A Systematic Review: Is Aedes albopictus an Efficient Bridge Vector for Zoonotic Arboviruses?
Abstract
1. Introduction
2. Results
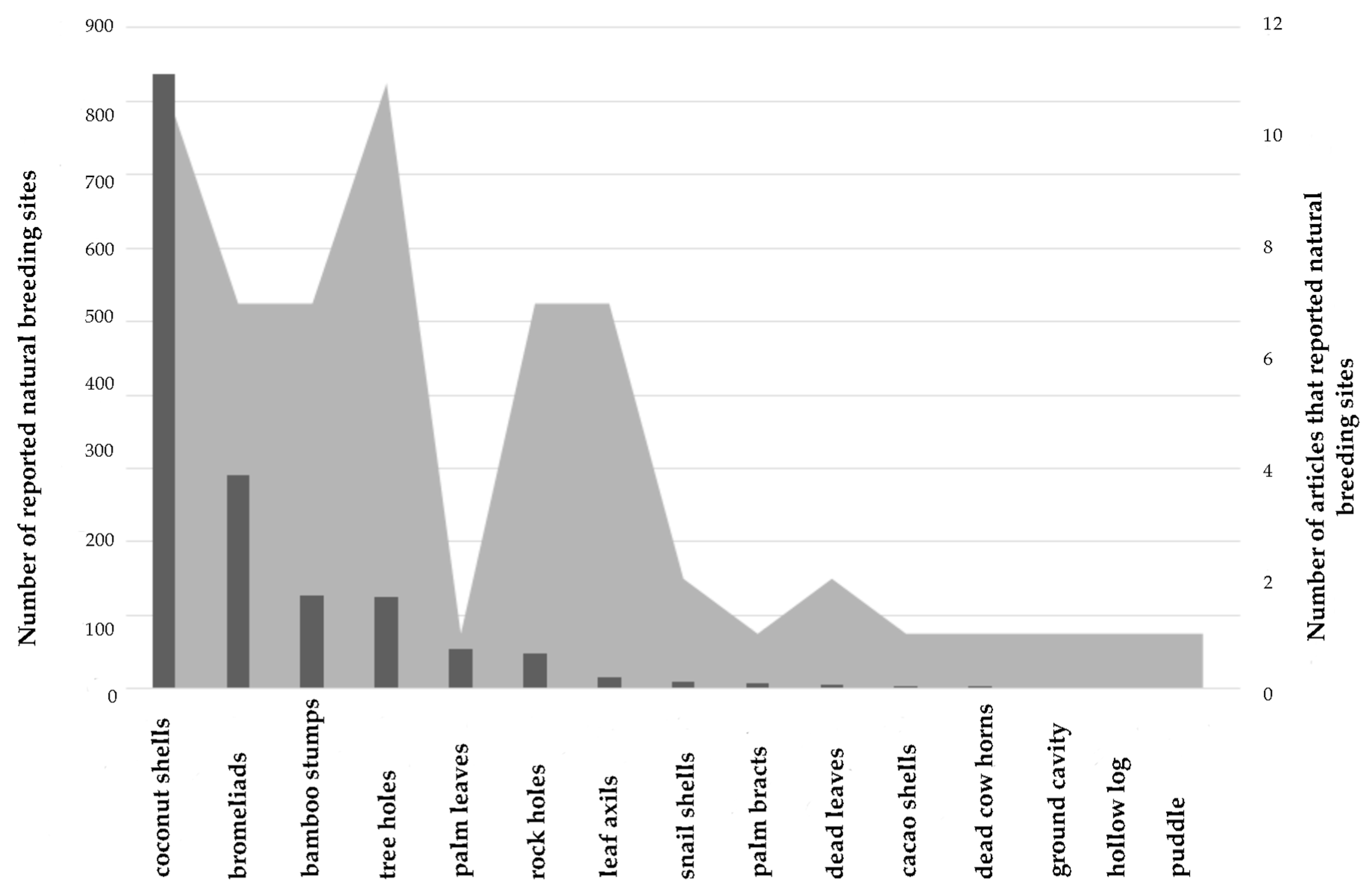
2.1. Natural Breeding Sites
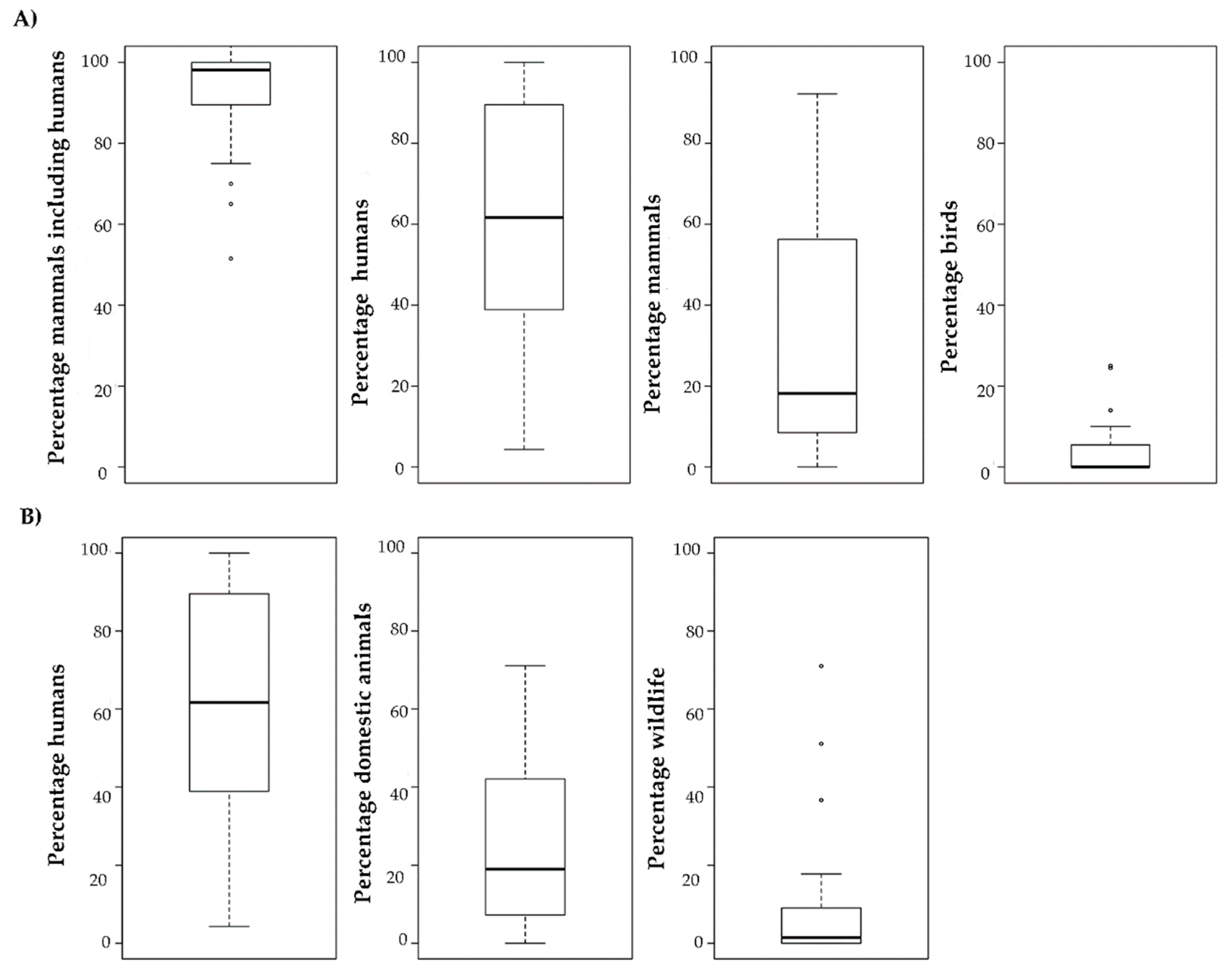
2.2. Feeding Behaviour
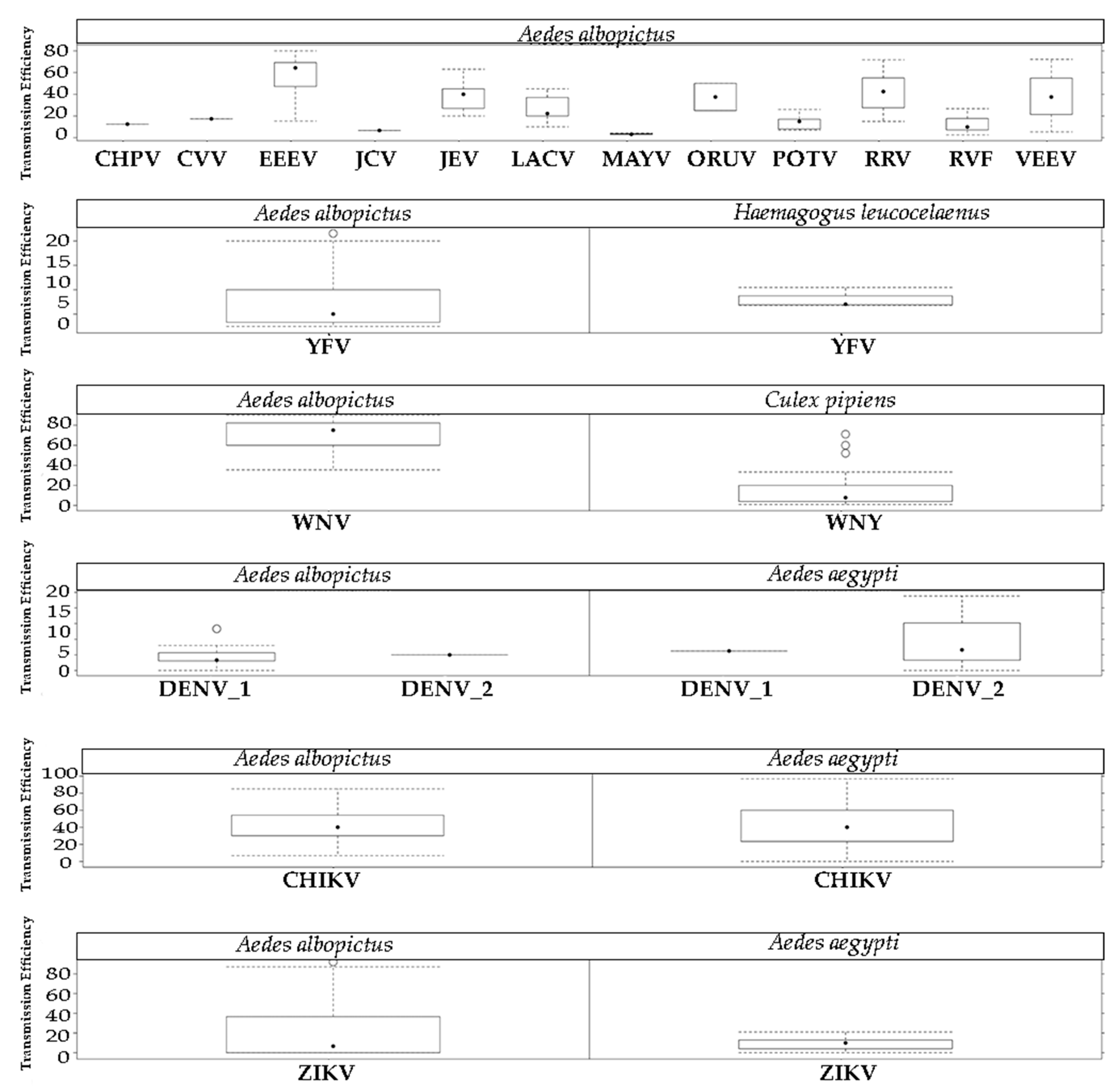
2.3. Arbovirus Transmission
3. Discussion
4. Material and Methods
4.1. Natural Breeding Sites
4.2. Feeding Behaviour
4.3. Arbovirus Transmission
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Whitmee, S.; Haines, A.; Beyrer, C.; Boltz, F.; Capon, A.G.; De Souza Dias, B.F.; Ezeh, A.; Frumkin, H.; Gong, P.; Head, P.; et al. Safeguarding human health in the Anthropocene epoch: Report of the Rockefeller Foundation-Lancet Commission on planetary health. Lancet 2015, 386, 1973–2028. [Google Scholar] [CrossRef]
- Taylor, L.H.; Latham, S.M.; Woolhouse, M.E.J.; Lathamt, S.M.; Bush, E. Risk factors for human disease emergence. R. Soc. 2001, 356, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.K.E.; Patel, N.G.N.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global trends in emerging infectious diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.; Murray, K.A.; Zambrana-Torrelio, C.; Morse, S.S.; Rondinini, C.; Di Marco, M.; Breit, N.; Olival, K.J.; Daszak, P. Global hotspots and correlates of emerging zoonotic diseases. Nat. Commun. 2017, 8, 1124. [Google Scholar] [CrossRef] [PubMed]
- Karesh, W.B.; Dobson, A.; Lloyd-Smith, J.O.; Lubroth, J.; Dixon, M.A.; Bennett, M.; Aldrich, S.; Harrington, T.; Formenty, P.; Loh, E.H.; et al. Ecology of zoonoses: Natural and unnatural histories. Lancet 2012, 380, 1936–1945. [Google Scholar] [CrossRef]
- Vasilakis, N.; Cardosa, J.; Hanley, K.A.; Holmes, E.C.; Weaver, S.C. Fever from the forest: Prospects for the continued emergence of sylvatic dengue virus and its impact on public health. Nat. Rev. Microbiol. 2011, 9, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, A.; Randolph, S. Drivers, dynamics, and control of emerging vector-borne zoonotic diseases. Lancet 2012, 380, 1946–1955. [Google Scholar] [CrossRef]
- Weaver, S.C.; Reisen, W.K. Present and future arboviral threats. Antivir. Res. 2010, 85, 328–345. [Google Scholar] [CrossRef]
- Go, Y.Y.; Balasuriya, U.B.R.; Lee, C. Zoonotic encephalitides caused by arboviruses: Transmission and epidemiology of alphaviruses and flaviviruses. Clin. Exp. Vaccine Res. 2014, 3, 58–77. [Google Scholar] [CrossRef]
- Weaver, S.C.; Barrett, A.D.T. Transmission cycles, host range, evolution and emergence of arboviral disease. Nat. Rev. Microbiol. 2004, 2, 789–801. [Google Scholar] [CrossRef]
- Gubler, D. Vector-borne diseases. Rev. Sci. Tech. 2009, 28, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Kamgang, B.; Happi, J.Y.; Boisier, P.; Njiokou, F.; Hervé, J.P.; Simard, F.; Paupy, C. Geographic and ecological distribution of the dengue and chikungunya virus vectors Aedes aegypti and Aedes albopictus in three major Cameroonian towns. Med. Vet. Entomol. 2010, 24, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Weaver, S.C. Host range, amplification and arboviral disease emergence. Arch. Virol. Suppl. 2005, 19, 33–44. [Google Scholar]
- Bhatt, S.; Gething, P.; Brady, O.; Messina, J.; Farlow, A.; Moyes, C. The global distribution and burden of dengue. Nature 2012, 496, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.G.; Harris, E. Dengue. Lancet 2015, 385, 453–465. [Google Scholar] [CrossRef]
- Staples, E.; Fischer, M. Chikungunya virus in the Americas-what a vectorborne pathogen can do. N. Engl. J. Med. 2014, 371, 887–889. [Google Scholar] [CrossRef] [PubMed]
- Hills, S.; Fischer, M.; Petersen, L. Epidemiology of Zika Virus Infection. J. Infect. Dis. 2017, 216, 868–874. [Google Scholar] [CrossRef]
- Zanluca, C.; De Melo, V.C.A.; Mosimann, A.L.P.; Dos Santos, G.I.V.; dos Santos, C.N.D.; Luz, K. First report of autochthonous transmission of Zika virus in Brazil. Mem. Inst. Oswaldo Cruz 2015, 110, 569–572. [Google Scholar] [CrossRef]
- Ahmed, Q.; Memish, Z. Yellow fever from Angola and Congo: A storm gathers. Trop. Doct. 2017, 47, 92–96. [Google Scholar] [CrossRef]
- Possas, C.; Martins, R.M.; de Oliveira, R.L.; Homma, A. Urgent call for action: Avoiding spread and re-urbanisation of yellow fever in Brazil. Mem. Inst. Oswaldo Cruz 2018, 113, 1. [Google Scholar] [CrossRef]
- Cordellier, R. L’epidemiologie de la fièvre jaune en Afrique de l’Ouest. Bull. World Health Organ. 1991, 69, 73–84. [Google Scholar] [PubMed]
- Klitting, R.; Fischer, C.; Drexler, J.; Gould, E.; Roiz, D.; Paupy, C.; de Lamballerie, X. What Does the Future Hold for Yellow Fever Virus? (II). Genes (Basel) 2018, 9, 425. [Google Scholar] [CrossRef] [PubMed]
- Paupy, C.; Delatte, H.; Bagny, L.; Corbel, V.; Fontenille, D. Aedes albopictus, an arbovirus vector: From the darkness to the light. Microbes Infect. 2009, 11, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Hawley, W. The biology of Aedes albopictus. J. Am. Mosq. Control Assoc. 1988, 4, 1–39. [Google Scholar]
- Eritja, R.; Palmer, J.; Roiz, D.; Sanpera-Calbet, I.; Bartumeus, F. Direct Evidence of Adult Aedes albopictus Dispersal by Car. Sci. Rep. 2017, 7, 1e4399. [Google Scholar] [CrossRef] [PubMed]
- Savage, H.; Niebylski, M.; Smith, G.; Mitchell, C.; Craig, G. Host-feeding patterns of Aedes albopictus (Diptera: Culicidae) at a temperate North American site. J. Med. Entomol. 1993, 30, 27–34. [Google Scholar] [CrossRef]
- Pereira dos Santos, T.; Roiz, D.; de Abreu, F.; Luz Bessa, S.; Santa Lucia, M.; Jiolle, D.; Neves, N.; Simard, F.; Lourenço De Oliveira, R.; Paupy, C. Potential of Aedes albopictus as a bridge vector for zoonotic pathogens at the urban-forest interface of Brazil. Emerg. Microbes Infect. 2018, 7, 191. [Google Scholar] [CrossRef]
- Grard, G.; Caron, M.; Mombo, I.M.; Nkoghe, D.; Mboui Ondo, S.; Jiolle, D.; Fontenille, D.; Paupy, C.; Leroy, E.M. Zika Virus in Gabon (Central Africa) - 2007: A New Threat from Aedes albopictus? PLoS Negl. Trop. Dis. 2014, 8, e2681. [Google Scholar] [CrossRef]
- Leroy, E.M.; Nkoghe, D.; Ollomo, B.; Nze-Nkogue, C.; Becquart, P.; Grard, G.; Pourrut, X.; Charrel, R.; Moureau, G.; Ndjoyi-Mbiguino, A.; et al. Concurrent chikungunya and dengue virus infections during simultaneous outbreaks, Gabon, 2007. Emerg. Infect. Dis. 2009, 15, 591–593. [Google Scholar] [CrossRef]
- Delatte, H.; Desvars, A.; Bouétard, A.; Bord, S.; Gimonneau, G.; Vourc’h, G.; Fontenille, D. Blood-Feeding Behavior of Aedes albopictus, a Vector of Chikungunya on La Réunion. Vector-Borne Zoonotic Dis. 2010, 10, 249–258. [Google Scholar] [CrossRef]
- Flahault, A.; Aumont, G.; Boisson, V.; Lamballerie, X.; Favier, F.; Fontenille, D.; Gauzere, B.; Journeaux, S.; Lotteau, V.; Paupy, C.; et al. Chikungunya, La Réunion et Mayotte, 2005–2006: Une épidémie sans histoire? Sante Publique (Paris) 2007, 19, 165–195. [Google Scholar]
