Monitoring of Pseudorabies in Wild Boar of Germany—A Spatiotemporal Analysis
Abstract
1. Introduction
2. Materials and Methods
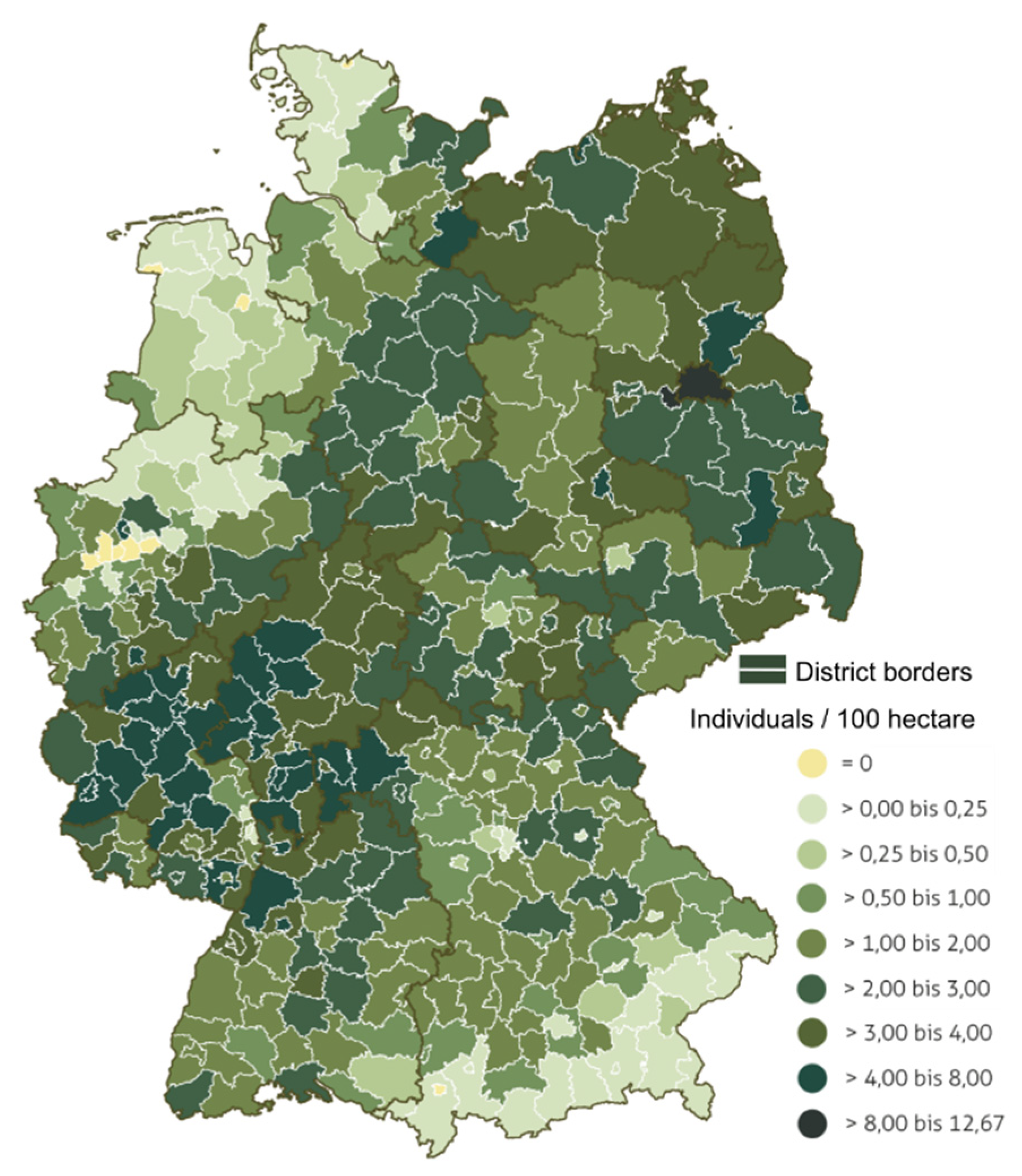
2.1. Study Area and Sample Collection
2.2. Diagnostic Assays
2.3. Spatiotemporal Analysis
2.3.1. Relative Risk
2.3.2. Seroprevalence in Administrative Units
2.3.3. Assessment of the Potential Effect of a Spatial Selection Bias
2.3.4. Evaluation of the Probability of Disease Presence
2.3.5. Hunting Statistics
3. Results
3.1. Nationwide PRV Monitoring in Wild Boar (2010–2015)
3.2. Spatiotemporal Analysis of the Combined Data Set (2000–2015)
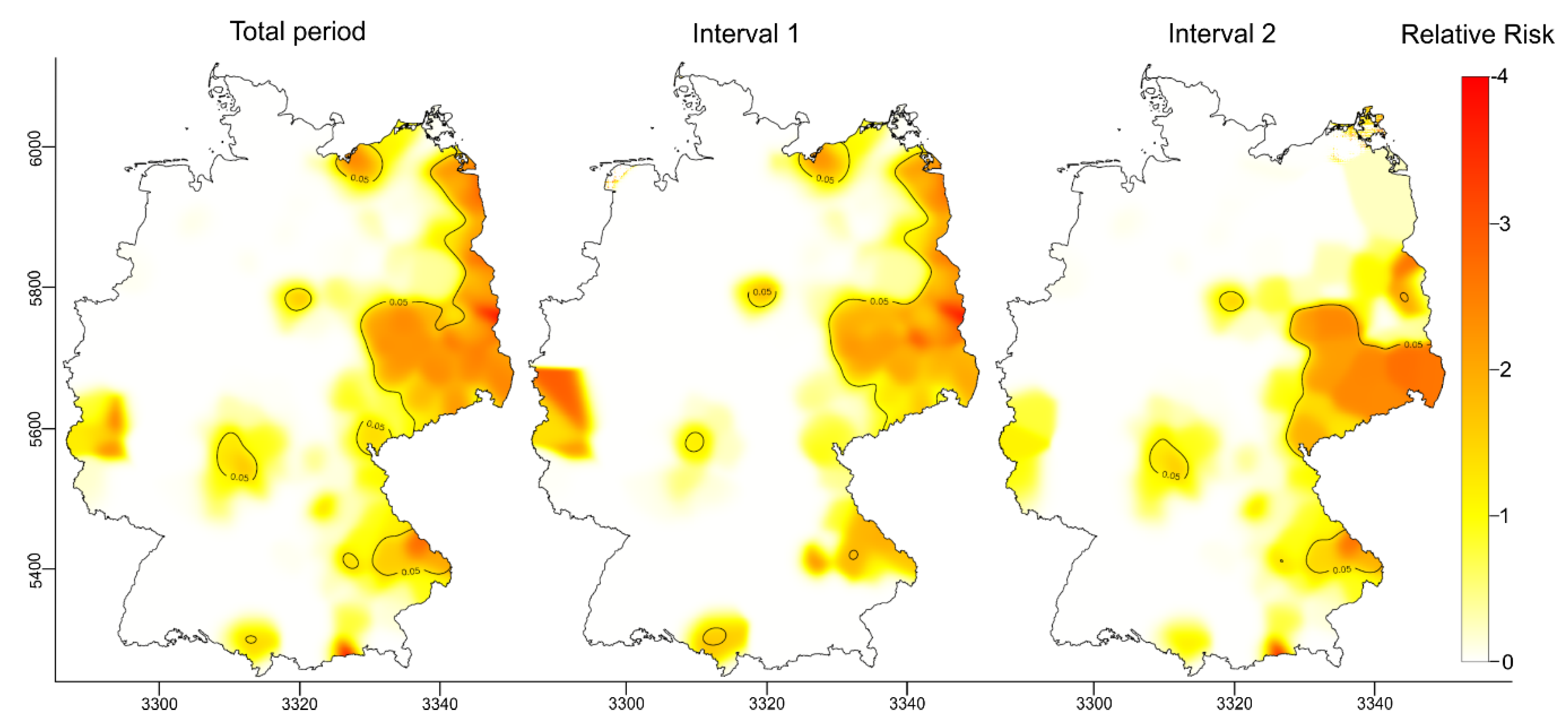
3.2.1. Spatiotemporal Analysis of the RR
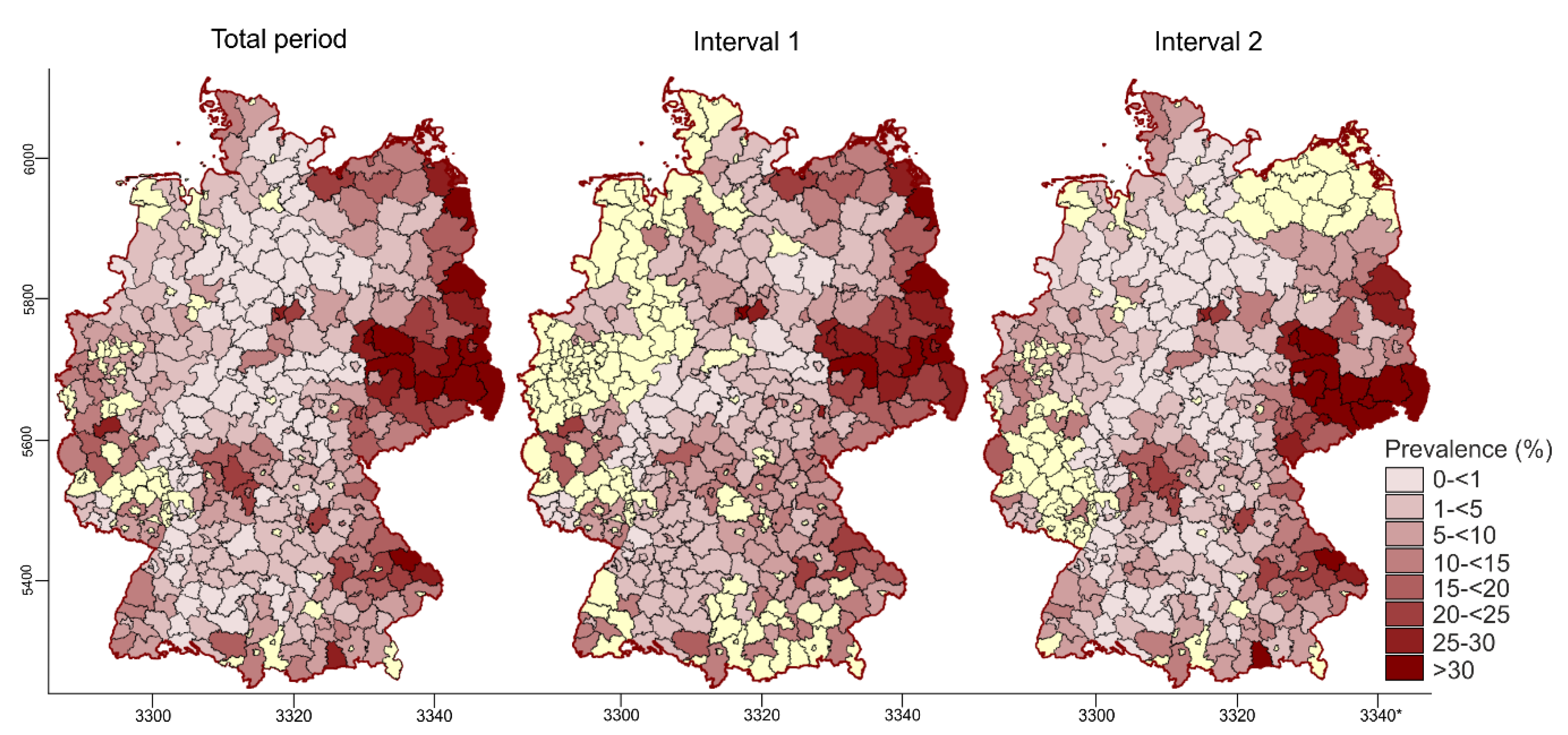
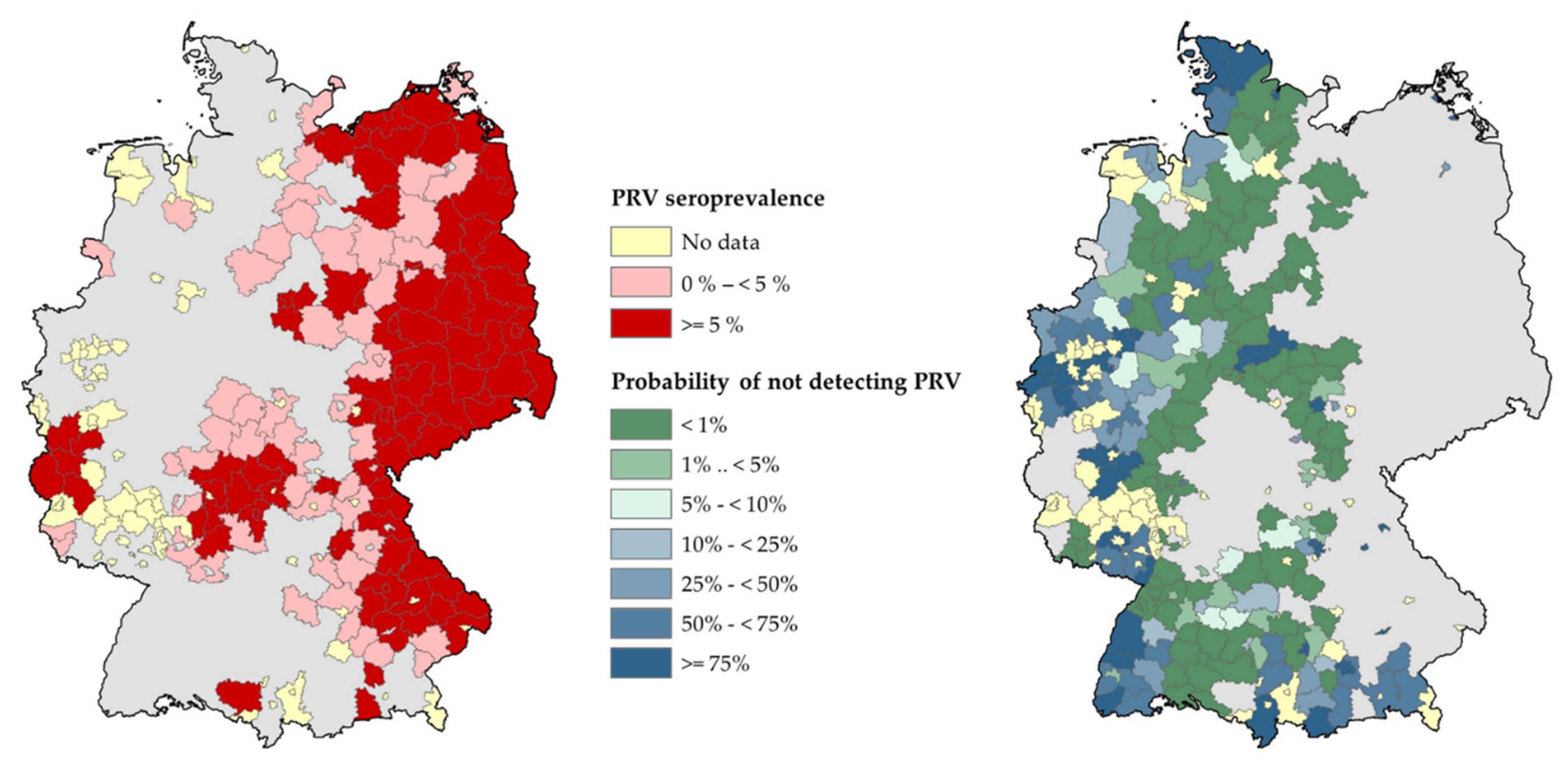
3.2.2. Spatiotemporal Analysis of the PRV Seroprevalence at District Level
3.2.3. The Probability of Endemicity and Absence of Disease
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mettenleiter, T.C.; Ehlers, B.; Müller, T.; Yoon, K.-J.; Teifke, J.P. Herpesviruses. In Diseases of Swine, 10th ed.; Zimmerman, J.J., Karriker, L., Ramirez, A., Schwartz, K., Stevenson, G., Eds.; John Wiley & Sons: Chichester, West Sussex, UK, 2012; pp. 412–444. [Google Scholar]
- Davison, A.J.; Eberle, R.; Ehlers, B.; Hayward, G.S.; McGeoch, D.J.; Minson, A.C.; Pellett, P.E.; Roizman, B.; Studdert, M.J.; Thiry, E. The order Herpesvirales. Arch. Virol. 2009, 154, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Hahn, E.C.; Fadl-Alla, B.; Lichtensteiger, C.A. Variation of Aujeszky’s disease viruses in wild swine in USA. Vet. Microbiol. 2010, 143, 45–51. [Google Scholar] [CrossRef] [PubMed]
- MacDiarmid, S.C. Aujeszky’s disease eradication in New Zealand. Aust. Vet. J. 2000, 78, 470–471. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.; Bätza, H.J.; Schlüter, H.; Conraths, F.J.; Mettenleiter, T.C. Eradication of Aujeszky’s disease in Germany. J. Vet. Med. B Infect. Dis. Vet. Public Health 2003, 50, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.; Conraths, F.J.; Hahn, E.C. Pseudorabies virus infection (Aujeszky’s disease) in wild swine. Infect. Dis. Rev. 2000, 2, 27–34. [Google Scholar]
