Haemoproteus spp. and Leucocytozoon californicus Coinfection in a Merlin (Falco colombarius)
Abstract
1. Introduction
2. Results
2.1. Blood Smears
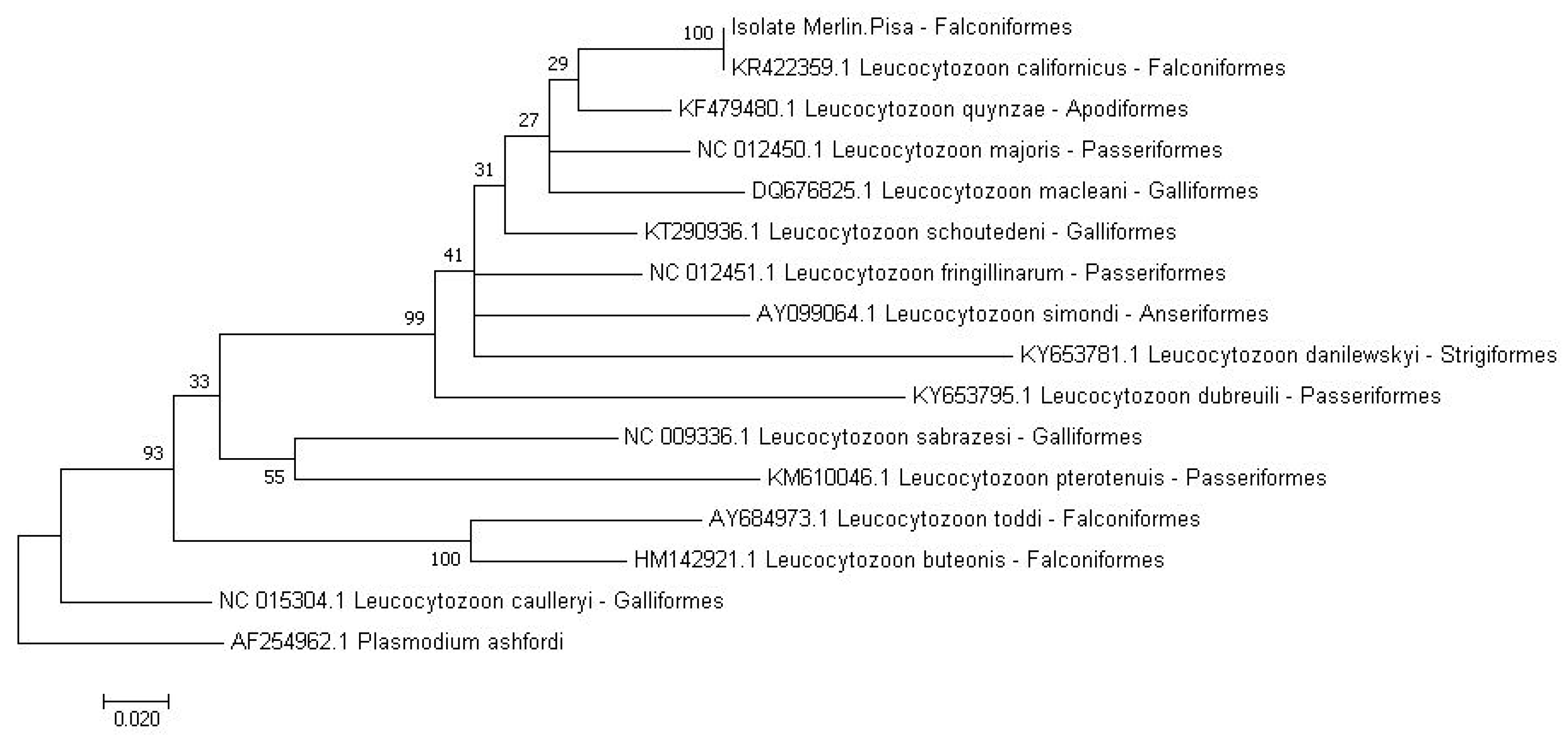
2.2. Molecular Analysis
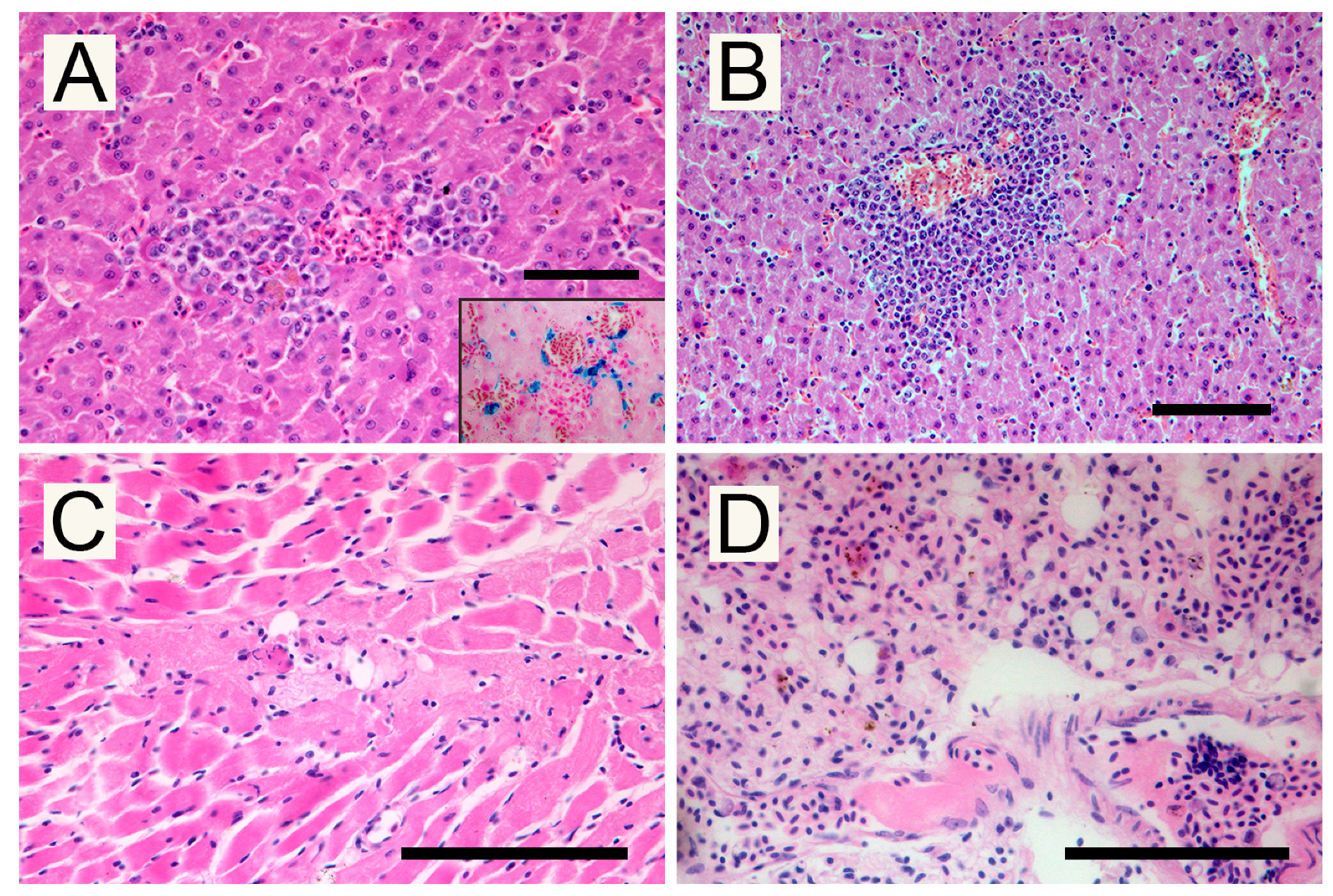
2.3. Pathological Investigations
3. Discussion
4. Materials and Methods
4.1. Case History
4.2. Blood Smears
4.3. Molecular Analysis
4.4. Pathological Investigations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ferrell, S.T.; Snowden, K.; Marlar, A.B.; Garner, M.; Lung, N.P. Fatal hemoprotozoal infections in multiple avian species in a zoological park. J. Zoo Wildl. Med. 2007, 38, 309–316. [Google Scholar] [CrossRef]
- Donovan, T.A.; Schrenzel, M.; Tucker, T.A.; Pessier, A.P.; Stalis, I.H. Hepatic hemorrhage, hemocoelom, and sudden death due to Haemoproteus infection in passerine birds: Eleven cases. J. Veter. Diagn. Investig. 2008, 20, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Valkiūnas, G. Avian Malaria Parasites and Other Haemosporidia; Informa UK Limited: Colchester, UK, 2004; pp. 36–45. [Google Scholar]
- Greiner, E.C.; Ritchie, B. Parasites. In Avian Medicine: Principles and Application; Ritchie, B., Harrison, G., Harrison, L., Eds.; Wingers: Lake Worth, FL, USA, 1994; pp. 1007–1029. [Google Scholar]
- Ojanen, U.; Rätti, O.; Adler, P.; Kuusela, K.; Malmqvist, B.; Helle, P. Blood feeding by black flies (Diptera: Simuliidae) on black grouse (Tetrao tetrix) in Finland. Èntomol. Fenn. 2002, 13, 153–158. [Google Scholar] [CrossRef][Green Version]
