Characterization of an MLP Homologue from Haemaphysalis longicornis (Acari: Ixodidae) Ticks
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval
2.2. Ticks and Tissue Collection
2.3. Cloning and Sequencing the Full-Length cDNA of MLP
2.4. Sequence Analysis
2.5. Phylogenetic Analysis
2.6. Expression of Recombinant MLP
2.7. Immunoblot Analysis
2.8. Temperature Treatments
2.9. RT-PCR Analysis of HLMLP Expression in Tissues at Different Developmental Stages and Blood during Feeding
2.10. Immunization and Challenge Infestation
3. Results
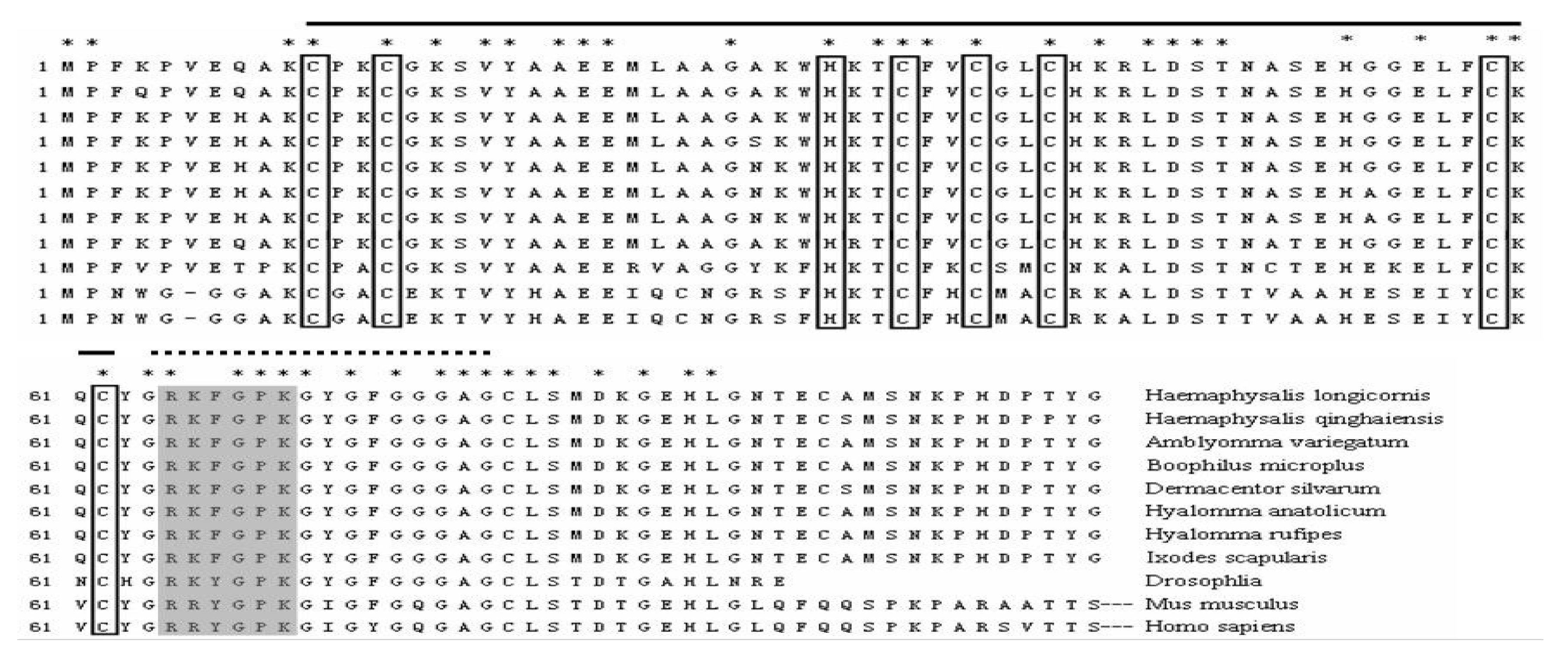
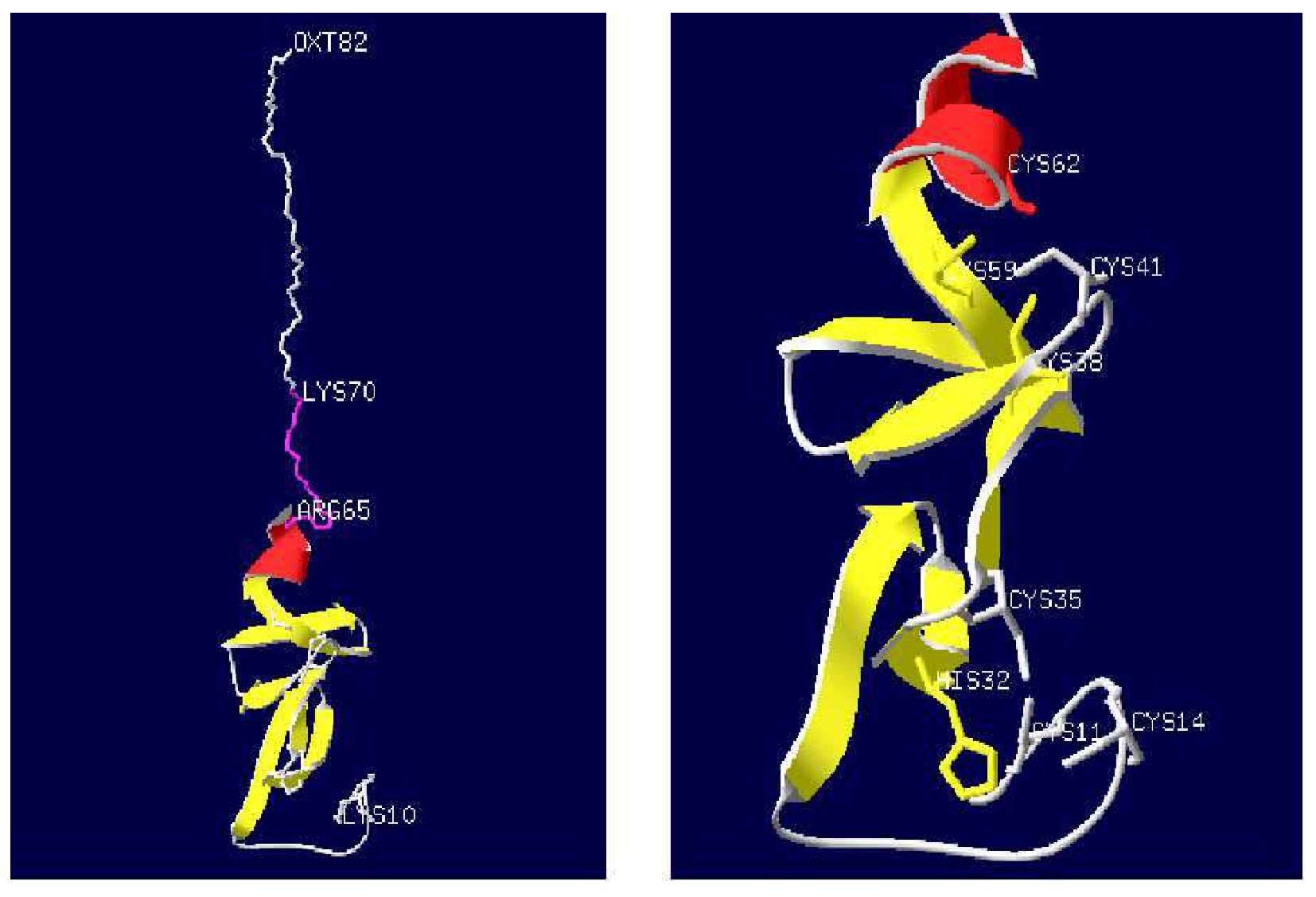
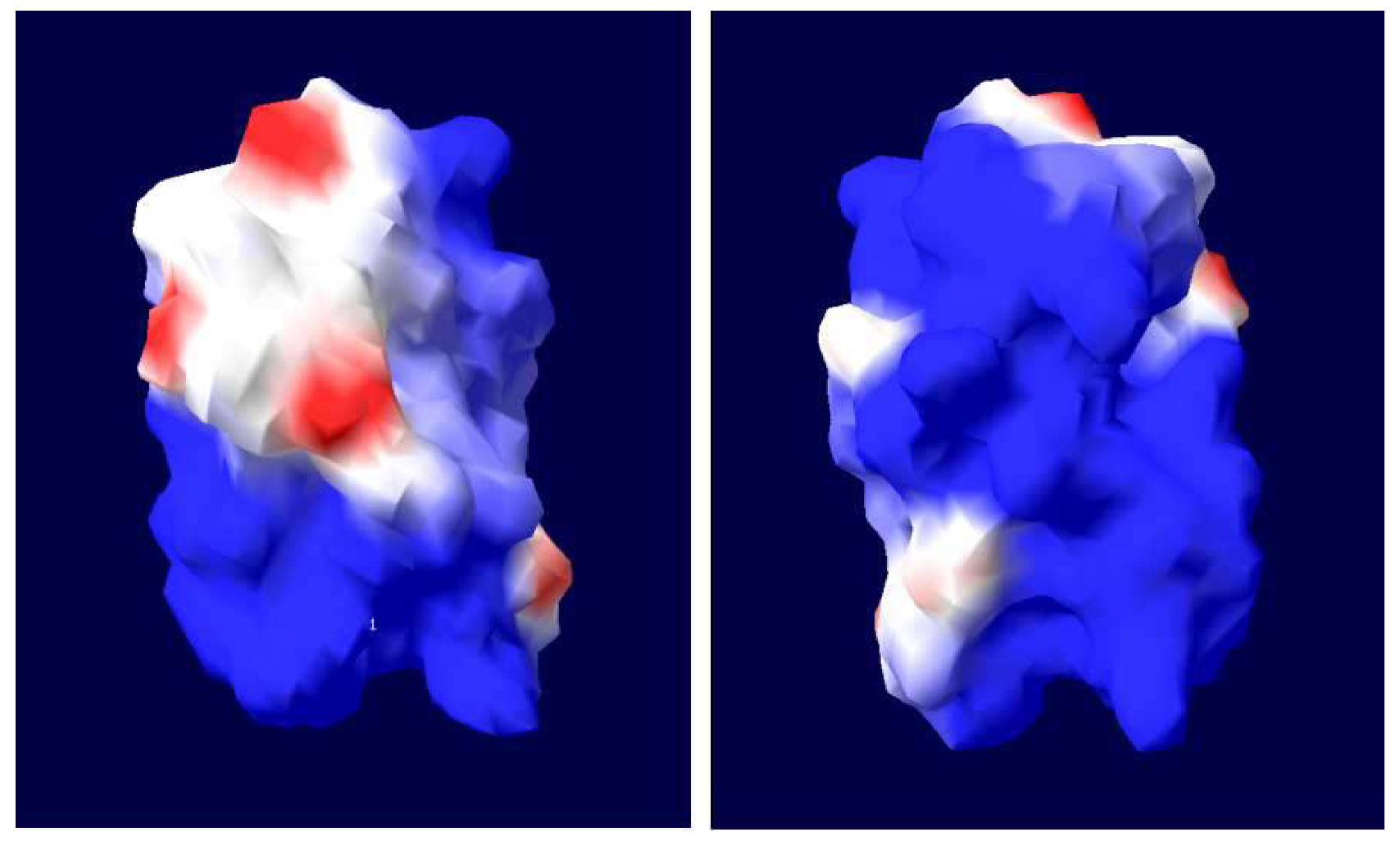
3.1. Nucleotide Sequence and Bioinformatics Analysis
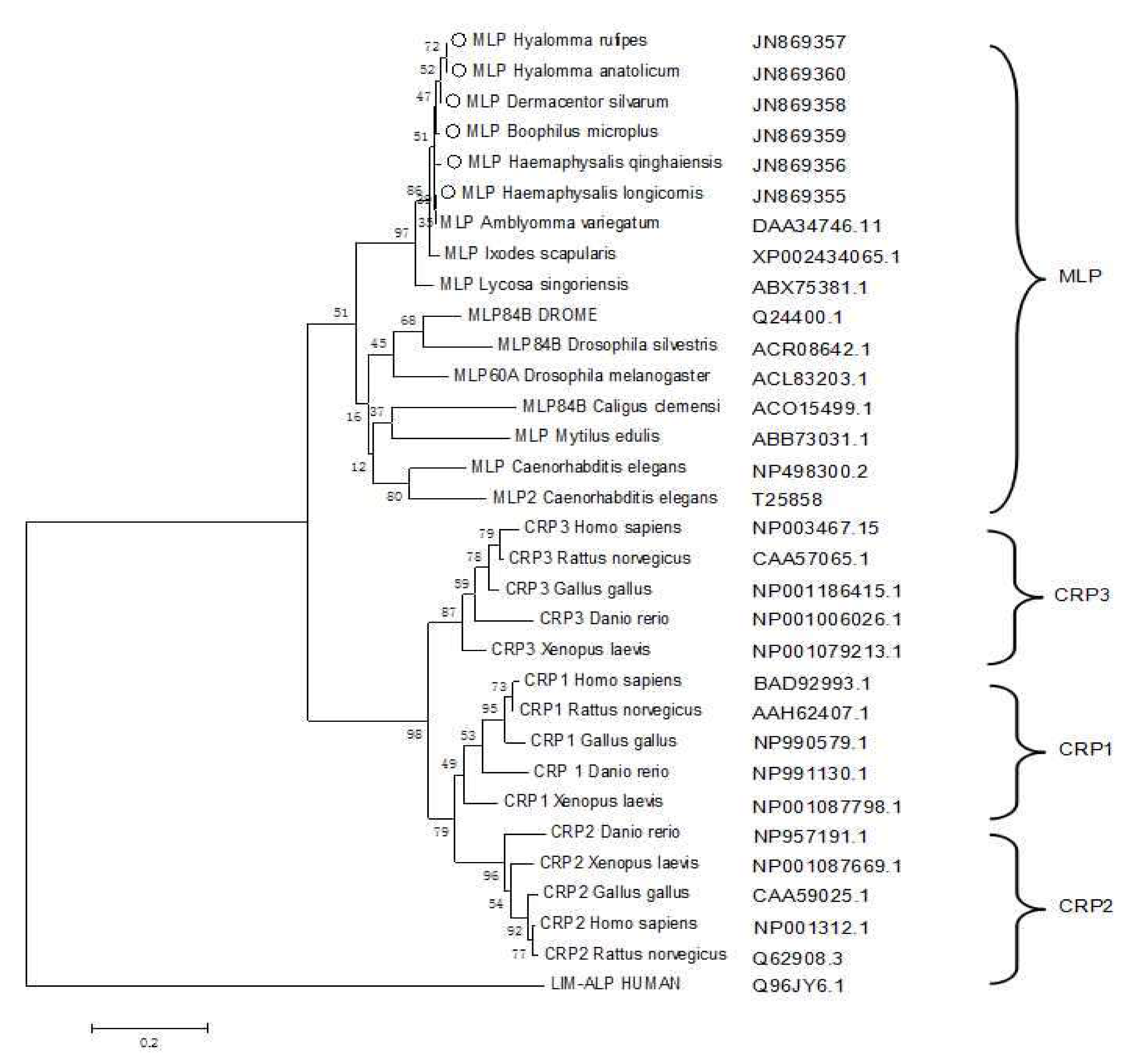
3.2. Phylogenetic Analysis
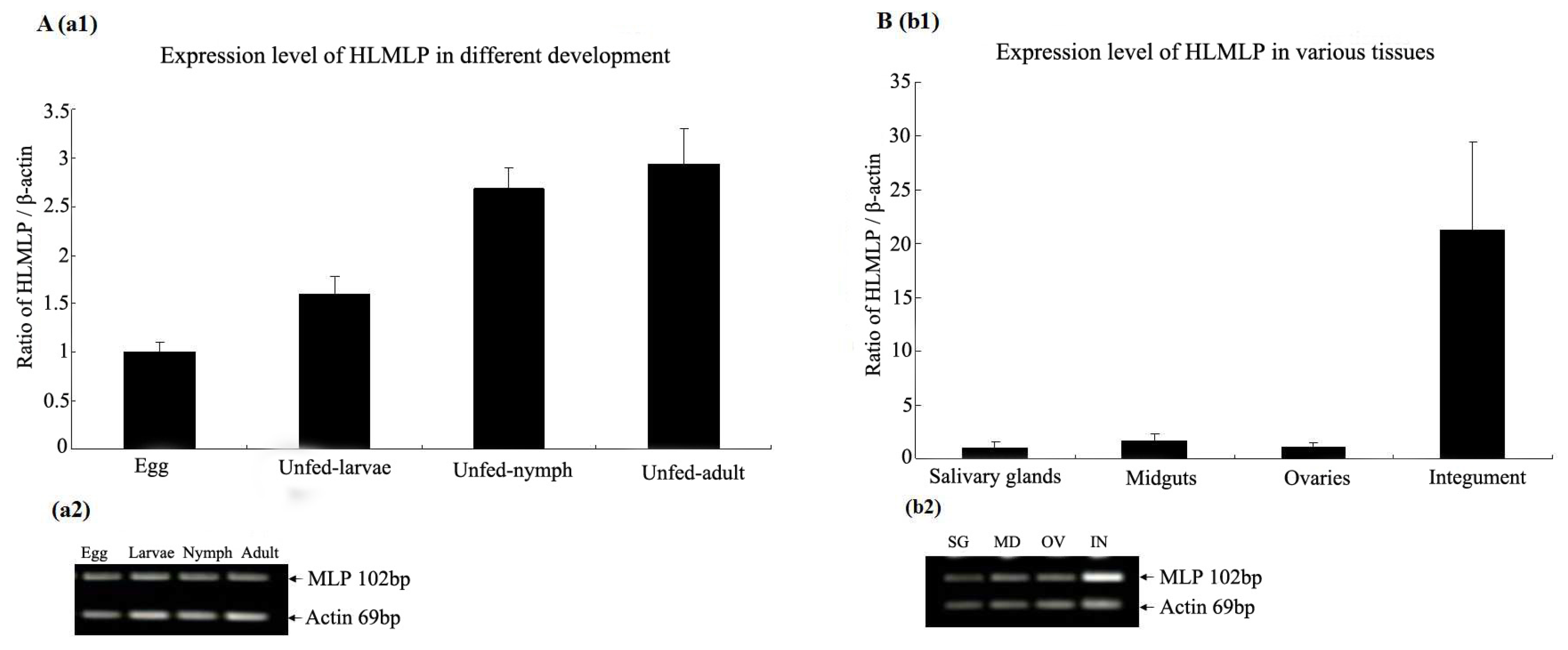
3.3. Expression Analysis of HLMLP in Tissues and Different Developmental Stages
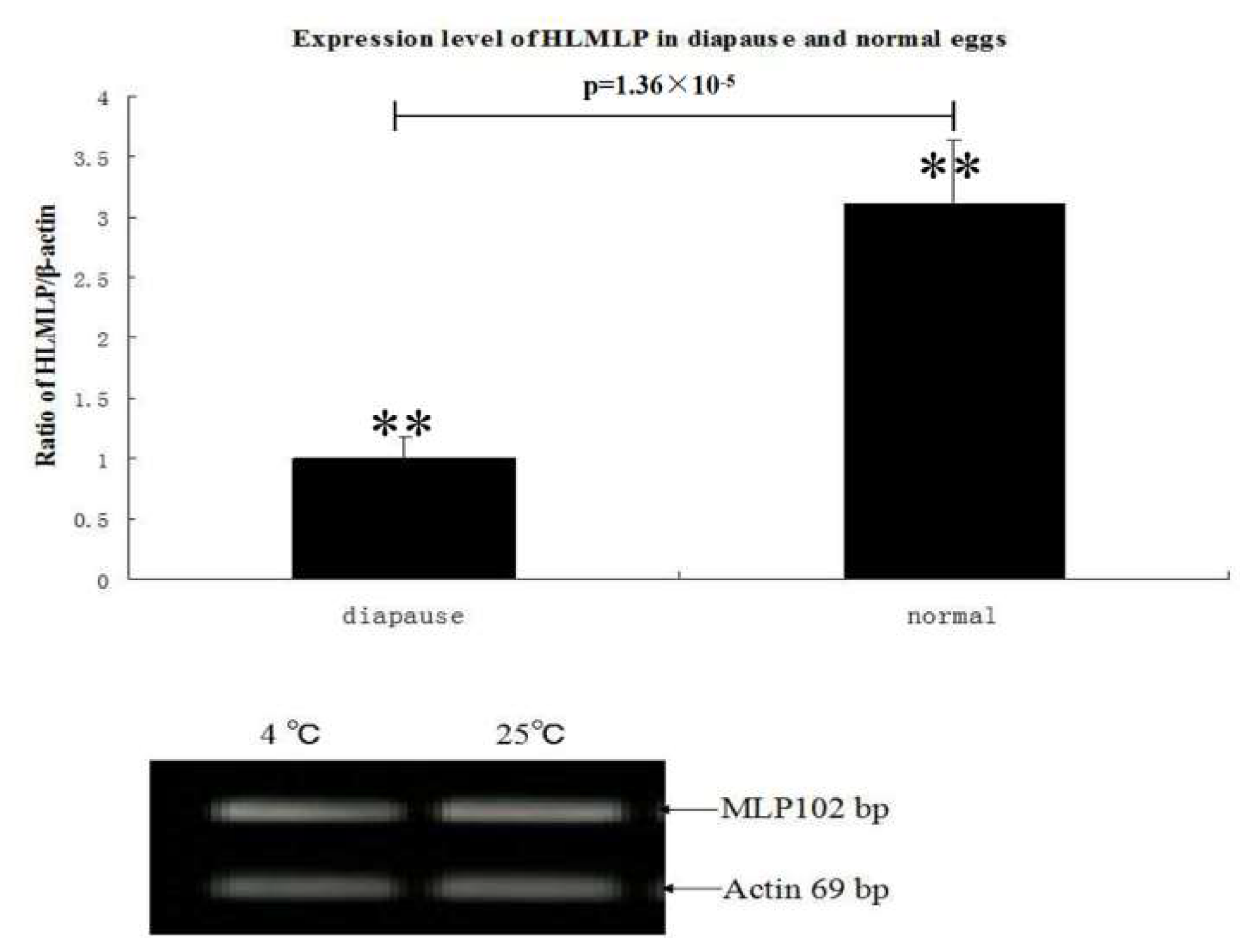
3.4. HLMLP Expression in Eggs in Response to Stress
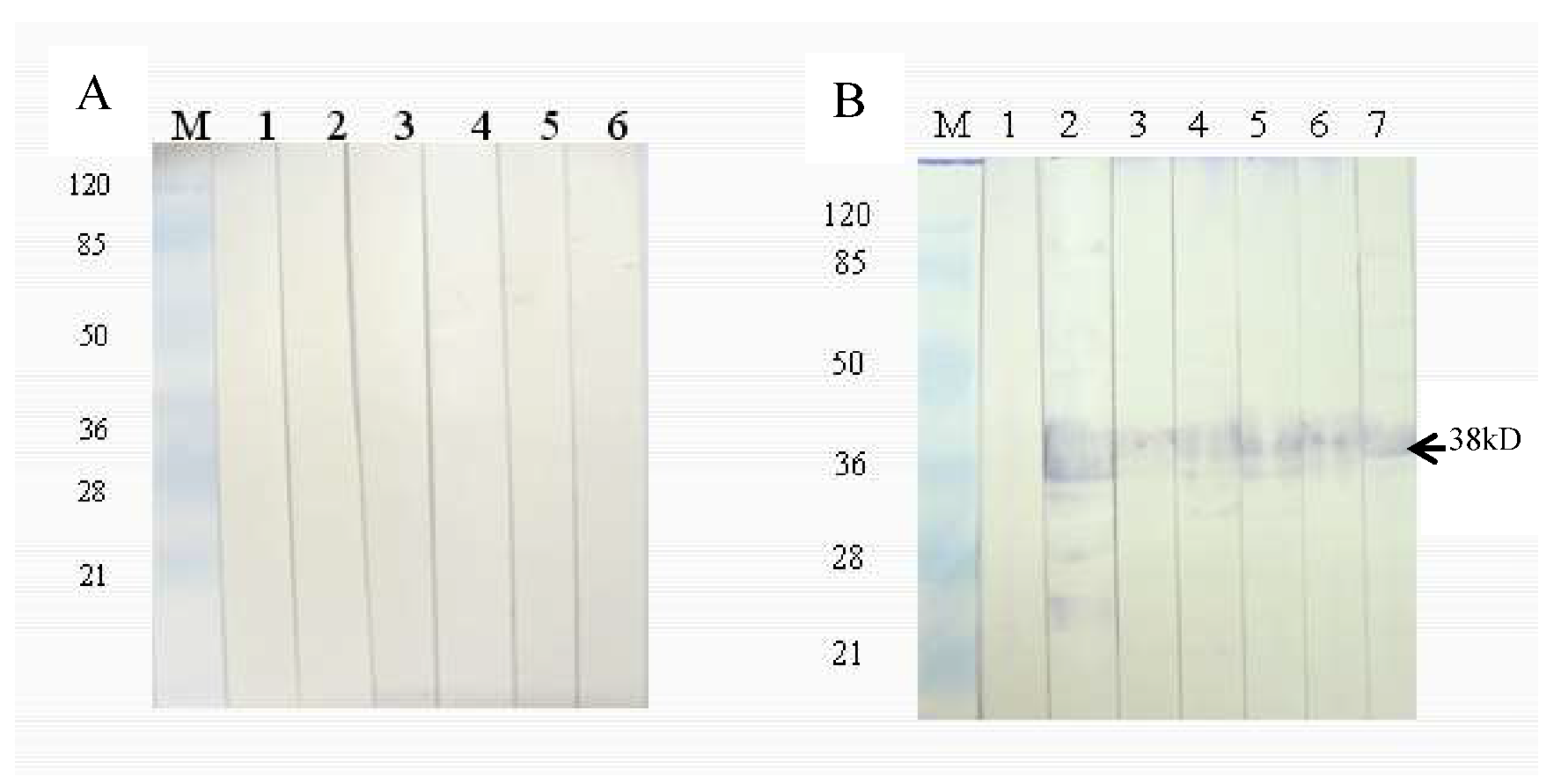
3.5. Immunogenicity Analysis of Purified rHLMLP
3.6. Protective Effects of rHLMLP Vaccination
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Availability of Data and Materials
Abbreviations
| CRPs | cysteine-rich proteins |
| MLP | muscle LIM protein |
| RACE | rapid amplification of cDNA ends |