- Müller, T.; Hahn, E.C.; Tottewitz, F.; Kramer, M.; Klupp, B.G.; Mettenleiter, T.C.; Freuling, C. Pseudorabies virus in wild swine: A global perspective. Arch. Virol. 2011, 156, 1691–1705. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K.; Bevins, S.N.; Baroch, J.A.; Cumbee, J.C., Jr.; Chandler, S.C.; Woodruff, B.S.; Bigelow, T.T.; DeLiberto, T.J. Pseudorabies in feral swine in the United States, 2009–2012. J. Wildl. Dis. 2013, 49, 709–713. [Google Scholar] [CrossRef]
- Meier, R.K.; Ruiz-Fons, F.; Ryser-Degiorgis, M.P. A picture of trends in Aujeszky’s disease virus exposure in wild boar in the Swiss and European contexts. BMC Vet. Res. 2015, 11, 277. [Google Scholar] [CrossRef]
- Jridi, M.; Bouzghaia, H.; Toma, B. Aujeszky’s disease in wild boar in Tunisia. Epidémiol. Santé Anim. 1996, 30, 99–105. [Google Scholar]
- Albayrak, H.; Ozan, E.; Cavunt, A. A serological survey of selected pathogens in wild boar (Sus scrofa) in northern Turkey. Eur. J. Wildl. Res. 2013, 59, 893–897. [Google Scholar] [CrossRef]
- Ishiguro, N.; Nishimura, M. Genetic profile and serosurvey for virus infections of Japanese wild boars in Shikoku Island. J. Vet. Med. Sci. 2005, 67, 563–568. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mahmoud, H.Y.; Suzuki, K.; Tsuji, T.; Yokoyama, M.; Shimojima, M.; Maeda, K. Pseudorabies virus infection in wild boars in Japan. J. Vet. Med. Sci. 2011, 73, 1535–1537. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lim, S.I.; Han, G.H.; Song, J.Y.; Lee, K.W.; Hyun, B.H. Serological survey of aujeszky’s disease in South Korean wild boar (Sus scrofa) population. In Proceedings of the 22nd Internation Pig Veterinray Society Congress, Jeju, Korea, 10–12 June 2012; p. 463. [Google Scholar]
- McLaughlin, A. An investigation into the Aujeszky’s disease status of South Island feral pigs. Surveillance 1996, 23, 22. [Google Scholar]