- Rezza, G.; Nicoletti, L.; Angelini, R.; Romi, R.; Finarelli, A.; Panning, M.; Cordioli, P.; Fortuna, C.; Boros, S.; Magurano, F.; et al. Infection with chikungunya virus in Italy: An outbreak in a temperate region. Lancet 2007, 370, 1840–1846. [Google Scholar] [CrossRef]
- Venturi, G.; Di Luca, M.; Fortuna, C.; Elena Remoli, M.; Riccardo, F.; Severini, F.; Toma, L.; Del Manso, M.; Benedetti, E.; Grazia Caporali, M.; et al. Detection of a chikungunya outbreak in Central Italy Detection of a chikungunya outbreak in Central. Euro Surveill. 2017, 22, 17–00646. [Google Scholar]
- Sawabe, K. Autochthonous dengue outbreak in Japan after a blank of 70 years and the future prediction of such cases. Med. Entomol. Zool. 2015, 66, 203–205. [Google Scholar] [CrossRef]
- Carvalho, R.G.; Lourenço-De-Oliveira, R.; Braga, I.A. Updating the geographical distribution and frequency of Aedes albopictus in Brazil with remarks regarding its range in the Americas. Mem. Inst. Oswaldo Cruz 2014, 109, 787–796. [Google Scholar] [CrossRef]
- Vanlandingham, D.; Higgs, S.; Huang, Y.-J. Aedes albopictus (Diptera: Culicidae) and Mosquito-Borne Viruses in the United States. J. Med. Entomol. 2016, 53, 1024–1028. [Google Scholar] [CrossRef]
- Bagny, L.; Delatte, H.; Elissa, N.; Quilici, S.; Fontenille, D.; Adhami, J.; Murati, N.; Adhami, J.; Reiter, P.; Beltrame, A.; et al. Aedes (Diptera: Culicidae) vectors of arboviruses in Mayotte (Indian Ocean): Distribution area and larval habitats. J. Med. Entomol. 2009, 46, 198–207. [Google Scholar] [CrossRef]
- Banerjee, S.; Aditya, G.; Saha, G.K. Household disposables as breeding habitats of dengue vectors: Linking wastes and public health. Waste Manag. 2013, 33, 233–239. [Google Scholar] [CrossRef]
- Dev, V. Dengue vectors in urban and suburban Assam, India: Entomological observations. Who South-East Asia J. Public Heal. 2014, 3, 5838. [Google Scholar] [CrossRef]
- Edillo, F.E.; Roble, N.D.; Otero, N.D. The key breeding sites by pupal survey for dengue mosquito vectors, Aedes aegypti (Linnaeus) and Aedes albopictus (Skuse), in Guba, Cebu City, Philippines. Southeast Asian J. Trop. Med. Public Health 2012, 43, 1365–1374. [Google Scholar]
- Rao, B.B.; George, B. Breeding patterns of aedes stegomyia albopictus in periurban areas of Calicut, Kerala, India. Southeast Asian J. Trop. Med. Public Health 2010, 41, 536–540. [Google Scholar] [PubMed]
- Shriram, A.N.; Sivan, A.; Sugunan, A.P. Spatial distribution of Aedes aegypti and Aedes albopictus in relation to geo-ecological features in South Andaman, Andaman and Nicobar Islands, India. Bull. Entomol. Res. 2018, 108, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Simard, F.; Nchoutpouen, E.; Toto, J.C.; Fontenille, D. Geographic distribution and breeding site preference of Aedes albopictus and Aedes aegypti (Diptera: Culicidae) in Cameroon, Central Africa. J. Med. Entomol. 2005, 42, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Thavara, U.; Tawatsin, A.; Chansang, C.; Kong-ngamsuk, W.; Paosriwong, S.; Boon-Long, J.; Rongsriyam, Y.; Komalamisra, N. Larval occurrence, oviposition behavior and biting activity of potential mosquito vectors of dengue on Samui Island, Thailand. J. Vector Ecol. 2001, 26, 172–180. [Google Scholar] [PubMed]
- Vijayakumar, K.; Sudheesh Kumar, T.K.; Nujum, Z.T.; Umarul, F.; Kuriakose, A. A study on container breeding mosquitoes with special reference to Aedes (Stegomyia) aegypti and Aedes albopictus in Thiruvananthapuram district, India. J. Vector Borne Dis. 2014, 51, 27–32. [Google Scholar]
- Forattini, O.P.; Brito, M. Brief Communication an Unusual Grround Larval Habitat of Aedes albopictus. Rev. Inst. Med. Trop. Sao Paulo 1998, 40, 121–122. [Google Scholar] [CrossRef]
- Marques, G.R.; Forattini, O.P. Aedes albopictus in soil bromeliads in Ilhabela, coastal area of Southeastern Brazil. Rev. Saude Publica 2005, 39, 548–552. [Google Scholar] [CrossRef]
- Mocellin, M.G.; Simões, T.C.; do Nascimento, T.F.S.; Teixeira, M.L.F.; Lounibos, L.P.; de Oliveira, R.L. Bromeliad-inhabiting mosquitoes in an urban botanical garden of dengue endemic Rio de Janeiro - Are bromeliads productive habitats for the invasive vectors Aedes aegypti and Aedes albopictus? Mem. Inst. Oswaldo Cruz 2009, 104, 1171–1176. [Google Scholar] [CrossRef]
- de Oliveira, V.C.; de Almeida Neto, L.C. Ocorrência de Aedes aegypti e Aedes albopictus em bromélias cultivadas no Jardim Botânico Municipal de Bauru, São Paulo, Brasil. Cad. Saude Publica 2017, 33, e00071016. [Google Scholar] [CrossRef]
- O’Meara, G.F.; Cutwa, M.M.; Evans, L.F. Bromeliad-inhabiting mosquitoes in south Florida: Native and exotic plants differ in species composition. J. Vector Ecol. 2003, 28, 37–46. [Google Scholar]
- Delatte, H.; Dehecq, J.S.; Thiria, J.; Domerg, C.; Paupy, C.; Fontenille, D. Geographic Distribution and Developmental Sites of Aedes albopictus (Diptera: Culicidae) During a Chikungunya Epidemic Event. Vector Control Southeast Asia 2008, 8, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Gilotra, S.K.; Rozeboom, L.E.; Bhattacharya, N.C. Observations on possible competitive displacement between populations of Aedes aegypti Linnaeus and Aedes albopictus Skuse in Calcutta. Bull. World Health Organ. 1967, 37, 437–446. [Google Scholar] [PubMed]
- Rozeboom, L.E.; Bridges, J.R. Relative population densities of Aedes albopictus and A. guamensis on Guam. Bull. World Health Organ. 1972, 46, 477–483. [Google Scholar] [PubMed]
- Sota, T.; Mogi, M.; Hayamizu, E. Seasonal distribution and habitat selection by Aedes albopictus and Aedes riversi (Diptera: Culicidae) in Northern Kyushu, Japan. J. Med. Entomol. 1992, 29, 296–304. [Google Scholar] [CrossRef]
- Gomes, A.; Forattini, O.; Kakitani, I.; Marques, G.; Marques, C.; Marucci, D.; Brito, M. Microhabitats de Aedes albopictus (Skuse) na região do Vale do Paraíba, Estado de São Paulo, Brasil. Rev. Saúde Pública S. Paulo 1992, 26, 108–118. [Google Scholar] [CrossRef]
- Müller, G.C.; Kravchenko, V.D.; Junnila, A.; Schlein, Y. Tree-hole breeding mosquitoes in Israel. J. Vector Ecol. 2012, 37, 102–109. [Google Scholar] [CrossRef]
- O’Meara, G.F.; Evans, L.F., Jr.; Womack, M.L. Colonization of rock holes by Aedes albopictus in the southeastern United States. J. Am. Mosq. Control Assoc. 1997, 13, 270–274. [Google Scholar]
- Sivan, A.; Shriram, A.N.; Sugunan, A.P.; Anwesh, M.; Muruganandam, N.; Kartik, C.; Vijayachari, P. Natural transmission of dengue virus serotype 3 by Aedes albopictus (Skuse) during an outbreak in Havelock Island: Entomological characteristics. Acta Trop. 2016, 156, 122–129. [Google Scholar] [CrossRef]
- Urbinatti, P.R.; Menezes, R.M.T.; Natal, D. Sazonalidade de Aedes albopictus em área protegida na cidade de São Paulo, Brasil. Rev. Saude Publica 2007, 41, 478–481. [Google Scholar] [CrossRef][Green Version]