| HL | Haemaphysalis longicornis |
| RT-PCR | real-time polymerase chain reaction |
| IPTG | isopropyl-β-D-thiogalactoside |
| GST | glutathione S-transferase |
| SDS-PAGE | sodium dodecyl sulphate–polyacrylamide gel electrophoresis |
| TBST | tris-buffered saline-Tween |
| RNAi | RNA interference |
| PBS | phosphate-buffered saline |
| NCBI | National Center for Biotechnology Information |
| BLAST | Basic Local Alignment Search Tool |
| MEGA | Molecular Evolutionary Genetics Analysis |
References
- Sadler, I.; Crawford, A.W.; Michelsen, J.W.; Beckerte, M.C. Zyxin and cCRP: Two interactive LIM domain proteins associated with the cytoskele. J. Cell Biol. 1992, 119, 1573–1587. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Garcia, I.; Rabbitts, T.H. The LIM domain: A new structural motif found in zinc-finger-like proteins. Trends Genet. 1994, 10, 315–320. [Google Scholar] [CrossRef]
- Bridge, K.S.; Shah, K.M.; Li, Y.; Foxler, D.E.; Wong, S.C.K.; Miller, D.C.; Davidson, K.M.; Foster, J.G.; Rose, R.; Hodgkinson, M.R.; et al. Argonaute utilization for miRNA silencing is determined by phosphorylation-dependent recruitment of LIM—Domain—Containing proteins. Cell Rep. 2017, 20, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Weiskirchen, R.; Pino, J.D.; Macalma, T.; Bister, K.; Beckerle, M.C. The cysteine-rich protein family of highly related LIM domain proteins. J. Biol. Chem. 1995, 270, 28946–28954. [Google Scholar] [CrossRef]