- Müller, T.; Klupp, B.G.; Freuling, C.; Hoffmann, B.; Mojcicz, M.; Capua, I.; Palfi, V.; Toma, B.; Lutz, W.; Ruiz-Fon, F.; et al. Characterization of pseudorabies virus of wild boar origin from Europe. Epidemiol. Infect. 2010, 138, 1590–1600. [Google Scholar] [CrossRef][Green Version]
- Romero, C.H.; Meade, P.N.; Homer, B.L.; Shultz, J.E.; Lollis, G. Potential sites of virus latency associated with indigenous pseudorabies viruses in feral swine. J. Wildl. Dis. 2003, 39, 567–575. [Google Scholar] [CrossRef][Green Version]
- Romero, C.H.; Meade, P.N.; Shultz, J.E.; Chung, H.Y.; Gibbs, E.P.; Hahn, E.C.; Lollis, G. Venereal transmission of pseudorabies viruses indigenous to feral swine. J. Wildl. Dis. 2001, 37, 289–296. [Google Scholar] [CrossRef]
- Hahn, E.C.; Page, G.R.; Hahn, P.S.; Gillis, K.D.; Romero, C.; Annelli, J.A.; Gibbs, E.P. Mechanisms of transmission of Aujeszky’s disease virus originating from feral swine in the USA. Vet. Microbiol. 1997, 55, 123–130. [Google Scholar] [CrossRef]
- Müller, T.; Klupp, B.; Zellmer, R.; Teuffert, J.; Ziedler, K.; Possardt, C.; Mewes, L.; Dresenkamp, B.; Conraths, F.J.; Mettenleiter, T.C. Characterisation of pseudorabies virus isolated from wild boar (Sus scrofa). Vet. Rec. 1998, 143, 337–340. [Google Scholar] [CrossRef]
- Oslage, U.; Dahle, J.; Müller, T.; Kramer, M.; Beier, D.; Liess, B. Prävalenz von Antikörpern gegen die Viren der Europäischen Schweinepest, der Aujeszky`schen Krankheit und des “Porcine reproductive and respiratory syndrome” (PRRS) bei Wildschweinen in den Bundesländern Sachsen-Anhalt und Brandenburg. Dtsch. Tierarztl. Wochenschr. 1994, 101, 1–40. [Google Scholar]
- Dedek, J.; Loepelmann, H.; Kokles, R. Results Obtained from Large-Scale Serological Studies into Black Game (Sus-Scrofa) in a GDR Region. In Diseases in Zoo Animals 1989, Verhandlungsbericht des 31. Internationalen Symposiums über die Erkrankungen der Zoo- und Wildtiere vom 24. Mai bis 28 Mai 1989 in Dortmund, Germany; Akad.-Verl.: Berlin, Germany, 1989; pp. 309–314. [Google Scholar]