- Pena, C.J.; Gonzalvez, G.; Chadee, D.D. Seasonal prevalence and container preferences of Aedes albopictus in Santo Domingo City, Dominican Republic. J. Vector Ecol. 2003, 28, 208–212. [Google Scholar]
- Kamgang, B.; Ngoagouni, C.; Manirakiza, A.; Nakouné, E.; Paupy, C.; Kazanji, M. Temporal Patterns of Abundance of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) and Mitochondrial DNA Analysis of Ae. albopictus in the Central African Republic. PLoS Negl. Trop. Dis. 2013, 7, e2590. [Google Scholar] [CrossRef] [PubMed]
- Marquetti, M.; Bisset, J.; Leyva, M.; Garcia, A.; Rodriguez, M. Comportamiento estacional y temporal de Aedes aegypti y Aedes albopictus en La Habana, Cuba. Rev. Cuba. De Med. Trop. 2008, 60, 62–67. [Google Scholar]
- Almeida, P.; Baptista, S.; Sousa, C.; Novo, T.; Ramos, H.; Panella, N.; Godsey, M.; Simões, M.J.; Anselmo, M.L.; Komar, N.; et al. Bioecology and Vectorial Capacity of Aedes albopictus (Diptera: Culicidae) in Macao, China, in Relation to Dengue Virus Transmission. J. Med. Entomol. 2005, 42, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Colless, D.H. Notes on the culicine mosquitoes of singapore. Ann. Trop. Med. Parasitol. 1959, 51, 87–101. [Google Scholar] [CrossRef]
- Dennett, J.A.; Bala, A.; Wuithiranyagool, T.; Randle, Y.; Sargent, C.B.; Guzman, H.; Siirin, M.; Hassan, H.K.; Reyna-Nava, M.; Unnasch, T.R.; et al. Associations between two mosquito populations and West Nile virus in Harris County, Texas, 2003-06. J. Am. Mosq. Control Assoc. 2007, 23, 264–275. [Google Scholar] [CrossRef]
- Faraji, A.; Egizi, A.; Fonseca, D.M.; Unlu, I.; Crepeau, T.; Healy, S.P.; Gaugler, R. Comparative Host Feeding Patterns of the Asian Tiger Mosquito, Aedes albopictus, in Urban and Suburban Northeastern USA and Implications for Disease Transmission. PLoS Negl. Trop. Dis. 2014, 8, e3037. [Google Scholar] [CrossRef] [PubMed]
- Gingrich, J.B.; Williams, G.M. Host-feeding patterns of suspected West Nile virus mosquito vectors in Delaware, 2001-2002. J. Am. Mosq. Control Assoc. 2005, 21, 194–200. [Google Scholar] [CrossRef]
- Gomes, A.; Silva, N.; Marques, G.; Brito, M. Host-feeding patterns of potential human disease vectors in the Paraiba Valley Region, State of Sao Paulo, Brazil. J. Vector Ecol. 2002, 28, 74–78. [Google Scholar]
- Kamgang, B.; Nchoutpouen, E.; Simard, F.; Paupy, C. Notes on the blood-feeding behavior of Aedes albopictus (Diptera: Culicidae) in Cameroon. Parasit. Vectors 2012, 5, 57. [Google Scholar] [CrossRef]
- Kek, R.; Hapuarachchi, H.C.; Chung, C.-Y.; Humaidi, M.B.; Razak, M.A.B.A.; Chiang, S.; Lee, C.; Tan, C.-H.; Yap, G.; Chong, C.-S.; et al. Feeding Host Range of Aedes albopictus (Diptera: Culicidae) Demonstrates Its Opportunistic Host-Seeking Behavior in Rural Singapore. J. Med. Entomol. 2014, 51, 880–884. [Google Scholar] [CrossRef]
- Kim, K.S.; Tsuda, Y.; Yamada, A. Bloodmeal Identification and Detection of Avian Malaria Parasite From Mosquitoes (Diptera: Culicidae) Inhabiting Coastal Areas of Tokyo Bay, Japan. J. Med. Entomol. 2009, 46, 1230–1234. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; mi Yu, H.; Lim, H.W.; Yang, S.C.; Roh, J.Y.; Chang, K.S.; Shin, E.H.; Ju, Y.R.; Lee, W.G. Host-feeding pattern and dengue virus detection of Aedes albopictus (Diptera: Culicidae) captured in an urban park in Korea. J. Asia. Pac. Entomol. 2017, 20, 809–813. [Google Scholar] [CrossRef]
- Muñoz, J.; Eritja, R.; Alcaide, M.; Montalvo, T.; Soriguer, R.C.; Figuerola, J. Host-Feeding Patterns of Native Culex pipiens and Invasive Aedes albopictus Mosquitoes (Diptera: Culicidae) in Urban Zones From Barcelona, Spain. J. Med. Entomol. 2011, 48, 956–960. [Google Scholar] [CrossRef] [PubMed]
- Niebylski, M.L.; Savage, H.M.; Nasci, R.S.; Craig, G.B. Blood hosts of Aedes albopictus in the United States. J. Am. Mosq. Control Assoc. 1994, 10, 447–450. [Google Scholar] [PubMed]
- Ponlawat, A.; Harrington, L.C. Blood Feeding Patterns of Aedes aegypti and Aedes albopictus in Thailand. J. Med. Entomol. 2005, 42, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.L.; Ponnusamy, L.; Unnasch, T.R.; Hassan, H.K.; Apperson, C.S. Host-Feeding Patterns of Aedes albopictus (Diptera: Culicidae) in Relation to Availability of Human and Domestic Animals in Suburban Landscapes of Central North Carolina. J. Med. Entomol. 2006, 43, 543–551. [Google Scholar] [CrossRef]
- Sawabe, K.; Isawa, H.; Hoshino, K.; Sasaki, T.; Roychoudhury, S.; Higa, Y.; Kasai, S.; Tsuda, Y.; Nishiumi, I.; Hisai, N.; et al. Host-Feeding Habits of Culex pipiens and Aedes albopictus (Diptera: Culicidae) Collected at the Urban and Suburban Residential Areas of Japan. J. Med. Entomol. 2010, 47, 442–450. [Google Scholar] [CrossRef]
- Sivan, A.; Shriram, A.; Sunish, I.; Vidhya, P. Host-feeding pattern of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in heterogeneous landscapes of South Andaman, Andaman and Nicobar Islands, India. Parasitol. Res. 2015, 114, 3539–3546. [Google Scholar] [CrossRef]
- Tandon, N.; Ray, S. Host feeding pattern of Aedes aegypti and Aedes albopictus in Kolkata, India. Denuge Bull. 2000, 24, 117–120. [Google Scholar]
- Tempelis, C.H.; Hayes, R.O.; Hess, A.D.; Reeves, W.C. Blood-feeding habits of four species of mosquito found in Hawaii. Am. J. Trop. Med. Hyg. 1970, 19, 335–341. [Google Scholar] [CrossRef]
- Valerio, L.; Francesca, M.; Bongiorno, G.; Facchinelli, L.; Pombi, M.; Caputo, B.; Maroli, M.; Torre, A. Della Host-Feeding Patterns of Aedes albopictus. Vector-Borne Zoonotic Dis. 2010, 10, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Egizi, A.; Healy, S.P.; Fonseca, D.M. Rapid blood meal scoring in anthropophilic Aedes albopictus and application of PCR blocking to avoid pseudogenes. Infect. Genet. Evol. 2013, 16, 122–128. [Google Scholar] [CrossRef]
- Tesh, R.B. Multiplication Phlebotomus phlebotomus fever group arboviruses in mosquitoes after intrathoracic inoculation. J. Med. Entomol. 1975, 12, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Tesh, R.B.; Shroyer, D.A. The mechanism of arbovirus transovarial transmission in mosquitoes: San Angelo virus in Aedes albopictus. Am. J. Trop. Med. Hyg. 1980, 29, 1394–1404. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, P.M.; Anderson, J.F.; Farajollahi, A.; Healy, S.P.; Unlu, I.; Crepeau, T.N.; Gaugler, R.; Fonseca, D.M.; Andreadis, T.G. Isolations of Cache Valley virus from Aedes albopictus (Diptera: Culicidae) in New Jersey and evaluation of its role as a regional arbovirus vector. J. Med. Entomol. 2013, 50, 1310–1314. [Google Scholar] [CrossRef]
- Ramachandra, R.; Sing, K.; Dhanda, V.; Bhattacharya, N. Experimental transmission of Chandipyura Virus by mosquitoes. Ind. J. Med. Res. 1967, 55, 1306–1310. [Google Scholar]
- Mitchell, C.J.; McLean, R.G.; Nasci, R.S.; Crans, W.J.; Smith, G.C.; Caccamise, D.F. Susceptibility parameters of Aedes albopictus to per oral infection with eastern equine encephalitis virus. J. Med. Entomol. 1993, 30, 233–235. [Google Scholar] [CrossRef][Green Version]