- Özmen, Ö.; Haligür, M.; Yukari, B.A. A study on the presence of leucocytozoonosis in wild birds of Burdur district. Turk. J. Vet. Anim. Sci. 2005, 9, 1273–1278. [Google Scholar]
- Norris, K.; Anwar, M.; Read, F. Reproductive effort influence the prevalence of haemoprotozoan parasites in Great Tits. J. Anim. Ecol. 1994, 63, 601–610. [Google Scholar] [CrossRef]
- Redig, P.T.; Cruz-Martinez, L. Raptors. In Handbook of Avian Medicine; Elsevier BV: Amsterdam, The Netherlands, 2009; pp. 209–242. [Google Scholar]
- Olias, P.; Wegelin, M.; Zenker, W.; Freter, S.; Gruber, A.D.; Klopfleisch, R. Avian Malaria Deaths in Parrots, Europe. Emerg. Infect. Dis. 2011, 17, 950–952. [Google Scholar] [CrossRef] [PubMed]
- Krone, O.; Waldenstrom, J.; Valkiunas, G.; Lessow, O.; Muller, K.; Iezhova, T.A.; Fickel, J.; Bensch, S. Haemosporidian blood parasites in European birds of prey and owls. J. Parasitol. 2008, 94, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Cai, B.; Qi, Y.; Liu, S.; Hong, L.; Lu, M.; Chen, X.; Qiu, C.; Peng, W.; Li, J.; et al. Multi-Strain Infections and ‘Relapse’ of Leucocytozoon sabrazesi Gametocytes in Domestic Chickens in Southern China. PLoS ONE 2014, 9, e94877. [Google Scholar] [CrossRef] [PubMed]