- Müller, T.; Teuffert, J.; Ziedler, K.; Possardt, C.; Kramer, M.; Staubach, C.; Conraths, F.J. Pseudorabies in the European wild boar from eastern Germany. J. Wildl. Dis. 1998, 34, 251–258. [Google Scholar] [CrossRef][Green Version]
- Kaden, V.; Lange, E.; Hänel, A.; Hlinak, A.; Mewes, L.; Hergarten, G.; Irsch, B.; Dedek, J.; Bruer, W. Retrospective serological survey on selected viral pathogens in wild boar populations in Germany. Eur. J. Wildl. Res. 2009, 55, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Pannwitz, G.; Freuling, C.; Denzin, N.; Schaarschmidt, U.; Nieper, H.; Hlinak, A.; Burkhardt, S.; Klopries, M.; Dedek, J.; Hoffmann, L.; et al. A long-term serological survey on Aujeszky’s disease virus infections in wild boar in East Germany. Epidemiol. Infect. 2012, 140, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Denzin, N.; Borgwardt, J.; Freuling, C.; Muller, T. Spatio-temporal analysis of the progression of Aujeszky’s disease virus infection in wild boar of Saxony-Anhalt, Germany. Geospat. Health 2013, 8, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Lutz, W.; Wurm, R. Serologische Untersuchungen zum Nachweis von Antikörpern gegen Viren des Seuchenhaften Spätaborts, der Aujeszkyschen Krankheit, der Europäischen Schweinepest und Porzine Parvoviren beim Wildschwein (Sus scrofa, L., 1758) in Nordrhein-Westfalen. Z. Jagdwissensch. 1996, 42, 123–133. [Google Scholar] [CrossRef]
- Lutz, W.; Junghans, D.; Schmitz, D.; Müller, T. A long-term survey of pseudorabies virus infections in European wild boar of western Germany. Z. Jagdwissensch. 2003, 49, 130–140. [Google Scholar] [CrossRef]
- Sattler, T.; Sailer, E.; Wodak, E.; Schmoll, F. Serological detection of emerging viral infections in wild boars from different hunting regions of Southern Germany. Tierarztl. Prax. Ausg. G Grosstiere Nutztiere 2012, 40, 27–32. [Google Scholar] [CrossRef]