- Moncayo, A.C.; Edman, J.D.; Turell, M.J. Effect of eastern equine encephalomyelitis virus on the survival of Aedes albopictus, Anopheles quadrimaculatus, and Coquillettidia perturbans (Diptera: Culicidae). J. Med. Entomol. 2000, 37, 701–706. [Google Scholar] [CrossRef]
- Scott, T.W.; Lorenz, L.H.; Weaver, S.C. Susceptibility of Aedes albopictus to infection with eastern equine encephalomyelitis virus. J. Am. Mosq. Control Assoc. 1990, 6, 274–278. [Google Scholar]
- Turell, M.; Beaman, J.; Neely, G. Experimental transmission of Eastern equine encephalitis virus by strains of Aedes albopictus and A. taeniorhynchus (Diptera: Culicidae). J. Med. Entomol. 1994, 31, 287–290. [Google Scholar] [CrossRef]
- Takashima, I.; Hashimoto, N. Getah virus in several species of mosquitoes. Trans. R. Soc. Trop. Med. Hyg. 1985, 79, 546–550. [Google Scholar] [CrossRef]
- Tesh, R.B. Experimental studies on the transovarial transmission of Kunjin and San Angelo viruses in mosquitoes. Am. J. Trop. Med. Hyg. 1980, 29, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Grimstad, P.R. Recently Introduced Aedes albopictus in the United States: Potential vector of La Crosse Virus (Bunyaviridae: California Serogroup). J. Am. Mosq. Control Assoc. 1989, 5, 422–427. [Google Scholar]
- Rosen, L.; Tesh, R.B.; Lien, J.; John, H. Transovarial Transmission of Japanese Encephalitis Virus by Mosquitoes. Am. Assoc. Adv. Sci. 1978, 199, 909–911. [Google Scholar] [CrossRef] [PubMed]
- Weng, M.H.; Lien, J.C.; Wang, Y.M.; Wu, H.L.; Chin, C. Susceptibility of three laboratory strains of Aedes albopictus (Diptera: Culicidae) to Japanese encephalitis virus from Taiwan. J. Med. Entomol. 1997, 34, 745–747. [Google Scholar] [CrossRef] [PubMed]
- Wispelaere, M.; Desprès, P.; Choumet, V. European Aedes albopictus and Culex pipiens Are Competent Vectors for Japanese Encephalitis Virus. PLoS Negl. Trop. Dis. 2017, 11, e0005294. [Google Scholar] [CrossRef]
- Cully, J.F., Jr.; Streit, T.G.; Heard, P.B. Transmission of La Crosse virus by four strains of Aedes albopictus to and from the eastern chipmunk (Tamias striatus). J. Am. Mosq. Control Assoc. 1992, 8, 237–240. [Google Scholar]
- Hughes, M.T.; Gonzalez, J.A.; Reagan, K.L.; Carol, D.; Beaty, B.J.; Beaty, B.J. Comparative Potential of Aedes triseriatus, Aedes albopictus, and Aedes aegypti (Diptera: Culicidae) to Transovarially Transmit La Crosse Virus Comparative Potential of Aedes triseriatus, Aedes albopictus, and Aedes aegypti (Diptera: Culicidae). Entomol. Soc. Am. 2006, 43, 757–761. [Google Scholar]
- Lambert, A.J.; Blair, C.D.; D’Anton, M.; Ewing, W.; Harborth, M.; Seiferth, R.; Xiang, J.; Lanciotti, R.S. La Crosse Virus in Aedes albopictus Mosquitoes, Texas, USA, 2009. Emerg. Infect. Dis. 2010, 16, 856–858. [Google Scholar] [CrossRef]
- Smith, G.C.; Francy, D.B. Laboratory studies of a Brazilian strain of Aedes albopictus as a potential vector of Mayaro and Oropouche viruses. J. Am. Mosq. Control Assoc. 1991, 7, 89–93. [Google Scholar]
- Tomori, O.; Aitken, T.H. Orungo virus: Transmission studies with Aedes albopictus and Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 1978, 14, 523–526. [Google Scholar] [PubMed]
- Francy, D.; Krabatsos, N.; Wesson, C.; Moore, J.; Lazuick, J.; Niebylski, T.; Tsai, T.; Craig, J. A new Arbovirus from Aedes albopictus, an Asian mosquito Established in the United States. Science 1990, 250, 1738–1740. [Google Scholar] [CrossRef] [PubMed]
- Heard, P.B.; Niebylski, M.L.; Francy, D.B. Transmission of a Newly Recognized Virus (Bunyaviridae, Bunyavirus) Isolated from Aedes albopictus (Diptera: Culicidae) in Potosi, Missouri. J. Med. Entomol. 1991, 28, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.J.; Smith, G.C.; Miller, B.R. Vector Competence of Aedes Albopictus for a Newly Recognized Bunyavirus From Mosquitoes Collected in Potosi, Missouri1. J. Am. Mosq. Control Assoc. 1990, 6, 523–527. [Google Scholar] [PubMed]
- Brustolin, M.; Talavera, S.; Nunez, A.; SantamarÍa, C.; Rivas, R.; Pujol, N.; Valle, M.; Verdun, M.; Brun, A.; Pages, N.; et al. Rift Valley fever virus and European mosquitoes: Vector competence of Culex pipiens and Stegomyia albopicta (= Aedes albopictus). Med. Vet. Entomol. 2017, 31, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Turell, M.J.; Bailey, C.L.; Beaman, J.R. Vector competence of a Houston, Texas strain of Aedes albopictus for Rift Valley fever virus. J. Am. Mosq. Control Assoc. 1988, 4, 94–96. [Google Scholar]
- Mitchell, C.J.; Gubler, D.J. Vector competence of geographic strains of Aedes albopictus and Aedes polynesiensis and certain other Aedes (Stegomyia) mosquitoes for Ross River virus. J. Am. Mosq. Control Assoc. 1987, 3, 142–147. [Google Scholar]
- Mitchell, C.J.; Miller, B.R.; Gubler, D.J. Vector competence of Aedes albopictus from Houston, Texas, for dengue serotypes 1 to 4, yellow fever and Ross River viruses. J. Am. Mosq. Control Assoc. 1987, 3, 460–465. [Google Scholar]
- Shroyer, D.A. Transovarial Maintenance of San Angelo Virus in Sequenctial Generations of Aedes albopictus. Am J Trop Med Hyg. 1986, 35, 408–417. [Google Scholar] [CrossRef]
- Hardy, J.L.; Rosen, L.; Kramer, L.D.; Presser, S.B.; Shroyer, D.A.; Turell, M.J. Effect of rearing temperature on transovarial transmission of St. Louis encephalitis virus in mosquitoes. Am. J. Trop. Med. Hyg. 1980, 29, 963–968. [Google Scholar] [CrossRef]
- Mitchell, C.J. Vector competence of North and South American strains of Aedes albopictus for certain arboviruses: A review. J. Am. Mosq. Control Assoc. 1991, 7, 446–451. [Google Scholar] [PubMed]
- Puggioli, A.; Bonilauri, P.; Calzolari, M.; Lelli, D.; Carrieri, M.; Urbanelli, S.; Pudar, D.; Bellini, R. Does Aedes albopictus (Diptera: Culicidae) play any role in Usutu virus transmission in Northern Italy? Experimental oral infection and field evidences. Acta Trop. 2017, 172, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Beaman, J.R.; Turell, M.J. Transmission of Venezuelan Equine Encephalomyelitis Virus by Strains of Aedes albopictus (Diptera: Culicidae) Collected from North and South America. J. Med. Entomol. 1991, 28, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, Z.; Moncayo, A.C.; Carrara, A.S.; Forattini, O.P.; Weaver, A.S.C. Vector Competence of Rural and Urban Strains of Aedes (Stegomyia) albopictus (Diptera: Culicidae) from São Paulo State, Brazil for IC, ID, and IF Subtypes of Venezuelan Equine Encephalitis Virus. J. Med. Entomol 2003, 40, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Turell, M.J.; Beaman, J.R. Experimental Transmission of Venezuelan Equine Encephalomyelitis Virus by a strain of Aedes albopictus (Diptera: Culicidae) from New Orleans, Lousiana. J. Med. Entomol. 1992, 29, 802–807. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Baqar, S.; Hayes, C.G.; Murphy, J.R.; Watts, D.M. Vertical transmission of West Nile virus by Culex and Aedes species mosquitoes. Am. J. Trop. Med. Hyg. 1993, 48, 757–762. [Google Scholar] [CrossRef]
