Persistent Calyxes in Postbloom Fruit Drop: A Microscopy and Microanalysis Perspective
Abstract
1. Introduction
2. Results
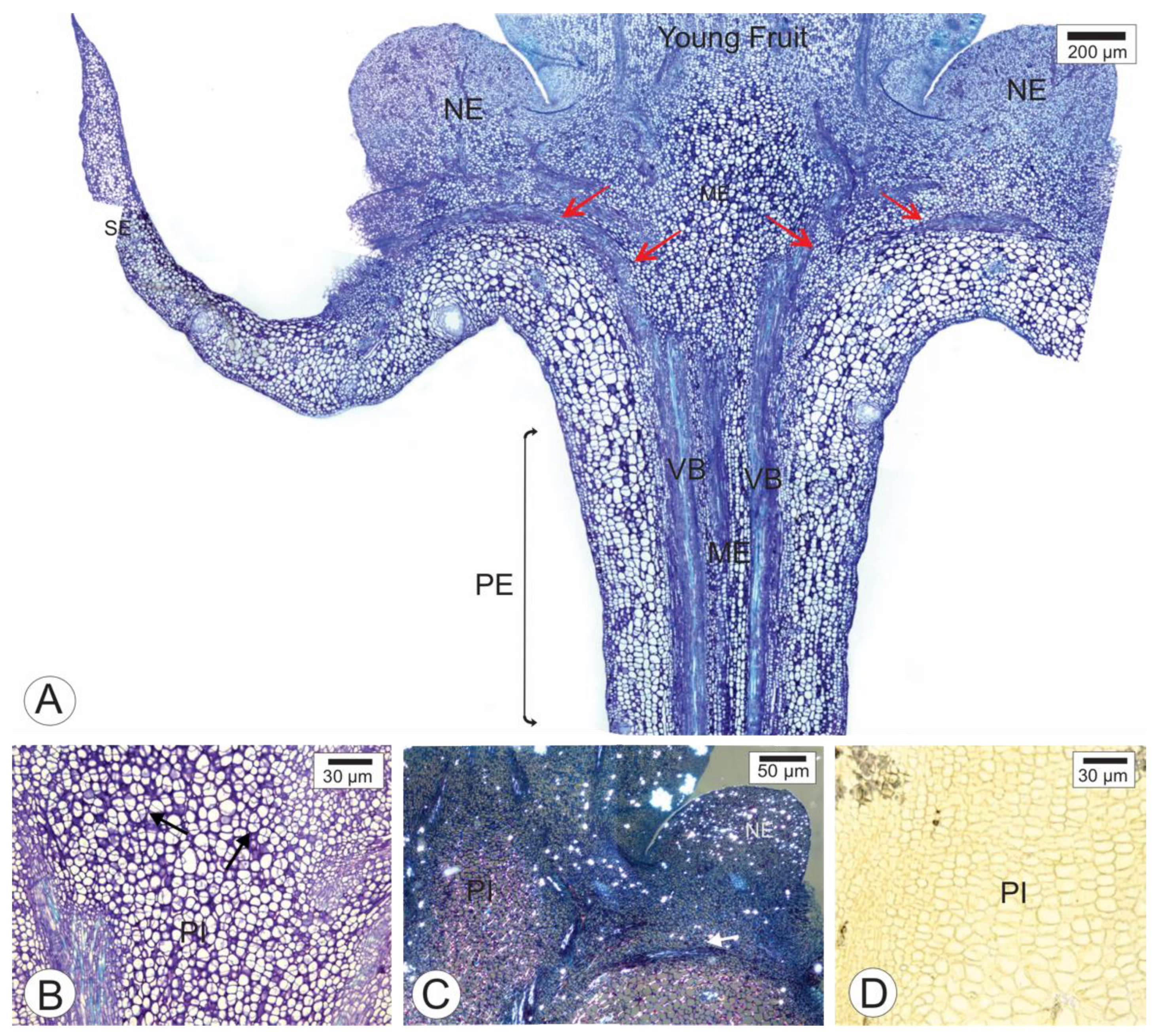
2.1. Anatomy of Healthy Receptacle and Peduncle
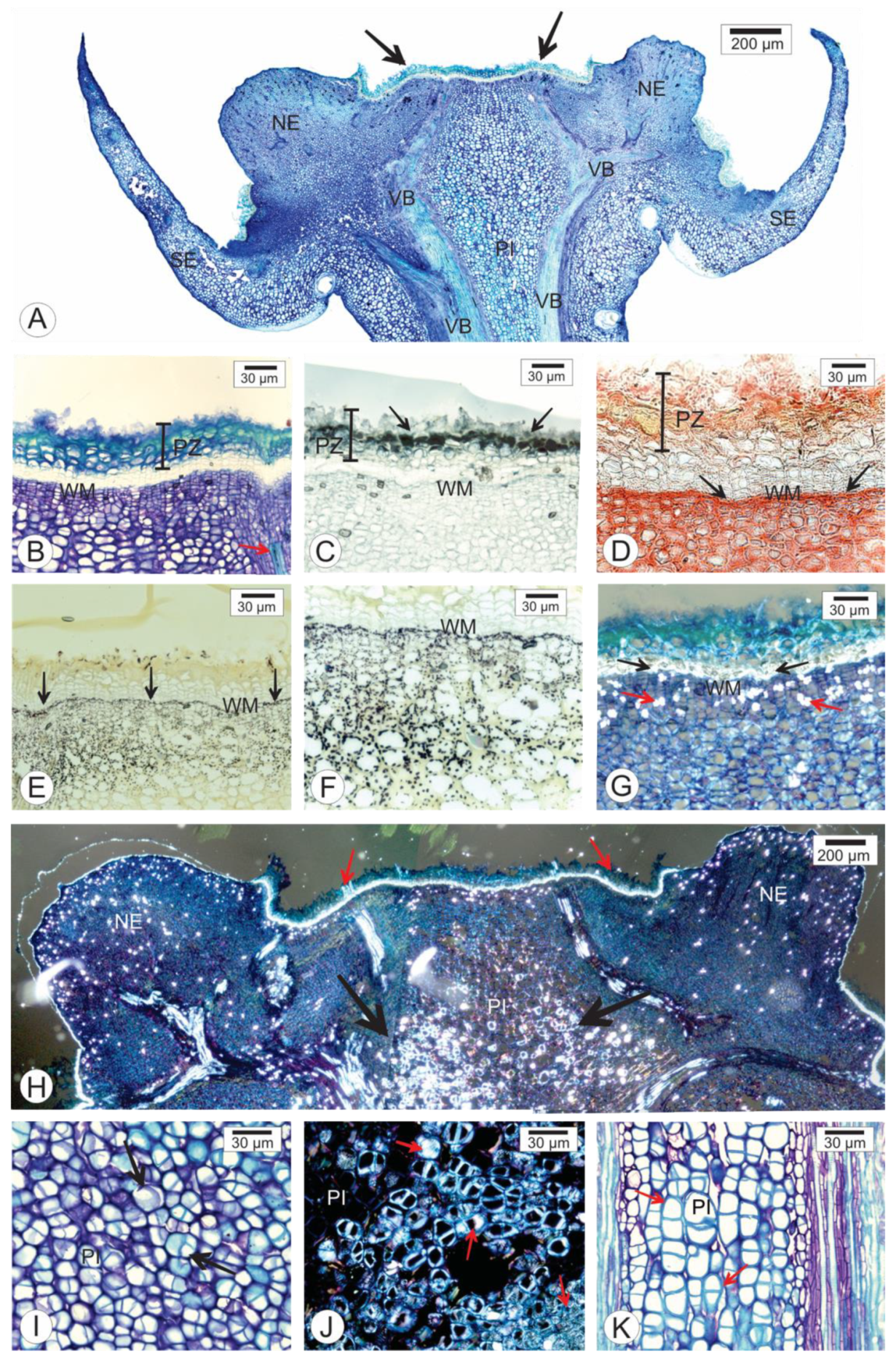
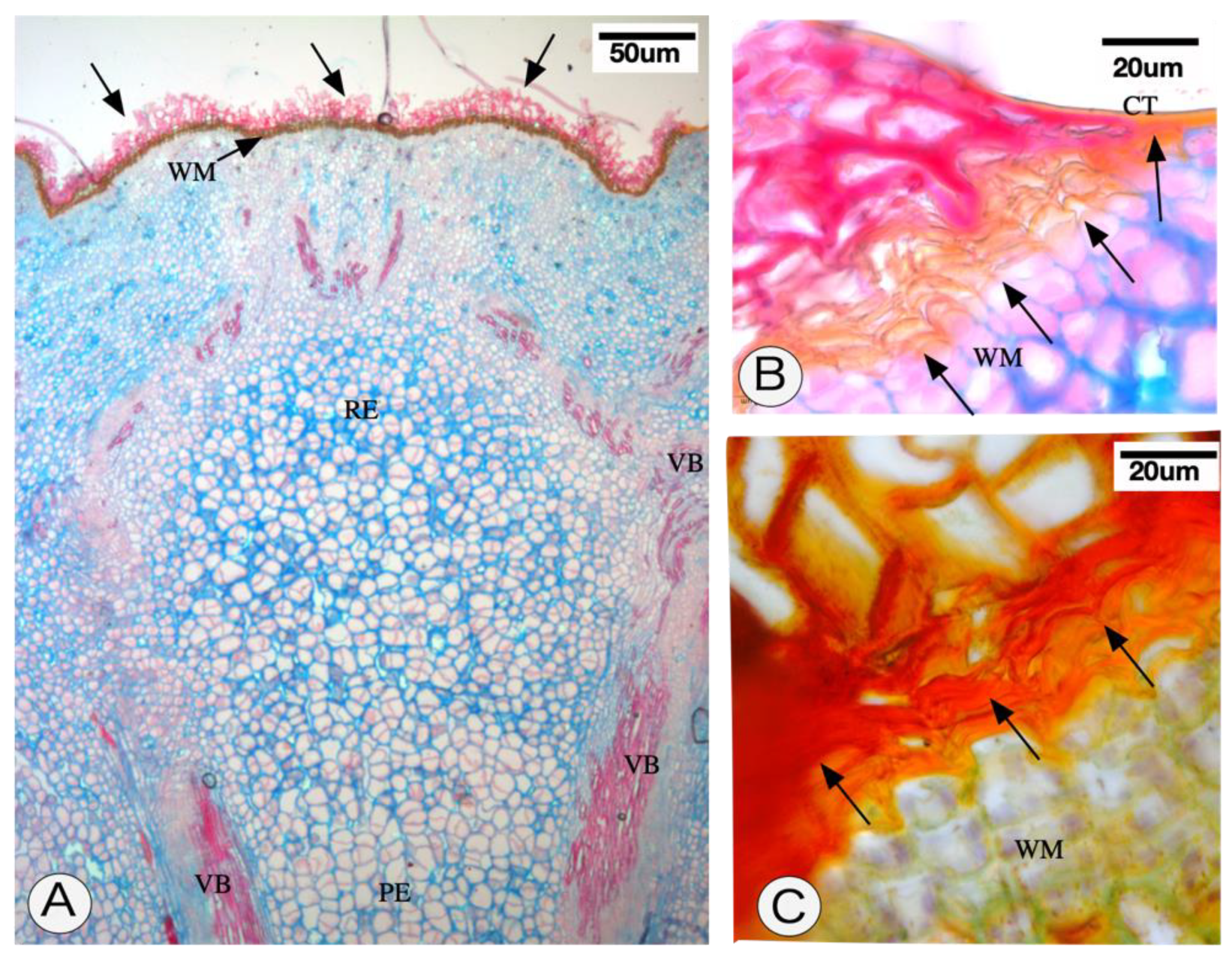
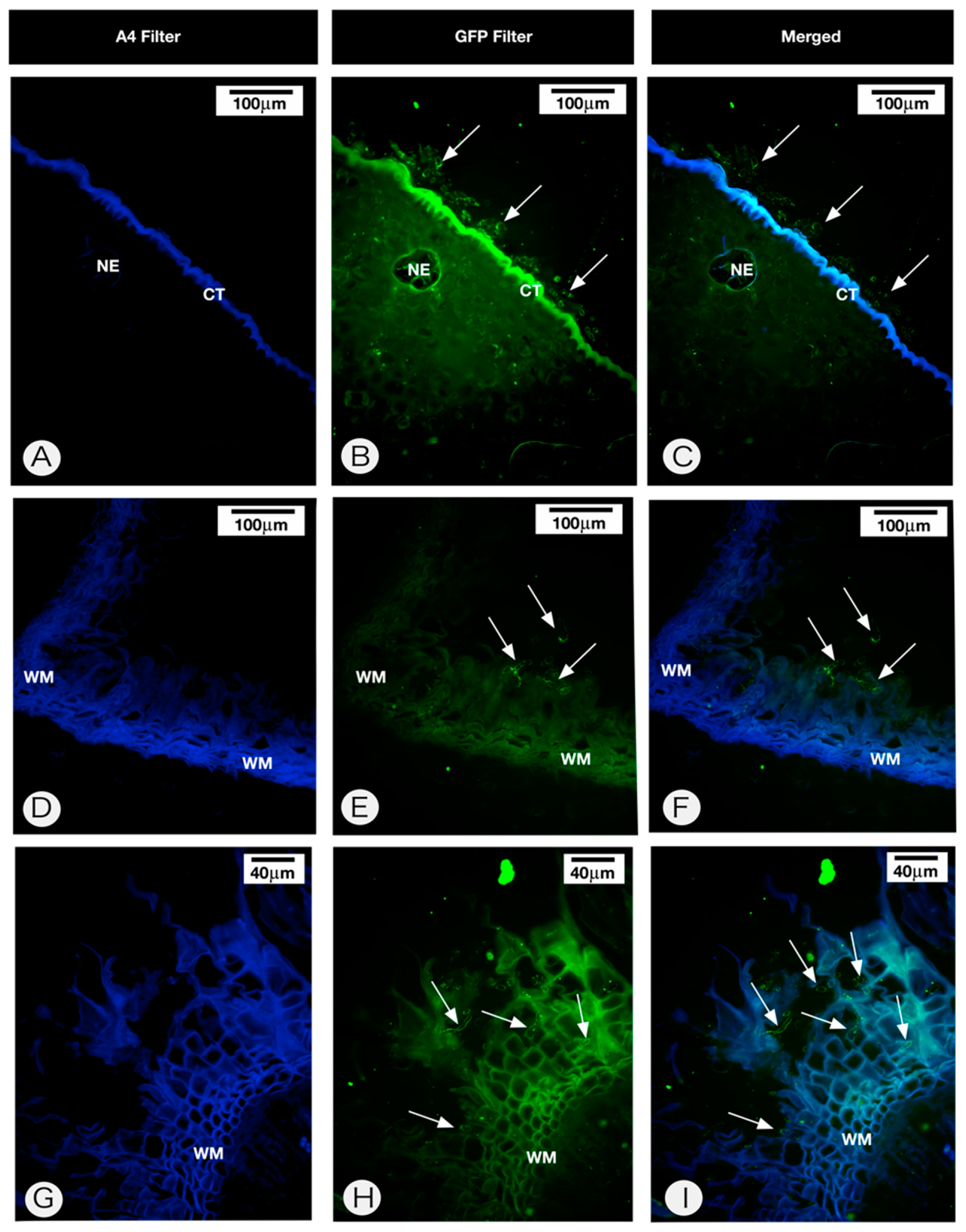
2.2. Anatomy of the Persistent Calyx
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Fungal Inoculation
4.3. Light Microscopy
4.4. Histochemical Tests
4.5. Fungal Detection
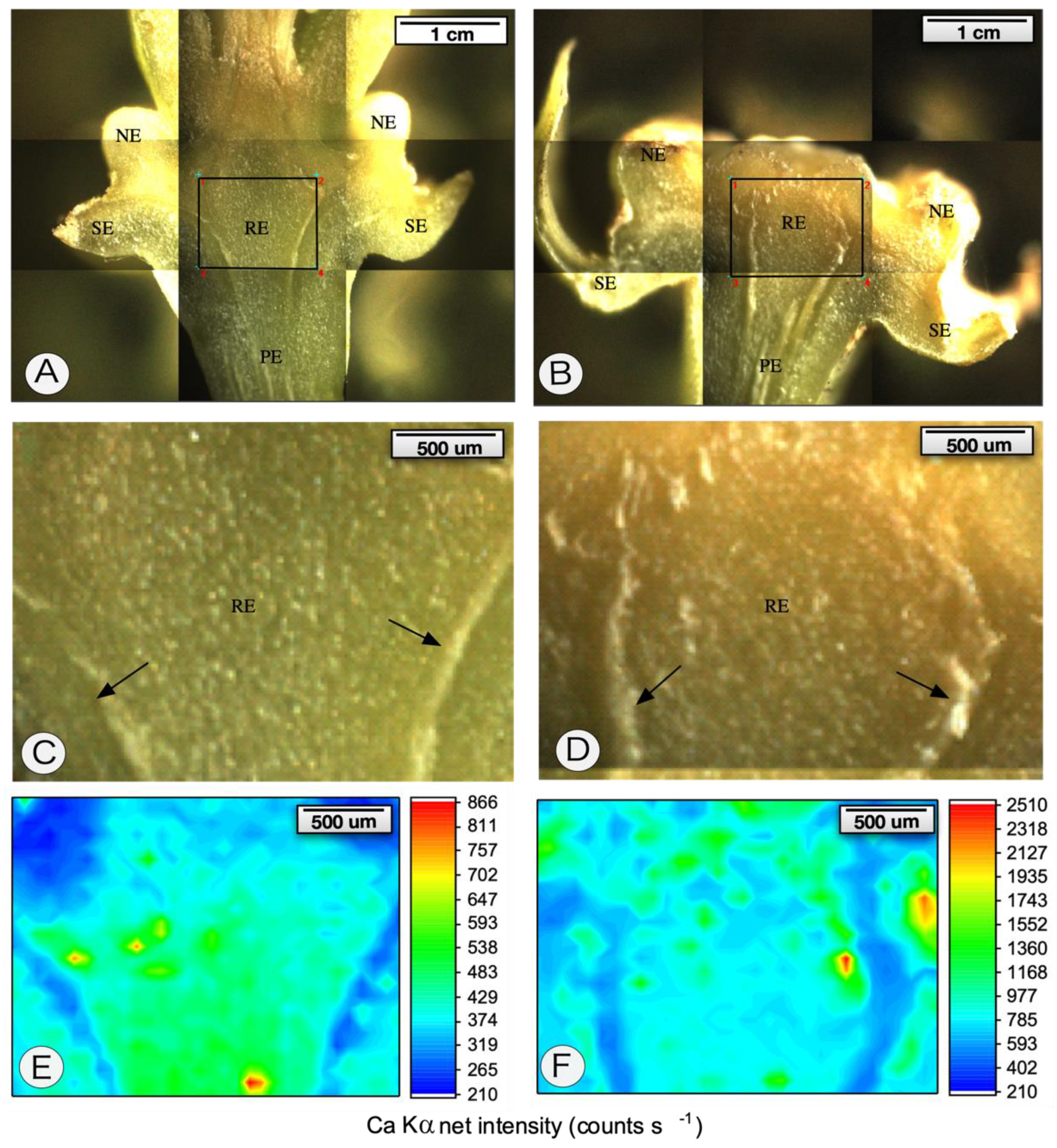
4.6. µ-XRF Spectroscopy
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lima, W.G.; Sposito, M.B.; Amorim, L.; Goncalves, F.P.; Melo, P.A. Colletotrichum gloeosporioides, a new causal agent of citrus post-bloom fruit drop. Eur. J. Plant Pathol. 2011, 131, 157–165. [Google Scholar] [CrossRef]
- Pereira, W.V.; Bertolini, E.; Cambra, M.; Massola, N.S. Multiplex real-time PCR for detection and quantification of Colletotrichum abscissum and C. gloeosporioides on citrus leaves. Eur. J. Plant Pathol. 2019, 155, 1047–1059. [Google Scholar] [CrossRef]