- Weyand, C.D. Epidemiologische Studie zur Seroprävalenz von Antikörpern Gegen Die Erreger von Aujeszky´scher Krankheit, Hepatitis E, Influenza A, Brucellose und Salmonellose bei Wildschweinen in Rheinland-Pfalz, Ph.D. Thesis, Justus-Liebig-Universität, Gießen, Germany, 2017. [Google Scholar]
- Brown, L.D.; Cai, T.T.; DasGupta, A. Interval Estimation for a Binomial Proportion. Stat. Sci. 2001, 16, 101. [Google Scholar] [CrossRef]
- Denzin, N.; Schliephake, A.; Wirth, A. Spatiotemporal analysis of the infection of the Red Fox (Vulpes vulpes L.) with Echinococcus multilocularis in Saxony-Anhalt. Berl. Munch. Tierarztl. Wochenschr. 2009, 122, 82–92. [Google Scholar] [CrossRef]
- Davies, T.M.; Marshall, J.C.; Hazelton, M.L. Tutorial on kernel estimation of continuous spatial and spatiotemporal relative risk. Stat. Med. 2018, 37, 1191–1221. [Google Scholar] [CrossRef]
- Silverman, B.W. Density Estimation for Statistics and Data Analysis; Chapman and Hall: London, UK, 1986. [Google Scholar]
- Bithell, J.F. Estimation of relative risk functions. Stat. Med. 1991, 10, 1745–1751. [Google Scholar] [CrossRef]
- Bithell, J.F. An application of density estimation to geographical epidemiology. Stat. Med. 1990, 9, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Kelsall, J.E.; Diggle, P.J. Kernel Estimation of Relative Risk. Bernoulli 1995, 1, 3. [Google Scholar] [CrossRef]
- Diggle, P.J.; Chetwynd, A.G.; Häggkvist, R.; Morris, S.E. Second-order analysis of space-time disease clustering. Stat. Methods Med. Res. 1995. [Google Scholar] [CrossRef] [PubMed]
- Diggle, P. A Kernel Method for Smoothing Point Process Data. J. R. Stat. Soc. C-Appl. 1985, 34, 138. [Google Scholar] [CrossRef]
- Davies, T.M.; Hazelton, M.L. Adaptive kernel estimation of spatial relative risk. Stat. Med. 2010, 29, 2423–2437. [Google Scholar] [CrossRef]
- Hazelton, M.L.; Davies, T.M. Inference Based on Kernel Estimates of the Relative Risk Function in Geographical Epidemiology. Biom. J. 2009, 51, 98–109. [Google Scholar] [CrossRef]
- R-Development-Core-Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 12 October 2019).