- Louis, H.A.; Pino, J.D.; Schmeichel, K.L.; Pomies, P.; Beckerle, M.C. Comparison of three members of the cysteine-rich protein family reveals functional conservation and divergent patterns of gene expression. J. Biol. Chem. 1997, 272, 27484–27491. [Google Scholar] [CrossRef]
- Harper, B.D.; Beckerle, M.C.; Pomies, P. Fine mapping of the alpha-actinin binding site within cysteine-rich protein. Biochem. J. 2000, 350, 269–274. [Google Scholar] [CrossRef]
- Arber, S.; Halder, G.; Caroni, P. Muscle LIM protein, a novel essential regulator of myogenesis, promotes myogenic differentiation. Cell 1994, 79, 221–231. [Google Scholar] [CrossRef]
- Arber, S.; Hunter, J.J.; Ross, J., Jr.; Hongo, M.; Sansig, G.; Borg, J.; Perriard, J.C.; Chien, K.R.; Caroni, P. MLP-deficient mice exhibit a disruption of cardiac cytoarchitectural organization, dilated cardiomyopathy, and heart failure. Cell 1997, 88, 393–403. [Google Scholar] [CrossRef]
- Bos, J.M.; Poley, R.N.; Ny, M.; Tester, D.J.; Xu, X.; Vatta, M.; Towbin, J.A.; Gersh, B.J.; Ommen, S.R.; Ackerman, M.J. Genotype-phenotype relationships involving hypertrophic cardiomyopathy-associated mutations in titin, muscle LIM protein, and telethonin. Mol. Genet. Metab. 2006, 88, 78–85. [Google Scholar] [CrossRef]