- Goulin, E.H.; Lima, T.A.; Deising, H.B.; Machado, M.A. RNA interference in Colletotrichum abscissum, causal agent of citrus postbloom fruit drop. In Proceedings of the 14th European Conference on Fungal Genetics, Haifa, Israel, 25–28 February 2018. [Google Scholar]
- Timmer, L.W.; Peres, N. Where have all the flowers gone? Postbloom fruit drop of citrus in the Americas. J. Citrus Pathol. 2015, 2, 1–6. [Google Scholar]
- Peres, N.A.; Timmer, L.W.; Adaskaveg, J.E.; Correll, J.C. Lifestyles of Colletotrichum acutatum. Plant Dis. 2005, 89, 784–796. [Google Scholar] [CrossRef]
- Timmer, L.W.; Brown, G.E. Biology and control of anthracnose diseases of citrus. In Colletotrichum: Host Specificity, Patholog, and Host-Pathogen Interaction; Prusky, D., Freeman, S., Dickman, M.B., Eds.; The American Phytopathological Society: Saint Paul, MN, USA, 2000; pp. 300–316. [Google Scholar]
- Porto, O.M. Queda anormal de frutos jovens de citros. Laranja 1993, 14, 341–356. [Google Scholar]
- De Goes, A.; Garrido, R.B.O.; Reis, R.F.; Baldassari, R.B.; Soares, M.A. Evaluation of fungicide applications to sweet orange at different flowering stages for control of postbloom fruit drop caused by Colletotrichum acutatum. Crop Prot. 2008, 27, 71–76. [Google Scholar] [CrossRef]
- Silva-Junior, G.J.; Spósito, M.B.; Marin, D.R.; Ribeiro-Junior, P.J.; Amorim, L. Spatiotemporal characterization of citrus postbloom fruit drop in Brazil and its relationship to pathogen dispersal. Plant Pathol. 2014, 63, 519–529. [Google Scholar] [CrossRef]
- Bassanezi, R.B.; Silva, G.J.; Feichtenberger, E.; Belasque, J., Jr.; Behlau, F.; Wulff, N.A. Doenças dos citros. In Manual de Fitopatologia: Doenças das Plantas Cultivadas; Amorim, L., Rezende, J.A.M., Bergamin Filho, A., Camargo, L.E.A., Eds.; Editora Ceres: Piracicaba, Brazil, 2016; Volume 2, pp. 271–306. [Google Scholar]
- Feichtenberger, E.; Bassanezi, R.B.; Sposito, M.B.; Belasque, J., Jr. Doenças dos citros (Citrus spp.). In Manual de Fitopatologia: Doenças das Plantas Cultivadas, 4th ed.; Kimati, H., Amorim, L., Rezende, J.A.M., Bergamin Filho, A., Camargo, L.E.A., Eds.; Agronômica Ceres: São Paulo, Brazil, 2005; Volume 2, pp. 239–269. [Google Scholar]