- Cressie, N.A.C. Statistics for Spatial Data, Rev, ed.; Wiley: New York, NY, USA, 1993. [Google Scholar]
- Marshall, R.J. Mapping Disease and Mortality Rates Using Empirical Bayes Estimators. J. R. Stat. Soc. C-Appl. 1991, 40, 283. [Google Scholar] [CrossRef]
- Berke, O. Choropleth mapping of regional count data of Echinococcus multilocularis among red foxes in Lower Saxony, Germany. Prev. Vet. Med. 2001, 52, 119–131. [Google Scholar] [CrossRef]
- Gortazar, C.; Ferroglio, E.; Hofle, U.; Frolich, K.; Vicente, J. Diseases shared between wildlife and livestock: A European perspective. Eur. J. Wildl. Res. 2007, 53, 241–256. [Google Scholar] [CrossRef]
- Office International des Epizooties. Chapter 8.2. Infection with Aujeszky’s disease virus. In OIE-Terrestrial Animal Health Code; Office International des Epizooties: Paris, France, 2019; pp. 1–8. Available online: https://www.oie.int/fileadmin/Home/eng/Health_standards/tahc/current/chapitre_aujeszky.pdf (accessed on 8 February 2020).
- Sedlak, K.; Bartova, E.; Machova, J. Antibodies to Selected Viral Disease Agents in Wild Boars from the Czech Republic. J. Wildl. Dis. 2008, 44, 777–780. [Google Scholar] [CrossRef]
- Payne, A.; Rossi, S.; Lacour, S.A.; Vallée, I.; Garin-Bastuji, B.; Simon, G.; Hervé, S.; Pavio, N.; Richomme, C.; Dunoyer, C.; et al. Bilan sanitaire du sanglier vis-à-vis de la trichinellose, de la maladie d’Aujeszky, de la brucellose, de l’hépatite E et des virus influenza porcins en France. Bull. Epidémiol. Santé Anim. Alim. 2011, 44, 2–8. [Google Scholar]
- Touloudi, A.; Valiakos, G.; Athanasiou, L.V.; Birtsas, P.; Giannakopoulos, A.; Papaspyropoulos, K.; Kalaitzis, C.; Sokos, C.; Tsokana, C.N.; Spyrou, V.; et al. A serosurvey for selected pathogens in Greek European wild boar. Vet. Rec. Open 2015, 2, e000077. [Google Scholar] [CrossRef]
- Vengust, G.; Valencak, Z.; Bidovec, A. Presence of Antibodies against Aujeszky’s Disease Virus in Wild Boar (Sus scrofa) in Slovenia. J. Wildl. Dis. 2005, 41, 800–802. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stukelj, M.; Toplak, I.; Vengust, G. Prevalence of Antibodies against Selected Pathogens in Wild Boars (Sus Scrofa) in Slovenia. Slov. Vet. Res. 2014, 51, 21–28. [Google Scholar]
- Lipowski, A.; Szczotka-Bochniarz, A.; Pejsak, Z. Prevalence of Antibodies to Aujeszky’s Disease Virus in Wild Boar in Poland, Between 2011 and 2014: A Retrospective Study. J. Vet. Res. 2017, 61, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Albina, E.; Mesplede, A.; Chenut, G.; Le Potier, M.F.; Bourbao, G.; Le Gal, S.; Leforban, Y. A serological survey on classical swine fever (CSF), Aujeszky’s disease (AD) and porcine reproductive and respiratory syndrome (PRRS) virus infections in French wild boars from 1991 to 1998. Vet. Microbiol. 2000, 77, 43–57. [Google Scholar] [CrossRef]
- Rossi, S.; Hars, J.; Garin-Bastuji, B.; Le Potier, M.F.; Boireau, P.; Aubry, P.; Hattenberger, A.M.; Louguet, Y.; Toma, B.; Boue, F. Resultats de l’enquete nationale serologique menee chez le sanglier sauvage (2000–2004). Bull. Epidemiol. Affsa 2008, 29, 5–7. [Google Scholar]
- Oggiano, A.; Patta, C.; Laddomada, A.; Caccia, A. Epidemiological Survey of Aujeszky’s Disease in Wild Boars in Sardinia. Atti Soc. Ital. Sci. Vet. 1991, 45, 1157–1161. [Google Scholar]