- Clark, K.A.; Bland, J.M.; Beckerle, M.C. The drosophila muscle LIM protein, Mlp84B, cooperates with D-titin to maintain muscle structural integrity. J. Cell Sci. 2007, 120, 2066–2077. [Google Scholar] [CrossRef]
- Niu, D.L.; Zhao, Y.E.; Yang, Y.N.; Yang, R.; Gong, X.J.; Hu, L. De novo RNA-seq and functional annotation of Haemaphysalis longicornis. Acta Parasitol. 2019, 64, 807–820. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.A.; Kadrmas, J.L. Drosophila melanogaster muscle LIM protein and alpha-actinin function together to stabilize muscle cytoarchitecture: A potential role for Mlp84B in actin-crosslinking. Cytoskeleton (Hoboken) 2013, 70, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Bughdadi, F.A. Ultrastructural study of muscles fibers in Tick Hyalomma (Hyalomma) anatolicum anatolicm (Ixodoidea: Ixodidae). Pak. J. Biol. Sci. 2010, 13, 828–834. [Google Scholar] [PubMed]
- da Silva Vaz, I., Jr.; Imamura, S.; Nakajima, C.; de Cardoso, F.C.; Ferreira, C.A.; Renard, G.; Masuda, A.; Ohashi, K.; Onuma, M. Molecular cloning and sequence analysis of cDNAs encoding for Boophilus microplus, Haemaphysalis longicornis and Rhipice-phalus appendiculatus actins. Vet. Parasitol. 2005, 127, 147–155. [Google Scholar] [CrossRef]
