hiscox, J.A. Cell cycle perturbations induced by infection with the coronavirus infectious bronchitis virus and their effect on virus replication. J. Virol. 2006, 80, 4147–4156. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.; Liu, D. The missing link in coronavirus assembly retention of the avian coronavirus infectious bronchitis virus envelope protein in the pre-golgi compartments and physical interaction between the envelope and membrane proteins. J. Biol. Chem. 2001, 276, 17515–17523. [Google Scholar] [CrossRef]
- Lin, G.Y.; Lamb, R.A. The paramyxovirus simian virus 5 V protein slows progression of the cell cycle. J. Virol. 2000, 74, 9152–9166. [Google Scholar] [CrossRef][Green Version]
- Lontok, E.; Corse, E.; Machamer, C.E. Intracellular targeting signals contribute to localization of coronavirus spike proteins near the virus assembly site. J. Virol. 2004, 78, 5913–5922. [Google Scholar] [CrossRef]
- Cornelis, S.; Bruynooghe, Y.; Denecker, G.; Van Huffel, S.; Tinton, S.; Beyaert, R. Identification and characterization of a novel cell cycle–regulated internal ribosome entry site. Mol. Cell 2000, 5, 597–605. [Google Scholar] [CrossRef]
- Honda, M.; Kaneko, S.; Matsushita, E.; Kobayashi, K.; Abell, G.A.; Lemon, S.M. Cell cycle regulation of hepatitis C virus internal ribosomal entry site–directed translation. Gastroenterology 2000, 118, 152–162. [Google Scholar] [CrossRef]
- O’Connor, J.B.; Brian, D.A. Downstream ribosomal entry for translation of coronavirus TGEV gene 3b. Virology 2000, 269, 172–182. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Qin, X.; Sarnow, P. Preferential translation of internal ribosome entry site-containing mRNAs during the mitotic cycle in mammalian cells. J. Biol. Chem. 2004, 279, 13721–13728. [Google Scholar] [CrossRef] [PubMed]
- Feuer, R.; Mena, I.; Pagarigan, R.; Slifka, M.K.; Whitton, J.L. Cell cycle status affects coxsackievirus replication, persistence, and reactivation in vitro. J. Virol. 2002, 76, 4430–4440. [Google Scholar] [CrossRef] [PubMed]
- Pyronnet, S.; Pradayrol, L.; Sonenberg, N. A cell cycle–dependent internal ribosome entry site. Mol. Cell 2000, 5, 607–616. [Google Scholar] [CrossRef]
- Bressy, C.; Droby, G.N.; Maldonado, B.D.; Steuerwald, N.; Grdzelishvili, V.Z. Cell cycle arrest in G2/M phase enhances replication of interferon-sensitive cytoplasmic RNA viruses via inhibition of antiviral gene expression. J. Virol. 2019, 93, e01885-18. [Google Scholar] [CrossRef] [PubMed]
- McNulty, M.S.; Curran, W.L.; Todd, D.; Mackie, D.P. Chicken anemia agent: An electron microscopic study. Avian Dis. 1990, 34, 736–743. [Google Scholar] [CrossRef]
- Crowther, R.A.; Berriman, J.A.; Curran, W.L.; Allan, G.M.; Todd, D. Comparison of the structures of three circoviruses: Chicken anemia virus, porcine circovirus type 2, and beak and feather disease virus. J. Virol. 2003, 77, 13036–13041. [Google Scholar] [CrossRef]
- Lai, G.-H.; Lin, M.-K.; Lien, Y.-Y.; Cheng, J.-H.; Sun, F.-C.; Lee, M.-S.; Chen, H.-J.; Lee, M.-S. Characterization of the DNA binding activity of structural protein VP1 from chicken anaemia virus. BMC Vet. Res. 2018, 14, 155. [Google Scholar] [CrossRef]
- Koch, G.; van Roozelaar, D.J.; Verschueren, C.A.; van der Eb, A.J.; Noteborn, M.H. Immunogenic and protective properties of chicken anaemia virus proteins expressed by baculovirus. Vaccine 1995, 13, 763–770. [Google Scholar] [CrossRef]
- Sun, F.; Pan, W.; Gao, H.; Qi, X.; Qin, L.; Wang, Y.; Gao, Y.; Wang, X. Identification of the interaction and interaction domains of chicken anemia virus VP2 and VP3 proteins. Virology 2018, 513, 188–194. [Google Scholar] [CrossRef]
- Panigrahi, S.; Stetefeld, J.; Jangamreddy, J.R.; Mandal, S.; Mandal, S.K.; Los, M. Modeling of molecular interaction between Apoptin, BCR-Abl and CrkL—An alternative approach to conventional rational drug design. PLoS ONE 2012, 7, e28395. [Google Scholar] [CrossRef] [PubMed]
- Kooistra, K.; Zhang, Y.-H.; Henriquez, N.V.; Weiss, B.; Mumberg, D.; Noteborn, M.H. TT virus-derived apoptosis-inducing protein induces apoptosis preferentially in hepatocellular carcinoma-derived cells. J. Gen. Virol. 2004, 85, 1445–1450. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, P. TT virus infection: A novel virus-host relationship. Med. Microbiol. 2002, 51, 455–458. [Google Scholar]