- Marques, J.P.R.; Amorim, L.; Sposito, M.B.; Appezzato-da-Gloria, B. Histopathology of postbloom fruit drop caused by Colletotrichum acutatum in citrus flowers. Eur. J. Plant Pathol. 2013, 135, 783–790. [Google Scholar] [CrossRef]
- Timmer, L.W. Diseases of fruit and foliage. In Citrus Health Masnagement; Timmer, L.W., Duncan, L.W., Eds.; APS Press: Saint Paul, MN, USA, 1999; pp. 107–115. [Google Scholar]
- Lin, Y.J.; Stover, E.; Sonoda, R.; Rosskopf, E. Stigma and style necrosis is associated with postbloom fruit drop disease in citrus following artificial inoculation. Hortscience 2001, 36, 1138. [Google Scholar] [CrossRef]
- Cintra, G.S. Podridão Floral Dos Citros: Variabilidade, Sobrevivência E Controle Do Agente Causal, Colletotrichum acutatum. Ph.D. Thesis, Universidade Estadual Paulista Júlio de Mesquita Filho, Jaboticabal, Brazil, 2009. [Google Scholar]
- Chen, H.Q.; Dekkers, K.L.; Cao, L.H.; Burns, J.K.; Timmer, L.W.; Chung, K.R. Evaluation of growth regulator inhibitors for controlling postbloom fruit drop (PFD) of citrus induced by the fungus Colletotrichum acutatum. Hortscience 2006, 41, 1317–1321. [Google Scholar] [CrossRef]