- Horigane, M.; Ogihara, K.; Nakajima, Y.; Honda, H.; Taylor, D.M. Identification and expression analysis of an actin gene fromthe soft tick, Ornithodoros moubata (Acari: Argasidae). Arch. Insect Biochem. Physiol. 2007, 64, 186–199. [Google Scholar] [CrossRef]
- Ferreira, C.A.; Barbosa, M.C.; Silveira, T.C.; Valenzuela, J.G.; da Vaz, I., Jr.; Masuda, A. cDNA cloning, expression and characterization of a Boophilus microplus paramyosin. Parasitology 2002, 125, 265–274. [Google Scholar] [CrossRef]
- You, M.; Xuan, X.; Tsuji, N.; Kamio, T.; Igarashi, I.; Nagasawa, H.; Mikami, T.; Fujisaki, K. Molecular characterization of a troponin I-like protein from the hard tick Haemaphysalis longicornis. Insect Biochem. Mol. Biol. 2001, 32, 67–73. [Google Scholar] [CrossRef]
- Tian, Z.C.; Zhang, P.; Luo, J.; Yin, H.; Luo, J.X.; Xie, J.; Zhang, L.; Liu, G. Cloning and characterization of a ribosomal protein L24 from Hemaphysalis longicornis eggs. Parasitol. Res. 2010, 107, 1213–1220. [Google Scholar] [CrossRef]
- Chai, H.P.; Liu, G.Y.; Zhang, L.; Gong, Z.L.; Xie, J.R.; Tian, Z.C.; Wang, L.; Jia, N. Construction of cDNA expression library of unfed female Haemaphysalis longicornis and immuno-screening. Chin. J. Parasitol. Parasit. Dis. 2009, 27, 6–10. (In Chinese) [Google Scholar]
- Tamura, K.; Dudley, J.; Nei, M.; Kumar, S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007, 24, 1596–1599. [Google Scholar] [CrossRef]