- Oggiano, A.; Sarria, A.; Cabras, P.; Manca, A.F.; Madrau, P.; Dci Guidici, S. Epidemiological Survey of Aujeszky`s Disease in free Ranging Pigs of Sardinia. Atti Soc. Ital. Sci. Vet. 1997, 51, 305–306. [Google Scholar]
- Firinu, A.; Ponti, M.N.; Patta, C.; Oggiano, A.; Ruiu, A.; Cabras, P.; Maestrale, C.; Cossu, P.; Pintore, A. Serologic survey on some transmissible diseases among wild boars and free ranging pigs in Sardinia. In Tradition and Innovation in Mediterranean Pig Production; Almeida, J.A., Tirapicos Nunes, J., Eds.; CIHEAM: Zaragoza, Spain, 2000; pp. 309–312. [Google Scholar]
- Nettles, V.F. Short-and Long-term Strategies for Resolving Problems of Pseudorabies and Swine Brucellosis in Feral Swine. Proc. Annu. Meet. USA Anim. Health Assoc. 1991, 95, 551–556. [Google Scholar]
- Thulke, H.H.; Selhorst, T.; Muller, T. Pseudorabies virus infections in wild boar: Data visualisation as an aid to understanding disease dynamics. Prev. Vet. Med. 2005, 68, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Czaplicki, G.; Dufey, J.; Saegerman, C. Is the Walloon European Wild boar a potential reservoir of Pseudorabies virus for porcine live stock? Epidémiol. Santé Anim. 2006, 49, 89–101. [Google Scholar]
- Tack, J. Wild Boar (Sus scrofa) Populations in Europe: A Scientific Review of Population Trends and Implications for Management; European Landowners’ Organization: Brussels, Belgium, 2018; p. 56. [Google Scholar]
- Massei, G.; Kindberg, J.; Licoppe, A.; Gacic, D.; Sprem, N.; Kamler, J.; Baubet, E.; Hohmann, U.; Monaco, A.; Ozolins, J.; et al. Wild boar populations up, numbers of hunters down? A review of trends and implications for Europe. Pest Manag. Sci. 2015, 71, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Pittiglio, C.; Khomenko, S.; Beltran-Alcrudo, D. Wild boar mapping using population-density statistics: From polygons to high resolution raster maps. PLoS ONE 2018, 13, e0193295. [Google Scholar] [CrossRef]
- Müller, T.; Teuffert, J.; Zellmer, R.; Staubach, C.; Klupp, B.; Otte, M.J.; Conraths, F.J. Pseudorabies virus infections in european wild boar—A potential danger for domestic pgis? In Proceedings of the VIII. ISVEE Conference, Paris, France, 8–11 July 1997; pp. 97–99. [Google Scholar]
- Müller, T.; Teuffert, J.; Zellmer, R.; Conraths, F.J. Experimental infection of European wild boars and domestic pigs with pseudorabies viruses with differing virulence. Am. J. Vet. Res. 2001, 62, 252–258. [Google Scholar] [CrossRef]
- Corn, J.L.; Stallknecht, D.E.; Mechlin, N.M.; Luttrell, M.P.; Fischer, J.R. Persistence of Pseudorabies Virus in Feral Swine Populations. J. Wildl. Dis. 2004, 40, 307–310. [Google Scholar] [CrossRef]
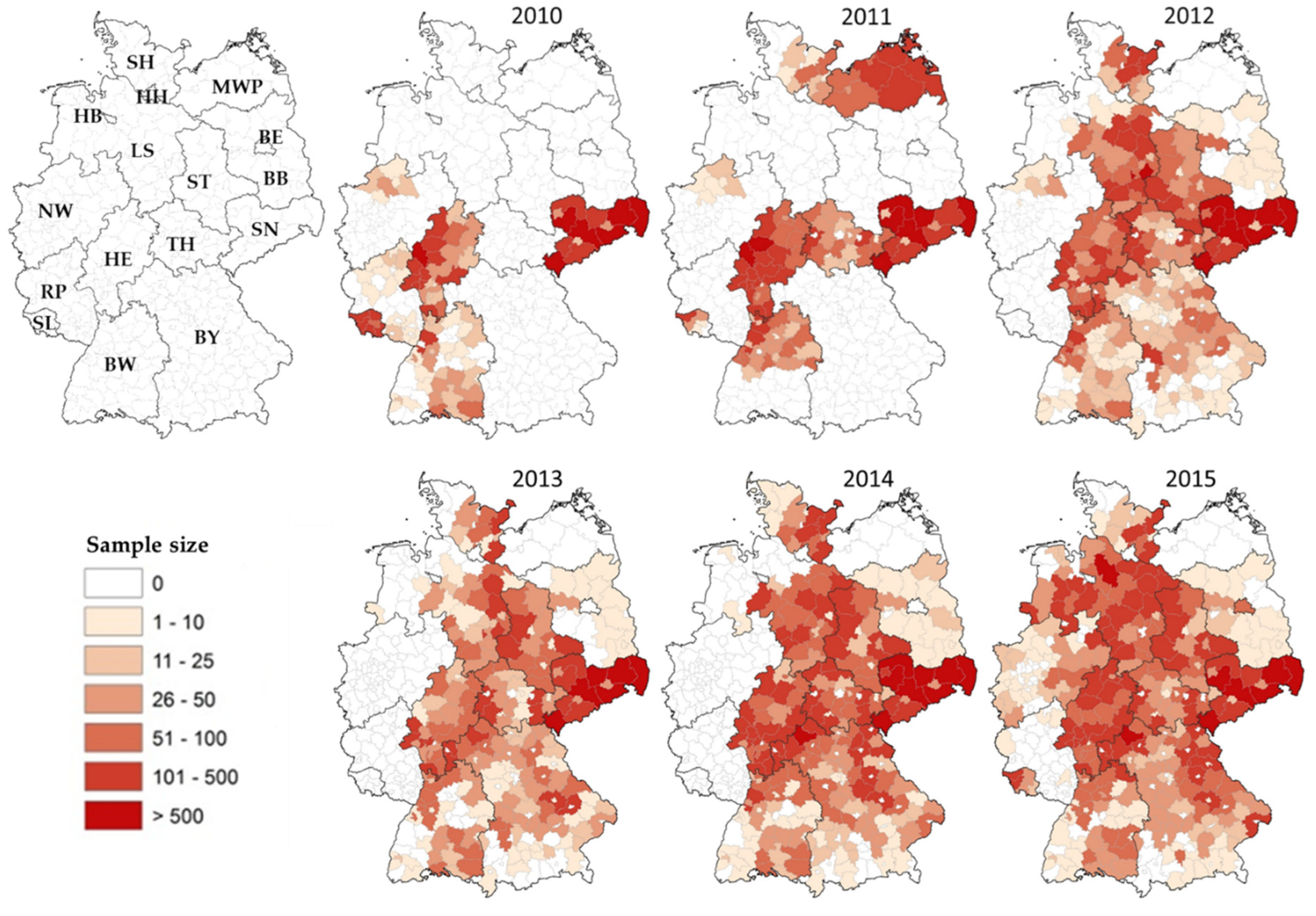
| Federal State | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | Total | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | pos | prev | N | pos | prev | N | pos | prev | N | pos | prev | N | pos | prev | N | pos | prev | N | pos | prev | 95% CI | |
| BE | 91 | 14 | 15.38 | 91 | 14 | 15.38 | 8.67–24.46 | |||||||||||||||
| BB | 73 | 6 | 8.22 | 52 | 4 | 7.69 | 131 | 13 | 9.92 | 97 | 0 | 0 | 353 | 23 | 6.52 | 4.17–9.62 | ||||||