- Brustolin, M.; Talavera, S.; Santamaría, C.; Rivas, R.; Pujol, N.; Aranda, C.; Marquès, E.; Valle, M.; Verdún, M.; Pagès, N.; et al. Culex pipiens and Stegomyia albopicta (=Aedes albopictus) populations as vectors for lineage 1 and 2 West Nile virus in Europe. Med. Vet. Entomol. 2016, 30, 166–173. [Google Scholar] [CrossRef]
- Cupp, E.W.; Hassan, H.K.; Yue, X.; Oldland, W.K.; Lilley, B.M.; Unnasch, T.R. West Nile Virus Infection in Mosquitoes in the Mid-South USA, 2002–2005. J. Med. Entomol. 2007, 44, 117–125. [Google Scholar] [CrossRef]
- Farajollahi, A.; Kesavaraju, B.; Price, D.C.; Williams, G.M.; Healy, S.P.; Gaugler, R.; Nelder, M.P. Field efficacy of BG-Sentinel and industry-standard traps for Aedes albopictus (Diptera: Culicidae) and West Nile virus surveillance. J. Med. Entomol. 2009, 46, 919–925. [Google Scholar] [CrossRef]
- Holick, J.; Kyle, A.; Ferraro, W.; Delaney, R.; Marta, I. Discovery of Aedes albopictus infected with west nile virus in Southeastern Pennsylvania. J. Am. Mosq. Control Assoc. Inc. 2002, 18, 131. [Google Scholar]
- Philip, C.B.; Smadel, J.E. Transmission of West Nile Virus by Infected Aedes albopictus. Proc. Soc. Exp. Biol. Med. 1943, 53, 49–50. [Google Scholar] [CrossRef]
- Sardelis, M.R.; Turell, M.J.; Guinn, M.L.O.; Andre, R.G.; Roberts, D.R. Vector Competence of Three North American Strains of Aedes Albopictus for West Nile Virus1. J. Am. Mosq. Control Assoc. 2002, 18, 284–289. [Google Scholar] [PubMed]
- Tiawsirisup, S.; Platt, K.B.; Evans, R.B.; Rowley, W.A. A Comparision of West Nile Virus Transmission by Ochlerotatus trivittatus (COQ.), Culex pipiens (L.), and Aedes albopictus (Skuse). Vector-Borne Zoonotic Dis. 2005, 5, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Vanlandingham, D.L.; McGee, C.E.; Klinger, K.A.; Vessey, N.; Fredregillo, C.; Higgs, S. Short report: Relative susceptibilties of South Texas mosquitoes to infection with West Nile virus. Am. J. Trop. Med. Hyg. 2007, 77, 925–928. [Google Scholar] [CrossRef]
- Fortuna, C.; Remoli, M.E.; Severini, F.; Di Luca, M.; Toma, L.; Fois, F.; Bucci, P.; Boccolini, D.; Romi, R.; Ciufolini, M.G. Evaluation of vector competence for West Nile virus in Italian Stegomyia albopicta (=Aedes albopictus) mosquitoes. Med. Vet. Entomol. 2015, 29, 430–433. [Google Scholar] [CrossRef]
- Amraoui, F.; Ayed, W.B.; Madec, Y.; Faraj, C.; Himmi, O.; Btissam, A.; Sarih, M.; Failloux, A.B. Potential of Aedes albopictus to cause the emergence of arboviruses in Morocco. PLoS Negl. Trop. Dis. 2019, 13, e0006997. [Google Scholar] [CrossRef]
- Couto-Lima, D.; Madec, Y.; Bersot, M.I.; Campos, S.S.; Motta, M.D.A.; Dos Santos, F.B.; Vazeille, M.; Da Costa Vasconcelos, P.F.; Lourenço-De-Oliveira, R.; Failloux, A.B. Potential risk of re-emergence of urban transmission of Yellow Fever virus in Brazil facilitated by competent Aedes populations. Sci. Rep. 2017, 7, 4848. [Google Scholar] [CrossRef]
- Johnson, B.; Chambers, T.; Grabtree, M.; Filippis, A.; Vilarinhos, P.; Resende, M.; Marcoris, L.; Miller, B. Vector competence of Brazilian Aedes aegypti and Ae. abopictus for a Brazilian yellow fever virus isolate *. Trans. R. Soc. Trop. Med. Hyg. 2002, 96, 611–613. [Google Scholar] [CrossRef]
- Lourenço de Oliveira, R.; Vazeille, M.; Maria, A.N.A.; Filippis, B.D.E.; Failloux, A. Large genetic differentiation and low variation in vector competence for dengue and yellow fever viruses of Aedes albopictus from Brazil, the United States, and the Cayman Islands. Am. J. Trop. Med. Hyg. 2003, 69, 105–114. [Google Scholar] [CrossRef]
- Miller, B.R.; Mitchell, C.J.; Ballinger, M.E. Replication, tissue tropisms and transmission of yellow fever virus in Aedes albopictus. Trans. R. Soc. Trop. Med. Hyg. 1989, 83, 252–255. [Google Scholar] [CrossRef]
- Ferreira-De-Lima, V.H.; Lima-Camara, T.N. Natural vertical transmission of dengue virus in Aedes aegypti and Aedes albopictus: A systematic review. Parasites Vectors 2018, 11, 77. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.J.; Haramis, L.D.; Karabatsos, N.; Smith, G.C.; Starwalt, V.J. Isolation of La Crosse, Cache Valley, and Potosi Viruses from Aedes Mosquitoes (Diptera: Culicidae) Collected at Used-Tire Sites in Illinois during 1994-1995. J. Med. Entomol. 1998, 35, 573–577. [Google Scholar] [CrossRef]
- Mitchell, C.J.; Niebylski, M.L.; Smith, G.C.; Karabatsos, N.; Martin, D.; Mutebi, J.P.; Craig, G.B.J.; Mahler, M.J. Isolation of eastern equine encephalitis virus from Aedes albopictus in Florida. Science 1992, 257, 526–527. [Google Scholar] [CrossRef] [PubMed]
- Westby, K.M.; Fritzen, C.; Paulsen, D.; Poindexter, S.; Moncayo, A.C. La Crosse Encephalitis Virus Infection in Field-Collected Aedes albopictus, Aedes japonicus, and Aedes triseriatus in Tennessee. J. Am. Mosq. Control Assoc. 2015, 31, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, R.R.; Gottfried, K.L.; Apperson, C.S.; Davis, B.S.; Erwin, P.C.; Smith, A.B.; Panella, N.A.; Powell, E.E.; Nasci, R.S. First isolation of La Crosse virus from naturally infected Aedes albopictus. Emerg. Infect. Dis. 2001, 7, 807–811. [Google Scholar] [CrossRef]
- Harrison, B.A.; Mitchell, C.J.; Apperson, C.S.; Smith, G.C.; Karabatsos, N.; Engber, B.R.; Newton, N.H. Isolation of potosi virus from Aedes albopictus in North Carolina. J. Am. Mosq. Control Assoc. 1995, 11, 225–229. [Google Scholar]
- Fortuna, C.; Remoli, M.E.; Di Luca, M.; Severini, F.; Toma, L.; Benedetti, E.; Bucci, P.; Montarsi, F.; Minelli, G.; Boccolini, D.; et al. Experimental studies on comparison of the vector competence of four Italian Culex pipiens populations for West Nile virus. Parasit. Vectors 2015, 8, 463. [Google Scholar] [CrossRef]
- Fros, J.J.; Miesen, P.; Vogels, C.B.; Gaibani, P.; Sambri, V.; Martina, B.E.; Koenraadt, C.J.; van Rij, R.P.; Vlak, J.M.; Takken, W.; et al. Comparative Usutu and West Nile virus transmission potential by local Culex pipiens mosquitoes in north-western Europe. One Heal. 2015, 1, 31–36. [Google Scholar] [CrossRef]
- Goddard, L.B.; Roth, A.E.; Reisen, W.K.; Scott, T.W.; States, U. Vector Competence of California Mosquitoes for. Emerg. Infect. Dis. 2002, 8, 1385–1391. [Google Scholar] [CrossRef]
- Ebel, G.D.; Rochlin, I.; Longacker, J.; Kramer, L.D. Culex restuans (Diptera: Culicidae) relative abundance and vector competence for West Nile Virus. J. Med. Entomol. 2005, 42, 838–843. [Google Scholar] [CrossRef]