- Tian, Z.; Liu, G.; Zhang, L.; Yin, H.; Wang, H.; Xie, J.; Zhang, P.; Luo, J. Identification of the heat shock protein 70 (HLHsp70) in Haemaphysalis Longicornis. Vet. Parasitol. 2011, 181, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.G.; Sultan, K.R.; Pette, D. Muscle lim protein: Expressed in slow muscle and induced in fast muscle by enhanced contractile activity. Am. J. Physiol. 1999, 276, C900–C906. [Google Scholar] [CrossRef] [PubMed]
- Willmann, R.; Kusch, J.; Sultan, K.R.; Schneider, A.G.; Pette, D. Muscle lim protein is upregulated in fast skeletal muscle during transition toward slower phenotypes. Am. J. Physiol. Cell Physiol. 2001, 280, C273–C279. [Google Scholar] [CrossRef] [PubMed]
- Konrat, R.; Weiskirchen, R.; Kräutler, B.; Bister, K. Solution structure of the carboxyl-terminal LIM domain from quail cysteine-rich protein CRP2. J. Biol. Chem. 1997, 272, 12001–12007. [Google Scholar] [CrossRef] [PubMed]
- Weiskirchen, R.; Erdel, M.; Utermann, G.; Bister, K. Cloning, structural analysis, and chromosomal localization of the human CSRP2 gene encoding the LIM domain protein CRP2. Genomics 1997, 44, 83–93. [Google Scholar] [CrossRef]
- Xu, X.; Qiu, H.; Du, Z.Q.; Fan, B.; Rothschild, M.F.; Yuan, F.; Liu, B. Porcine CSRP3: Polymorphism and association analyses with meat quality traits and comparative analyses with CSRP1 and CSRP2. Mol. Biol. Rep. 2010, 37, 451–459. [Google Scholar] [CrossRef]