- Goren, R. Anatomical, physiological, and hormonal aspects of abscission in citrus. In Horticultural Reviews; Janick, J., Ed.; John Wiley & Sons, Inc.: Toronto, ON, Canada, 1993; Volume 15, pp. 145–182. [Google Scholar]
- Li, W.; Yuan, R.C.; Burns, J.K.; Timmer, L.W.; Chung, K.R. Genes for hormone biosynthesis and regulation are highly expressed in citrus flowers infected with the fungus Colletotrichum acutatum, causal agent of postbloom fruit drop. J. Am. Soc. Hortic. Sci. 2003, 128, 578–583. [Google Scholar]
- Lahey, K.A.; Yuan, R.; Burns, J.K.; Ueng, P.P.; Timmer, L.W.; Chung, K.R. Induction of phytohormones and differential gene expression in citrus flowers infected by the fungus Colletotrichum acutatum. Mol. Plant-Microbe Interact. 2004, 17, 1394–1401. [Google Scholar] [CrossRef] [PubMed]
- Thomson, N.; Evert, R.F.; Kelman, A. Wound healing in whole potato tubers: A cytochemical, fluorescence, and ultrastructural analysis of cut and bruise wounds. Can. J. Bot. 1995, 73, 1436–1450. [Google Scholar] [CrossRef]