- Biagini, P. Classification of TTV and related viruses (anelloviruses). In TT Viruses; Springer: Berlin, Germany, 2009; pp. 21–33. [Google Scholar]
- Prasetyo, A.A.; Kamahora, T.; Kuroishi, A.; Murakami, K.; Hino, S. Replication of chicken anemia virus (CAV) requires apoptin and is complemented by VP3 of human torque teno virus (TTV). Virology 2009, 385, 85–92. [Google Scholar] [CrossRef][Green Version]
- Sauvage, V.; Cheval, J.; Foulongne, V.; Gouilh, M.A.; Pariente, K.; Manuguerra, J.C.; Richardson, J.; Dereure, O.; Lecuit, M.; Burguiere, A. Identification of the first human gyrovirus, a virus related to chicken anemia virus. J. Virol. 2011, 85, 7948–7950. [Google Scholar] [CrossRef][Green Version]
- Bullenkamp, J.; Cole, D.; Malik, F.; Alkhatabi, H.; Kulasekararaj, A.; Odell, E.; Farzaneh, F.; Gäken, J.; Tavassoli, M. Human Gyrovirus Apoptin shows a similar subcellular distribution pattern and apoptosis induction as the chicken anaemia virus derived VP3/Apoptin. Cell Death Dis. 2012, 3, e296. [Google Scholar] [CrossRef]
- Chaabane, W.; Ghavami, S.; Małecki, A.; Łos, M.J. Human gyrovirus-apoptin interferes with the cell cycle and induces G2/M arrest prior to apoptosis. Arch. Immunol. Ther. Exp. 2017, 65, 545–552. [Google Scholar] [CrossRef]
- Rosario, K.; Breitbart, M.; Harrach, B.; Segalés, J.; Delwart, E.; Biagini, P.; Varsani, A. Revisiting the taxonomy of the family Circoviridae: Establishment of the genus Cyclovirus and removal of the genus Gyrovirus. Arch. Virol. 2017, 162, 1447–1463. [Google Scholar] [CrossRef]
- Hamel, A.L.; Lin, L.L.; Nayar, G.P. Nucleotide sequence of porcine circovirus associated with postweaning multisystemic wasting syndrome in pigs. J. Virol. 1998, 72, 5262–5267. [Google Scholar] [CrossRef]
- Hough, K.P.; Rogers, A.M.; Zelic, M.; Paris, M.; Heilman, D.W. Transformed cell-specific induction of apoptosis by porcine circovirus type 1 viral protein 3. J. Gen. Virol. 2015, 96, 351–359. [Google Scholar] [CrossRef]
- Liu, J.; Chen, I.; Kwang, J. Characterization of a previously unidentified viral protein in porcine circovirus type 2-infected cells and its role in virus-induced apoptosis. J. Virol. 2005, 79, 8262–8274. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Guo, K.; Zhang, G.; Zhang, Y. The ORF4 protein of porcine circovirus type 2 antagonizes apoptosis by stabilizing the concentration of ferritin heavy chain through physical interaction. J. Gen. Virol. 2016, 97, 1636–1646. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Maddika, S.; Booy, E.P.; Johar, D.; Gibson, S.B.; Ghavami, S.; Los, M. Cancer-specific toxicity of apoptin is independent of death receptors but involves the loss of mitochondrial membrane potential and the release of mitochondrial cell-death mediators by a Nur77-dependent pathway. J. Cell Sci. 2005, 118, 4485–4493. [Google Scholar] [CrossRef] [PubMed]
- Teras, M.; Viisileht, E.; Pahtma-Hall, M.; Rump, A.; Paalme, V.; Pata, P.; Pata, I.; Langevin, C.; Boudinot, S.R. Porcine circovirus type 2 ORF3 protein induces apoptosis in melanoma cells. BMC Cancer 2018, 18, 1237. [Google Scholar] [CrossRef]
- Karuppannan, A.K.; Kwang, J. ORF3 of porcine circovirus 2 enhances the in vitro and in vivo spread of the of the virus. Virology 2011, 410, 248–256. [Google Scholar] [CrossRef]
- Chaiyakul, M.; Hsu, K.; Dardari, R.; Marshall, F.; Czub, M. Cytotoxicity of ORF3 proteins from a nonpathogenic and a pathogenic porcine circovirus. J. Virol. 2010, 84, 11440–11447. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, C.; Liang, Y.; Teodoro, J.G. The Role of Apoptin in Chicken Anemia Virus Replication. Pathogens 2020, 9, 294. https://doi.org/10.3390/pathogens9040294
Feng C, Liang Y, Teodoro JG. The Role of Apoptin in Chicken Anemia Virus Replication. Pathogens. 2020; 9(4):294. https://doi.org/10.3390/pathogens9040294
Chicago/Turabian StyleFeng, Cynthia, Yingke Liang, and Jose G. Teodoro. 2020. "The Role of Apoptin in Chicken Anemia Virus Replication" Pathogens 9, no. 4: 294. https://doi.org/10.3390/pathogens9040294
APA StyleFeng, C., Liang, Y., & Teodoro, J. G. (2020). The Role of Apoptin in Chicken Anemia Virus Replication. Pathogens, 9(4), 294. https://doi.org/10.3390/pathogens9040294