- Xu, L.; Liu, J.T.; Li, K.; Wang, S.Y.; Xu, S. Genistein inhibits Ang II-induced CRP and MMP-9 generations via the ER-p38/ERK1/2-PPARγ-NF-κB signaling pathway in rat vascular smooth muscle cells. Life Sci. 2019, 216, 140–146. [Google Scholar] [CrossRef]
- Clark, K.A.; Lesage-Horton, H.; Zhao, C.; Beckerle, M.C.; Swank, D.M. Deletion of drosophila muscle LIM protein decreases flight muscle stiffness and power generation. Am. J. Physiol. Cell Physiol. 2011, 301, C373–C382. [Google Scholar] [CrossRef]
- Knöll, R.; Kostin, S.; Klede, S.; Savvatis, K.; Klinge, L. A common MLP (muscle LIM protein) variant is associated with cardiomyopathy. Circ. Res. 2010, 106, 695–704. [Google Scholar] [CrossRef]

| Gene | Tick Species | Accession No. | No. of Amino Acids | Molecular Weight | pI |
|---|---|---|---|---|---|
| HLMLP | Haemaphysalis longicornis | JN869355 | 106 | 11.3 | 8.58 |
| HqMLP | Haemaphysalis qinghaiensis | JN869356 | 106 | 11.4 | 8.37 |
| HaMLP | Hyalomma anatolicum | JN869360 | 106 | 11.4 | 8.58 |
| HrMLP | Hyalomma rufipes | JN869357 | 106 | 11.4 | 8.58 |
| BmMLP | Boophilus microplus | JN869359 | 106 | 11.4 | 8.58 |
| DsMLP | Dermacentor silvarum | JN869358 | 106 | 11.4 | 8.58 |
| Parameter | Immunization Treatment | |
|---|---|---|
| rHLMLP | PBS | |
| Duration of feeding (days) | 7–9 | 7–9 |