- Greiner, E.C.; Kocan, A.A. Leucocytozoon (Haemosporida; Leucocytozoidae) of the Falconiformes. Can. J. Zool. 1977, 55, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Valkiūnas, G.; Sehgal, R.N.; Iezhova, T.A.; Hull, A.C. Identification of Leucocytozoon toddi Group (Haemosporida: Leucocytozoidae), with Remarks on the Species Taxonomy of Leucocytozoids. J. Parasitol. 2010, 96, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Sacchi, L.; Prigioni, C. Occurrence of Leucocytozoon and Haemoproteus (Apicomplexa, Haemosporina) in Falconiformes and Strigiformes of Italy. Annal. Parasitol. Hum. Compar. 1984, 59, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Walther, E.; Valkiūnas, G.; Wommack, E.A.; Bowie, R.C.K.; Iezhova, T.A.; Sehgal, R.N.M. Description and molecular characterization of a new Leucocytozoon parasite (Haemosporidae: Leucocytozoidae) Leucocytozoon californicus sp. nov., found in American kestrel (Falco sparverius sparverius). Parasitol. Res. 2016, 115, 1853–1862. [Google Scholar] [CrossRef] [PubMed]
- Silveira, P.; Belo, N.O.; Lacorte, G.A.; Kolesnikovas, C.K.; Vanstreels, R.E.; Steindel, M.; Catao-Dias, J.; Valkiūnas, G.; Braga, É.M. Parasitological and new molecular-phylogenetic characterization of the malaria parasite Plasmodium tejerai in South American penguins. Parasitol. Int. 2013, 62, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Vanstreels, R.E.T.; Da Silva-Filho, R.P.; Kolesnikovas, C.K.M.; Bhering, R.C.C.; Ruoppolo, V.; Epiphanio, S.; Amaku, M.; Junior, F.F.; Braga, É.M.; Catao-Dias, J. Epidemiology and pathology of avian malaria in penguins undergoing rehabilitation in Brazil. Veter. Res. 2015, 46, 30. [Google Scholar] [CrossRef] [PubMed]