- Kilpatrick, A.; Fonseca, D.M.; Ebel, G.D.; Reddy, M.R.; Kramer, L.D. Spatial and temporal variation in vector competence of Culex pipiens and Cx. restuans mosquitoes for West Nile virus. Am. J. Trop. Med. Hyg. 2010, 83, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.L.; Mores, C.N.; Lord, C.C.; Tabachnick, W.J. Impact of Extrinsic Incubation Temperature and Virus Exposure on Vector Competence of Culex pipiens quinquefasciatus Say (Diptera: Culicidae) for West Nile Virus. Vector-Borne Zoonotic Dis. 2007, 7, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.L.; Anderson, S.L.; Lord, C.C. Vector competence of Culex pipiens quinquefasciatus (Diptera: Culicidae) for West Nile virus isolates from Florida. Trop. Med. Int. Heal. 2014, 19, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.L.; Richards, S.L.; Tabachnick, W.J.; Smartt, C.T. Effects of West Nile Virus Dose and Extrinsic Incubation Temperature on Temporal Progression of Vector Competence in Culex pipiens quinquefasciatus. J. Am. Mosq. Control Assoc. 2010, 26, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Turell, M.J.; Guinn, M.L.O.; Dohm, D.J.; Jones, J.W. Vector Competence of North American Mosquitoes (Diptera: Culicidae) for West Nile Virus Vector Competence of North American Mosquitoes (Diptera: Culicidae) for West Nile Virus. J. Med. Entomol. 2001, 38, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, R.; Fleisher, A.E.; Minnick, S.L.; Simmons, K.A.; Scott, T.W. Nutritional Stress Affects Mosquito Survival and Vector Competence for West Nile Virus. Vector-Borne Zoonotic Dis. 2008, 8, 727–732. [Google Scholar] [CrossRef]
- Paupy, C.; Ollomo, B.; Kamgang, B.; Moutailler, S.; Rousset, D.; Demanou, M.; Hervé, J.-P.; Leroy, E.; Simard, F. Comparative role of Aedes albopictus and Aedes aegypti in the emergence of Dengue and Chikungunya in central Africa. Vector Borne Zoonotic Dis. 2010, 10, 259–266. [Google Scholar] [CrossRef]
- Vega-Rúa, A.; Lourenço-De-Oliveira, R.; Mousson, L.; Vazeille, M.; Fuchs, S.; Yébakima, A.; Gustave, J.; Girod, R.; Dusfour, I.; Leparc-Goffart, I.; et al. Chikungunya Virus Transmission Potential by Local Aedes Mosquitoes in the Americas and Europe. PLoS Negl. Trop. Dis. 2015, 9, e0003780. [Google Scholar] [CrossRef]
- Vega-Rua, A.; Zouache, K.; Caro, V.; Diancourt, L.; Delaunay, P.; Grandadam, M.; Failloux, A.B. High Efficiency of Temperate Aedes albopictus to Transmit Chikungunya and Dengue Viruses in the Southeast of France. PLoS ONE 2013, 8, e59716. [Google Scholar] [CrossRef]
- Honório, N.A.; Wiggins, K.; Câmara, D.C.P.; Eastmond, B.; Alto, B.W. Chikungunya virus vector competency of Brazilian and Florida mosquito vectors. PLoS Negl. Trop. Dis. 2018, 12, e0006521. [Google Scholar] [CrossRef]
- Fortuna, C.; Toma, L.; Remoli, M.E.; Amendola, A.; Severini, F.; Boccolini, D.; Romi, R.; Venturi, G.; Rezza, G.; Di Luca, M. Vector competence of Aedes albopictus for the Indian Ocean Lineage (IOL) chikungunya viruses of the 2007 and 2017 outbreaks in italy: A comparison between strains with and without the E1:A226V mutation. Eurosurveillance 2018, 23, 1800246. [Google Scholar] [CrossRef] [PubMed]
- Ngoagouni, C.; Kamgang, B.; Kazanji, M.; Paupy, C.; Nakouné, E. Potential of Aedes aegypti and Aedes albopictus populations in the Central African Republic to transmit enzootic chikungunya virus strains. Parasites Vectors 2017, 10, 164. [Google Scholar] [CrossRef] [PubMed]
- Calvez, E.; Guillaumot, L.; Girault, D.; Richard, V.; O’Connor, O.; Paoaafaite, T.; Teurlai, M.; Pocquet, N.; Cao-Lormeau, V.M.; Dupont-Rouzeyrol, M. Dengue-1 virus and vector competence of Aedes aegypti (Diptera: Culicidae) populations from New Caledonia. Parasites Vectors 2017, 10, 381. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, C.M.; Melo, F.F.; Bezerra, J.M.T.; Chaves, B.A.; Silva, B.M.; Silva, L.D.; Pessanha, J.E.M.; Arias, J.R.; Secundino, N.F.C.; Norris, D.E.; et al. Distinct variation in vector competence among nine field populations of Aedes aegypti from a Brazilian dengue-endemic risk city. Parasites Vectors 2014, 7, 320. [Google Scholar] [CrossRef]
- Poole-Smith, B.K.; Hemme, R.R.; Delorey, M.; Felix, G.; Gonzalez, A.L.; Amador, M.; Hunsperger, E.A.; Barrera, R. Comparison of Vector Competence of Aedes mediovittatus and Aedes aegypti for Dengue Virus: Implications for Dengue Control in the Caribbean. PLoS Negl. Trop. Dis. 2015, 9, e0003462. [Google Scholar] [CrossRef]
- Richards, S.L.; Anderson, S.L.; Alto, B.W. Vector Competence of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) for Dengue Virus in the Florida Keys. J. Med. Entomol. 2012, 49, 942–946. [Google Scholar] [CrossRef]
- Brustolin, M.; Santamaria, C.; Napp, S.; VerdÚn, M.; Rivas, R.; Pujol, N.; Talavera, S.; Busquets, N. Experimental study of the susceptibility of a European Aedes albopictus strain to dengue virus under a simulted Mediterranean temperature regime. Med. Vet. Entomol. 2018, 32, 393–398. [Google Scholar] [CrossRef]
- Chepkorir, E.; Lutomiah, J.; Mutisya, J.; Mulwa, F.; Limbaso, K.; Orindi, B.; Ng’Ang’a, Z.; Sang, R. Vector competence of Aedes aegypti populations from Kilifi and Nairobi for dengue 2 virus and the influence of temperature. Parasites Vectors 2014, 7, 435. [Google Scholar] [CrossRef]
- Diallo, M.; Ba, Y.; Faye, O.; Soumare, L.; Dia, I.; Sall, A.A. Vector competence of Aedes aegypti populations from Senegal for sylvatic and epidemic dengue 2 virus isolated in West Africa. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, 493–498. [Google Scholar] [CrossRef]
- Heitmann, A.; Jansen, S.; Lühken, R.; Leggewie, M.; Badusche, M.; Pluskota, B.; Becker, N.; Vapalahti, O.; Schmidt-Chanasit, J.; Tannich, E. Experimental transmission of zika virus by mosquitoes from central Europe. Eurosurveillance 2017, 22, 14–17. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, T.; Lai, Z.; Zhang, Z.; Jia, Z.; Zhou, G.; Williams, T. Competence of Aedes aegypti, Aedes albopictus, and Culex quinquefasciatus mosquitoes as Zika Virus Vectors, China. Emerg. Infect. Dis. 2017, 23, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Di Luca, M.; Severini, F.; Toma, L.; Boccolini, D.; Romi, R.; Remoli, M.E.; Sabbatucci, M.; Rizzo, C.; Venturi, G.; Rezza, G. Experimental studies of susceptibility of Italian Aedes albopictus to Zika virus. Euro Surveill. 2016, 21, 18. [Google Scholar]
- Hugo, L.E.; Stassen, L.; La, J.; Gosden, E.; Ekwudu, O.; Winterford, C.; Viennet, E.; Faddy, H.M.; Devine, G.J.; Frentiu, F.D. Vector competence of Australian Aedes aegypti and Aedes albopictus for an epidemic strain of Zika virus. PLoS Negl. Trop. Dis. 2019, 13, e0007281. [Google Scholar] [CrossRef] [PubMed]
- González, M.A.; Pavan, M.G.; Fernandes, R.S.; Busquets, N.; David, M.R.; Lourenço-Oliveira, R.; García-Pérez, A.L.; MacIel-De-Freitas, R. Limited risk of Zika virus transmission by five Aedes albopictus populations from Spain. Parasites Vectors 2019, 12, 150. [Google Scholar] [CrossRef]
- Bonica, M.B.; Goenaga, S.; Martin, M.L.; Feroci, M.; Luppo, V.; Muttis, E.; Fabbri, C.; Morales, M.A.; Enria, D.; Micieli, M.V.; et al. Vector competence of Aedes aegypti for different strains of Zika virus in Argentina. PLoS Negl. Trop. Dis. 2019, 13, e0007433. [Google Scholar] [CrossRef]