- Marques, J.P.R.; Astúa, J.F.; Kitajima, E.W.; da Glória, B.A. Lesões foliares e de ramos de laranjeira-doce causadas pela leprose-dos-citros. Pesq. Agropec. Bras. 2007, 42, 1531–1536. [Google Scholar] [CrossRef][Green Version]
- Pascholati, S.F.; Leite, B. Hospedeiro: Mecanismos de resistência. In Manual de Fitopatologia—Princípios e Conceitos, 3rd ed.; Filho, A.B., Kimati, H., Amorim, L., Eds.; Agronômica Ceres: São Paulo, Brazil, 1995; Volume 1, pp. 417–453. [Google Scholar]
- Marques, J.P.R.; Spósito, M.B.; Mello, A.F.S.; Amorin, L.; Mondin, M.; Appezzato-da-Glória, B. Histopathology of black spot symptoms in sweet oranges. Eur. J. Plant Pathol. 2012, 133, 439–448. [Google Scholar] [CrossRef]
- Carpita, N.C.; Ralph, J.; McCann, M.C. The cell wall. In Biochemistry and Molecular Biology of Plants, 2nd ed.; Buchanan, B.B., Gruissem, W., Jones, R.L., Eds.; Wiley Blackwell: New York, NY, USA, 2015; pp. 45–189. [Google Scholar]
- Caffall, K.H.; Mohnen, D. The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohyd. Res. 2009, 344, 1879–1900. [Google Scholar] [CrossRef]