| BW | 768 | 18 | 2.34 | 1131 | 4 | 0.35 | 1118 | 15 | 1.34 | 1062 | 14 | 1.32 | 1212 | 22 | 1.82 | 1286 | 26 | 2.02 | 6577 | 99 | 1.51 | 1.23–1.83 |
| BY | 1575 | 81 | 5.14 | 2798 | 287 | 10.26 | 4606 | 550 | 11.94 | 4125 | 405 | 9.82 | 13,104 | 1323 | 10.10 | 9.59–10.62 | ||||||
| HB | ||||||||||||||||||||||
| HE | 2895 | 35 | 1.21 | 4900 | 64 | 1.31 | 3015 | 54 | 1.79 | 1751 | 33 | 1.88 | 2909 | 68 | 2.34 | 3073 | 72 | 2.34 | 18,543 | 326 | 1.76 | 1.57–1.96 |
| HH | ||||||||||||||||||||||
| LS | 2760 | 222 | 8.04 | 1305 | 56 | 4.29 | 1939 | 11 | 0.57 | 4430 | 22 | 0.50 | 10,434 | 311 | 2.98 | 2.66–3.33 | ||||||
| MWV | 1104 | 135 | 12.23 | 1104 | 135 | 12.23 | 10.35–14.31 | |||||||||||||||
| NW | 78 | 0 | 0 | 68 | 0 | 0 | 53 | 0 | 0 | 799 | 3 | 0.38 | 998 | 3 | 0.30 | 0.06–0.88 | ||||||
| RP | 100 | 5 | 5.00 | ,_ | _ | _ | _ | _ | _ | _ | _ | _ | 1936 ** | 47 ** | 2.43 ** | 9 | 1 | 11.11 | 2092 | 6 | 0.29 | 0.11–0.62 |
| SH | 279 | 2 | 0.72 | 848 | 0 | 0 | 619 | 0 | 0 | 1184 | 3 | 0.25 | 599 | 0 | 0 | 3529 | 5 | 0.14 | 0.05–0.33 | |||
| SL | 1397 | 2 | 0.14 | 330 | 1 | 0.30 | 373 | 25 | 6.70 | 2100 | 28 | 1.33 | 0.89–1.92 | |||||||||
| SN | 5695 | 1624 | 28.52 | 5464 | 1665 | 30.47 | 6889 | 2048 | 29.73 | 6542 | 2171 | 33.19 | 7527 | 2585 | 34.34 | 6258 | 2.126 | 33.97 | 38,375 | 12,219 | 31.84 | 31.38–32.31 |
| ST | 996 | 79 | 7.93 | 936 | 122 | 13.03 | 1101 | 138 | 12.53 | 1855 | 182 | 9.81 | 4888 | 521 | 10.66 | 9.81–11.56 | ||||||
| TH | 917 | 18 | 1.96 | 1343 | 38 | 2.83 | 1028 | 41 | 3.99 | 1983 | 88 | 4.44 | 1336 | 66 | 4.94 | 6607 | 251 | 3.80 | 3.35–4.29 | |||
| Total | 10,933 | 1684 | 15.40 | 14,193 | 1889 | 11.71 | 18,670 | 2543 | 13.62 | 16,093 | 2728 | 16.59 | 22,592 | 3478 | 15.39 | 24,331 | 2.942 | 12.09 | 108,748 | 15,311 | 14.08 | 13.87–14.29 |
| Administrative Unit | Town/Village | Municipality | District | Total |
|---|---|---|---|---|
| No. of Cases * | 5274 | 380 | 14,898 | 20,552 |
| No. of Controls # | 43,906 | 16,023 | 89,665 | 149,594 |
| 95% CI | |||||
|---|---|---|---|---|---|
| Data Set | Time Interval | Sample Size | Prevalence (%) | LCL 1 | UCL 1 |
| Original | Total | 149,594 | 13.73 | 13.55 | 13.90 |
| 1 | 74,797 | 12.91 | 12.67 | 13.15 | |
| 2 | 74,797 | 14.54 | 14.29 | 14.80 | |
| Adjusted | 1 | 74,797 | 8.71 | -- | -- |
| 2 | 74,797 | 13.40 | -- | -- | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Denzin, N.; Conraths, F.J.; Mettenleiter, T.C.; Freuling, C.M.; Müller, T. Monitoring of Pseudorabies in Wild Boar of Germany—A Spatiotemporal Analysis. Pathogens 2020, 9, 276. https://doi.org/10.3390/pathogens9040276
Denzin N, Conraths FJ, Mettenleiter TC, Freuling CM, Müller T. Monitoring of Pseudorabies in Wild Boar of Germany—A Spatiotemporal Analysis. Pathogens. 2020; 9(4):276. https://doi.org/10.3390/pathogens9040276
Chicago/Turabian StyleDenzin, Nicolai, Franz J. Conraths, Thomas C. Mettenleiter, Conrad M. Freuling, and Thomas Müller. 2020. "Monitoring of Pseudorabies in Wild Boar of Germany—A Spatiotemporal Analysis" Pathogens 9, no. 4: 276. https://doi.org/10.3390/pathogens9040276
APA StyleDenzin, N., Conraths, F. J., Mettenleiter, T. C., Freuling, C. M., & Müller, T. (2020). Monitoring of Pseudorabies in Wild Boar of Germany—A Spatiotemporal Analysis. Pathogens, 9(4), 276. https://doi.org/10.3390/pathogens9040276