- Main, B.J.; Nicholson, J.; Winokur, O.C.; Steiner, C.; Riemersma, K.K.; Stuart, J.; Takeshita, R.; Krasnec, M.; Barker, C.M.; Coffey, L.L. Vector competence of Aedes aegypti, Culex tarsalis, and Culex quinquefasciatus from California for Zika virus. PLoS Negl. Trop. Dis. 2018, 12, e0006524. [Google Scholar] [CrossRef]
- Calvez, E.; O’Connor, O.; Pol, M.; Rousset, D.; Faye, O.; Richard, V.; Tarantola, A.; Dupont-Rouzeyrol, M. Differential transmission of Asian and African Zika virus lineages by Aedes aegypti from New Caledonia. Emerg. Microbes Infect. 2018, 7, 159. [Google Scholar] [CrossRef]
- Chouin-Carneiro, T.; Vega-Rua, A.; Vazeille, M.; Yebakima, A.; Girod, R.; Goindin, D.; Dupont-Rouzeyrol, M.; Lourenço-de-Oliveira, R.; Failloux, A.B. Differential Susceptibilities of Aedes aegypti and Aedes albopictus from the Americas to Zika Virus. PLoS Negl. Trop. Dis. 2016, 10, e4543. [Google Scholar] [CrossRef]
- Hery, L.; Boullis, A.; Delannay, C.; Vega-Rúa, A. Transmission potential of African, Asian and American Zika virus strains by Aedes aegypti and Culex quinquefasciatus from Guadeloupe (French West Indies). Emerg. Microbes Infect. 2019, 8, 699–706. [Google Scholar] [CrossRef]
- Richard, V.; Paoaafaite, T.; Cao-Lormeau, V.M. Vector Competence of French Polynesian Aedes aegypti and Aedes polynesiensis for Zika Virus. PLoS Negl. Trop. Dis. 2016, 10, e0005024. [Google Scholar] [CrossRef]
- Hall-Mendelin, S.; Pyke, A.T.; Moore, P.R.; Ritchie, S.A.; Moore, F.A.J.; Van Den Hurk, A.F. Characterization of a Western Pacific Zika Virus Strain in Australian Aedes aegypti. Vector-Borne Zoonotic Dis. 2018, 18, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Ellis, B.R.; Sang, R.C.; Horne, K.M.; Higgs, S.; Wesson, D.M. Yellow fever virus susceptibility of two mosquito vectors from Kenya, East Africa. Trans. R. Soc. Trop. Med. Hyg. 2012, 106, 387–389. [Google Scholar] [CrossRef] [PubMed]
- Yen, P.S.; Amraoui, F.; Rúa, A.V.; Failloux, A.B. Aedes aegypti mosquitoes from guadeloupe (French west indies) are able to transmit yellow fever virus. PLoS ONE 2018, 13, e0204710. [Google Scholar] [CrossRef] [PubMed]
- Van Den Hurk, A.F.; McElroy, K.; Pyke, A.T.; McGee, C.E.; Hall-Mendelin, S.; Day, A.; Ryan, P.A.; Ritchie, S.A.; Vanlandingham, D.L.; Higgs, S. Vector competence of Australian mosquitoes for yellow fever virus. Am. J. Trop. Med. Hyg. 2011, 85, 446–451. [Google Scholar] [CrossRef]
- Jupp, P.G.; Kemp, A. Laboratory vector competence experiments with yellow fever virus and five South African mosquito species including Aedes aegypti. Trans R Soc Trop Med Hyg 2002, 96, 493–498. [Google Scholar] [CrossRef]
- Focks, D.A. Review of Entomological Sampling Methods and Indicators for Dengue Vectors; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- De Abreu, F.V.S.; Delatorre, E.; Dos Santos, A.A.C.; Ferreira-De-Brito, A.; De Castro, M.G.; Ribeiro, I.P.; Furtado, N.D.; Vargas, W.P.; Ribeiro, M.S.; Meneguete, P.; et al. Combination of surveillance tools reveals that yellow fever virus can remain in the same atlantic forest area at least for three transmission seasons. Mem. Inst. Oswaldo Cruz 2019, 114, e190076. [Google Scholar] [CrossRef]
- Silver, J.B. Mosquito Ecology: Field Sampling Methods, 3rd ed.; Springer Netherlands: Dordrecht, The Netherlands, 2008. [Google Scholar]
- De Abreu, F.V.S.; Ribeiro, I.P.; Ferreira-de-Brito, A.; dos Santos, A.A.C.; de Miranda, R.M.; Bonelly, I.; Neves, M.S.A.S.; Bersot, M.I.; dos Santos, T.P.; Gomes, M.Q.; et al. Haemagogus leucocelaenus and Haemagogus janthinomys are the primary vectors in the major yellow fever outbreak in Brazil, 2016–2018. Emerg. Microbes Infect. 2019, 8, 218–231. [Google Scholar] [CrossRef]
- Kilpatrick, A.; Kramer, L.; Campbell, S.; Alleyne, O.; Dobson, A.; Daszak, P. West Nile Virus Risk Assessment and the Bridge Vector Paradigm. Emerg. Infect. Dis. 2005, 11, 425–429. [Google Scholar] [CrossRef]
- Muñoz, J.; Ruiz, S.; Soriguer, R.; Alcaide, M.; Viana, D.S.; Roiz, D.; Vázquez, A.; Figuerola, J. Feeding patterns of potential West Nile virus vectors in south-west Spain. PLoS ONE 2012, 7, 3954. [Google Scholar] [CrossRef]
- Failloux, A.; Vazeille, M.; Rodhain, F. Geographic genetic variation in populations of the dengue virus vector Aedes aegypti. J. Mol. Evol. 2002, 55, 653–663. [Google Scholar] [CrossRef]
- Lambrechts, L.; Chevillon, C.; Albright, R.; Thaisomboonsuk, B.; Richardson, J.; Jarman, R.; Scott, T. Genetic specificity and potential for local adaptation between dengue viruses and mosquito vectors. Biomed Cent. Evol. Biol. 2009, 9, 160. [Google Scholar] [CrossRef] [PubMed]
- Zouache, K.; Fontaine, A.; Vega-Rua, A.; Mousson, L.; Thiberge, J.M.; Lourenco-De-Oliveira, R.; Caro, V.; Lambrechts, L.; Failloux, A.B. Three-way interactions between mosquito population, viral strain and temperature underlying chikungunya virus transmission potential. Proc. R. Soc. Bbiol. Sci. 2014, 281, 20141078. [Google Scholar] [CrossRef] [PubMed]
- Possas, C.; Lourenço de Oliveira, R.; Tauil, P.L.; Pinheiro, F.D.P.; Pissinatti, A.; Venancio da Cunha, R.; Freire, M.; Menezes Martins, R.; Homma, A. Yellow Fever outbreak in Brazil: The puzzle of rapid viral spread and challenges for immunization. Mem. Inst. Oswaldo Cruz 2018, 113, e180278. [Google Scholar] [CrossRef] [PubMed]
- Amraoui, F.; Pain, A.; Piorkowski, G.; Vazeille, M.; Couto-Lima, D.; de Lamballerie, X.; Lourenço-de-Oliveira, R.; Failloux, A.B. Experimental Adaptation of the Yellow Fever Virus to the Mosquito Aedes albopictus and Potential risk of urban epidemics in Brazil, South America. Sci. Rep. 2018, 8, 14337. [Google Scholar] [CrossRef]
- Tsetsarkin, K.A.; Weaver, S.C. Sequential adaptive mutations enhance efficient vector switching by chikungunya virus and its epidemic emergence. Plos Pathog. 2011, 7, e1002412. [Google Scholar] [CrossRef]
- Forattini, O.P.; Marques, G.R.A.M.; Kakitani, I.; De Brito, M.; Sallum, M.A.M. Significado epidemiológico dos criadouros de Aedes albopictus em bromélias. Rev. Saude Publica 1998, 32, 186–188. [Google Scholar] [CrossRef]
- Li, Y.; Kamara, F.; Zhou, G.; Puthiyakunnon, S.; Li, C.; Liu, Y.; Zhou, Y.; Yao, L.; Yan, G.; Chen, X.G. Urbanization Increases Aedes albopictus Larval Habitats and Accelerates Mosquito Development and Survivorship. PLoS Negl. Trop. Dis. 2014, 8, e3301. [Google Scholar] [CrossRef]
- Hiscox, A.; Kaye, A.; Vongphayloth, K.; Banks, I.; Piffer, M.; Khammanithong, P.; Sananikhom, P.; Kaul, S.; Hill, N.; Lindsay, S.W.; et al. Risk factors for the presence of Aedes aegypti and Aedes albopictus in domestic water-holding containers in areas impacted by the Nam Theun 2 hydroelectric project, Laos. Am. J. Trop. Med. Hyg. 2013, 88, 1070–1078. [Google Scholar] [CrossRef]