- Waldenström, J.; Bensch, S.; Hasselquist, D.; Östman, Ö. A New Nested Polymerase Chain Reaction Method Very Efficient in Detecting Plasmodium and Haemoproteus Infections from Avian Blood. J. Parasitol. 2004, 90, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Hellgren, O.; Waldenström, J.; Bensch, S. A new Pcr assay for simultaneous studies of Leucocytozoon, Plasmodium, and Haemoproteus from Avian blood. J. Parasitol. 2004, 90, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Valkiūnas, G. Bird Haemosporida; Institute of Ecology: Vilnius, Lithuania, 1997; p. 608. [Google Scholar]
- Bensch, S.; Stjernman, M.; Hasselquist, D.; Hansson, B.; Westerdahl, H.; Pinheiro, R.T. Host specificity in avian blood parasites: A study of Plasmodium and Haemoproteus mitochondrial DNA amplified from birds. Proc. R. Soc. B Biol. Sci. 2000, 267, 1583–1589. [Google Scholar] [CrossRef] [PubMed]
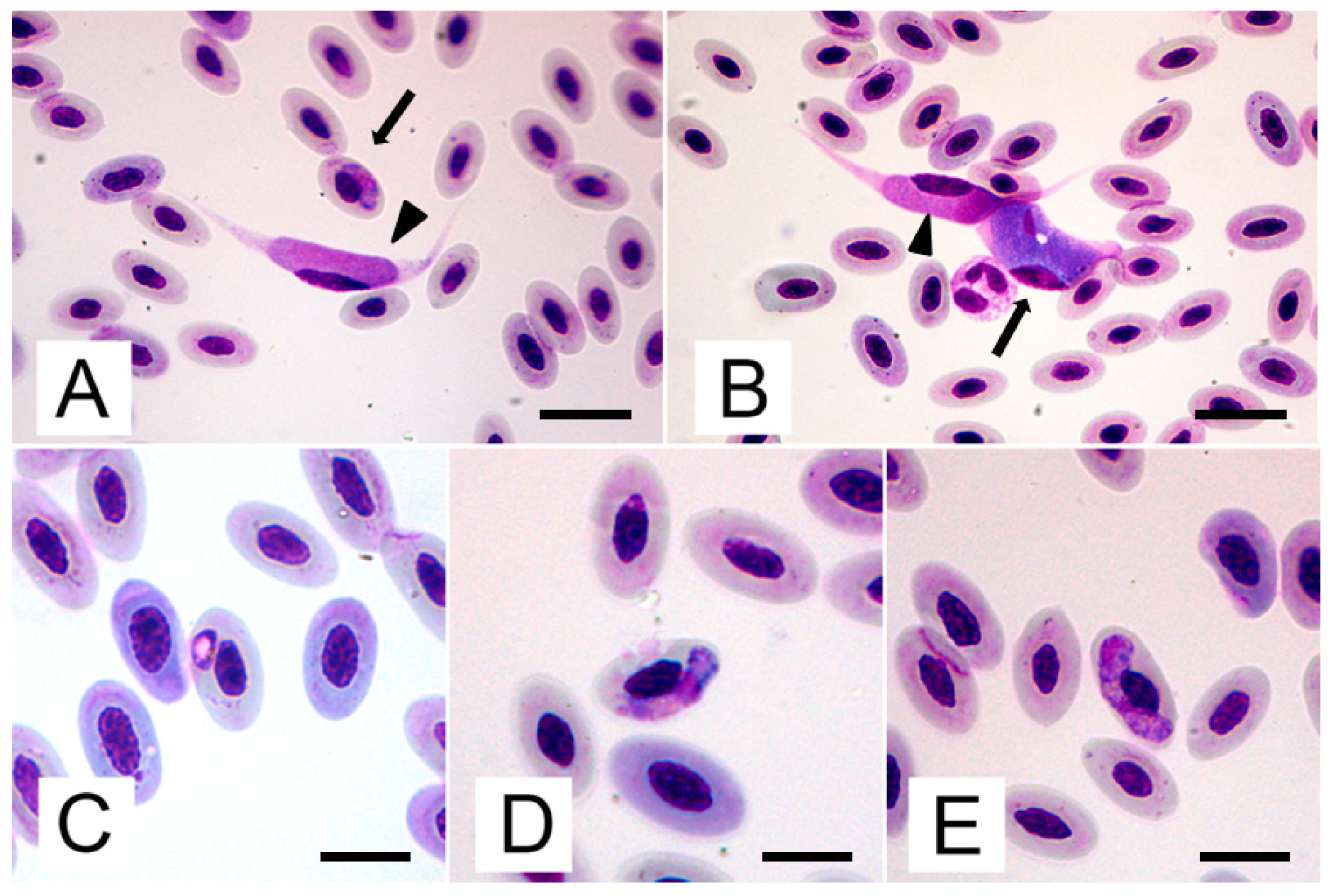
| Figure . | Macrogametocytes (n=10) | Microgametocytes (n=10) | |
|---|---|---|---|
| Min – Max (Mean ± SD) µm | Min – Max (Mean ± SD) µm | ||
| Lenght | 19.0 – 21.6 (20.5 ± 0.7) | 18.0 – 20.9 (19.4 ± 1.2) | |
| Parasite | Width | 8.2 – 10.2 (9.1 ± 0.9) | 5.8 – 7.2 (6.5 ± 0.6) |