| Engorged weight, mean (mg) a | 232 ± 12 * | 291 ± 23 |
| Mortality (%) b | 8 ± 2.3 | 10 ± 1.8 |
| Average egg weight (mg) | 47.35 ± 4.5 | 48.51 ± 3.8 |
| Oviposition rate (%) | 85.34 | 79.68 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, J.; Shen, H.; Ren, Q.; Guan, G.; Zhao, B.; Yin, H.; Chen, R.; Zhao, H.; Luo, J.; Li, X.; et al. Characterization of an MLP Homologue from Haemaphysalis longicornis (Acari: Ixodidae) Ticks. Pathogens 2020, 9, 284. https://doi.org/10.3390/pathogens9040284
Luo J, Shen H, Ren Q, Guan G, Zhao B, Yin H, Chen R, Zhao H, Luo J, Li X, et al. Characterization of an MLP Homologue from Haemaphysalis longicornis (Acari: Ixodidae) Ticks. Pathogens. 2020; 9(4):284. https://doi.org/10.3390/pathogens9040284
Chicago/Turabian StyleLuo, Jin, Hui Shen, Qiaoyun Ren, Guiquan Guan, Bo Zhao, Hong Yin, Ronggui Chen, Hongying Zhao, Jianxun Luo, Xiangrui Li, and et al. 2020. "Characterization of an MLP Homologue from Haemaphysalis longicornis (Acari: Ixodidae) Ticks" Pathogens 9, no. 4: 284. https://doi.org/10.3390/pathogens9040284
APA StyleLuo, J., Shen, H., Ren, Q., Guan, G., Zhao, B., Yin, H., Chen, R., Zhao, H., Luo, J., Li, X., & Liu, G. (2020). Characterization of an MLP Homologue from Haemaphysalis longicornis (Acari: Ixodidae) Ticks. Pathogens, 9(4), 284. https://doi.org/10.3390/pathogens9040284