- Amerasinghe, F.P.; Alagoda, T.S.B. Mosquito oviposition in bamboo traps, with special reference to Aedes albopictus, Aedes novalbopictus and Armigeres subalbatus. Insect Sci. Applic. 1984, 5, 493–500. [Google Scholar] [CrossRef]
- Brito, M.D.; Forattini, O.P. Produtividade de criadouros de Aedes aegypti no Vale do ParaIba, SP, Brasil. Rev. Saúde Pública 2004, 38, 209–215. [Google Scholar] [CrossRef]
- Chan, K.L.; Ho, B.C.; Chan, Y.C. Aedes aegypti (L.) and Aedes albopictus (Skuse) in Singapore City. 2. Larval habitats. Bull. World Health Organ. 1971, 44, 629–633. [Google Scholar] [PubMed]
- Delatte, H.; Toty, C.; Boyer, S.; Bouetard, A.; Bastien, F.; Fontenille, D. Evidence of Habitat Structuring Aedes albopictus Populations in Réunion Island. PLoS Negl. Trop. Dis. 2013, 7, e2111. [Google Scholar] [CrossRef] [PubMed]
- Tuten, H.C.; Bridges, W.C.; Paul, K.S.; Adler, P.H. Blood-feeding ecology of mosquitoes in zoos. Med. Vet. Entomol. 2012, 26, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Hess, A.D.; Hayes, R.O.; Tempelis, C.H. The use of th forage ratio technique in mosquito host preference studies. Mosq. News 1968, 28, 386–389. [Google Scholar]
| Biological Class | Biological Family | Mean Frequency (%) |
|---|---|---|
| Aves | Phasianidae | 10.08 |
| Passeridae | 7.78 | |
| Anatidae | 7.5 | |
| Columbidae | 5.83 | |
| Sulidae | 2.33 | |
| Thamnophilidae | 1.49 | |
| Pycnonotidae | 1.39 | |
| Corvidae | 1.11 | |
| Ciconiidae | 1.0 | |
| Mammalia | Hominidae (Humans) | 59.83 |
| Muridae | 15.34 | |
| Canidae | 11.6 | |
| Herpestidae | 9.53 | |
| Bovidae | 8.9 | |
| Felidae | 8.49 | |
| Leporidae | 8.27 | |
| Sciuridae | 5.07 | |
| Suidae | 4.99 | |
| Didelphidae | 4.6 | |
| Equidae | 4.39 | |
| Cervidae | 4.15 | |
| Muridae/Soricidae | 3.43 | |
| Phyllostomidae | 2.99 | |
| Procyonidae | 2.71 | |
| Furipteridae | 1.49 | |
| Cricetidae | 0.61 | |
| Actinopterygii | Cobitidae | 1.11 |
| Amphibia | Salamandridae | 2.22 |
| Infection Method | Virus | Infection or Infection Rate | Dissemination Rate | Dissemination Efficiency | Transmission Rate | Transmission Efficiency | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| Host feeding | CHPV | 25.00 | 0.00 | ND | ND | ND | ND | ND | ND | 12.50 | 0.00 |
| EEEV | 75.36 | 35.35 | 85.19 | 25.66 | 76.99 | 33.58 | 40.00 | 0 | 57.17 | 20.15 | |
| JEV | ND | ND | ND | ND | ND | ND | ND | ND | 37.00 | 9.17 | |
| LACV | ND | ND | ND | ND | ND | ND | ND | ND | 23.86 | 6.69 | |
| MAYV | 11.88 | 3.31 | 20.00 | 0.00 | 4.07 | 1.32 | ND | ND | 3.46 | 0.69 | |
| OROV | 6.67 | 5.20 | ND | ND | ND | ND | ND | ND | ND | ND | |
| POTV | 26.26 | 17.06 | ND | ND | ND | ND | ND | ND | ND | ND | |
| RRV | 80.66 | 23.02 | ND | ND | ND | ND | ND | ND | 41.40 | 16.57 | |
| RVFV | 69.26 | 27.24 | 60.04 | 6.34 | 40.72 | 11.96 | 15.00 | 7.07 | 6.54 | 4.67 | |
| VEEV | 71.20 | 20.49 | 89.48 | 10.48 | 64.78 | 22.53 | 59.94 | 26.57 | 38.09 | 23.53 | |
| WNV | 73.41 | 23.81 | 94.39 | 3.91 | 69.80 | 23.98 | 82.72 | 11.49 | 68.63 | 18.62 | |
| Intrathoracic injection | AMTV | 100.00 | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| BUJV | 100.00 | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| CHIV | 96.88 | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| ICOV | 40.91 | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| ITPV | 81.25 | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| KARV | 94.12 | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| ORUV | 37.50 | 17.68 | ND | ND | ND | ND | ND | ND | 37.50 | 17.68 | |
| PACV | 100.00 | 0.00 | ND | ND | ND | ND | ND | ND | ND | ND | |
| RVFV | ND | ND | ND | ND | ND | ND | ND | ND | 15.93 | 7.35 | |
| SALV | 92.86 | 0.00 | ND | ND | ND | ND | ND | ND | ND | ND | |
| URUV | 94.12 | 0.00 | ND | ND | ND | ND | ND | ND | ND | ND | |
| Membrane feeding | CHIKV | 58.92 | 28.23 | 77.58 | 22.60 | 79.06 | 23.45 | 53.49 | 33.98 | 42.68 | 23.78 |
| CVV | 56.50 | 0.00 | 100.00 | 0.00 | ND | ND | 29.60 | 0.00 | 17.39 | 0.00 | |
| DENV-1 | 60.18 | 16.01 | 63.79 | 23.97 | 39.56 | 23.90 | 8.33 | 0.00 | 6.25 | 0.00 | |
| DENV-2 | 58.10 | 30.93 | 53.12 | 22.93 | 34.83 | 18.81 | 12.47 | 13.20 | 10.13 | 12.29 | |
| GETV | 100.00 | 0.00 | ND | ND | ND | ND | ND | ND | ND | ND | |
| JCV | 96.67 | 0.00 | 89.66 | 0.00 | 86.67 | 0.00 | 7.69 | 0.00 | 6.67 | 0.00 | |
| JEV | 91.98 | 10.72 | 90.79 | 14.56 | 84.63 | 19.92 | ND | ND | 40.50 | 15.98 | |
| KEYV | 91.89 | 0.00 | 91.18 | 0.00 | 83.78 | 0.00 | ND | ND | ND | ND | |
| LACV | 89.72 | 7.38 | 86.83 | 13.70 | 71.03 | 22.93 | 35.84 | 14.25 | 29.93 | 16.75 | |
| POTV | 93.55 | 6.59 | 96.13 | 3.21 | 89.86 | 5.96 | ND | ND | 14.67 | 7.00 | |
| RVFV | 10.53 | 0.00 | 25.00 | 0.00 | 2.63 | 0.00 | 100.00 | 0.00 | 2.63 | 0.00 | |
| TVTV | 28.00 | 0.00 | 85.71 | 0.00 | 24.00 | 0.00 | ND | ND | ND | ND | |
| USUV | 64.40 | 31.2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| WNV | 32.61 | 24.53 | 64.59 | 25.58 | 20.33 | 16.96 | ND | ND | ND | ND | |
| YFV | 33.18 | 21.18 | 55.52 | 20.97 | 20.86 | 10.90 | 36.52 | 26.17 | 7.68 | 5.94 | |
| ZIKV | 67.19 | 23.70 | 38.71 | 21.76 | 29.25 | 22.80 | 24.62 | 22.46 | 9.21 | 6.91 | |
| Mosquito Species | Virus | IR (%) | DE (%) | TE (%) |
|---|---|---|---|---|
| Aedes aegypti | CHIKV | NA | 98.3 ± 3.8 | 42.92 ± 20.19 |
| DENV-1 | 37.7 ± 27 | 34.4 ± 24.9 | 4.9 ± 4.6 | |
| DENV-2 | 44.4 ± 33.4 | 33.3 ± 24.2 | 5 ± 0 | |
| ZIKV | 69.0 ± 27.4 | 44.0 ± 28.3 | 20.48 ± 26.87 | |
| YFV | 46.4.0±23.6 | 21.3 ± 19.0 | 16.5 ± 17.7 | |
| Aedes albopictus | CHIKV | 58.9 ± 28.2 | 79.0 ± 23.4 | 42.68 ± 23.7 |
| DENV-1 | 60.2 ± 16 | 39.5 ± 24.2 | 6.25 ± 0 | |
| DENV-2 | 58.0 ± 30.9 | 34.8 ± 18.8 | 10.13 ± 12.28 | |
| WNV | 63.8 ± 29.2 | 58.1 ± 30.8 | 68.6 ± 18.6 | |
| YFV | 33.1 ± 21.1 | 20.8 ± 10.8 | 7.68 ± 5.9 | |
| ZIKV | 67.1 ± 23.7 | 29.2 ± 22.8 | 9.21 ± 6.9 | |
| Culex pipiens | WNV | 47.7 ± 33.7 | 30.4 ± 29.7 | 13.49 ± 14.8 |
| Haemagogus leucocelenus | YFV | 50.9 ± 4.0 | 30.06 ± 1.6 | 8.08 ± 2.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira-dos-Santos, T.; Roiz, D.; Lourenço-de-Oliveira, R.; Paupy, C. A Systematic Review: Is Aedes albopictus an Efficient Bridge Vector for Zoonotic Arboviruses? Pathogens 2020, 9, 266. https://doi.org/10.3390/pathogens9040266
Pereira-dos-Santos T, Roiz D, Lourenço-de-Oliveira R, Paupy C. A Systematic Review: Is Aedes albopictus an Efficient Bridge Vector for Zoonotic Arboviruses? Pathogens. 2020; 9(4):266. https://doi.org/10.3390/pathogens9040266
Chicago/Turabian StylePereira-dos-Santos, Taissa, David Roiz, Ricardo Lourenço-de-Oliveira, and Christophe Paupy. 2020. "A Systematic Review: Is Aedes albopictus an Efficient Bridge Vector for Zoonotic Arboviruses?" Pathogens 9, no. 4: 266. https://doi.org/10.3390/pathogens9040266
APA StylePereira-dos-Santos, T., Roiz, D., Lourenço-de-Oliveira, R., & Paupy, C. (2020). A Systematic Review: Is Aedes albopictus an Efficient Bridge Vector for Zoonotic Arboviruses? Pathogens, 9(4), 266. https://doi.org/10.3390/pathogens9040266