| Area | 87.0 – 147.8 (123.9 ± 24.4) | 69.1 – 98.0 (83.7 ± 11.4) | |
| Lenght | 3.1 – 4.8 (4.1 ± 0.7) | 6.9 – 8.9 (7.7 ± 0.8) | |
| Parasite nucleus | Width | 2.0 – 2.9 (2.5 ± 0.4) | 3.3 – 5.4 (4.2 ± 0.9) |
| Area | 6.7 – 11.7 (8.8 ± 2.0) | 20.8 – 32.0 (27.2 ± 4.4) | |
| Cell-parasite complex | Area | 128.7 – 230.0 (189.6 ± 40.7) | 130.0 – 197.50 (163.2 ±25.6) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nardoni, S.; Parisi, F.; Rocchigiani, G.; Ceccherelli, R.; Mancianti, F.; Poli, A. Haemoproteus spp. and Leucocytozoon californicus Coinfection in a Merlin (Falco colombarius). Pathogens 2020, 9, 263. https://doi.org/10.3390/pathogens9040263
Nardoni S, Parisi F, Rocchigiani G, Ceccherelli R, Mancianti F, Poli A. Haemoproteus spp. and Leucocytozoon californicus Coinfection in a Merlin (Falco colombarius). Pathogens. 2020; 9(4):263. https://doi.org/10.3390/pathogens9040263
Chicago/Turabian StyleNardoni, Simona, Francesca Parisi, Guido Rocchigiani, Renato Ceccherelli, Francesca Mancianti, and Alessandro Poli. 2020. "Haemoproteus spp. and Leucocytozoon californicus Coinfection in a Merlin (Falco colombarius)" Pathogens 9, no. 4: 263. https://doi.org/10.3390/pathogens9040263
APA StyleNardoni, S., Parisi, F., Rocchigiani, G., Ceccherelli, R., Mancianti, F., & Poli, A. (2020). Haemoproteus spp. and Leucocytozoon californicus Coinfection in a Merlin (Falco colombarius). Pathogens, 9(4), 263. https://doi.org/10.3390/pathogens9040263