- Schneider, H. The anatomy of citrus. In The Citrus Industry; Reuther, W., Batchelor, L.D., Webber, H.J., Eds.; University of California: Berkeley, CA, USA, 1968; Volume 2, pp. 1–86. [Google Scholar]
- Vance, C.P.; Kirk, T.K.; Sherwood, R.T. Lignification as a mechanism of disease resistance. Annu. Rev. Phytopathol. 1980, 18, 259–288. [Google Scholar] [CrossRef]
- Nicholson, R.L.; Hammerschmidt, R. Phenolic-compounds and their role in disease resistance. Annu. Rev. Phytopathol. 1992, 30, 369–389. [Google Scholar] [CrossRef]
- Bhuiyan, N.H.; Selvaraj, G.; Wei, Y.; King, J. Role of lignification in plant defense. Plant Signal. Behav. 2009, 4, 158–159. [Google Scholar] [CrossRef]
- Karnovsky, M.J. A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron microscopy. J. Cell Biol. 1965, 27, 137–138. [Google Scholar]
- Sakai, W.S. Simple method for differential staining of paraffin embedded plant material using toluidine blue o. Stain Technol. 1973, 48, 247–249. [Google Scholar] [CrossRef] [PubMed]
- Pearse, A.G.E. Histochemistry, Theoretical and Applied, 3rd ed.; Churchill: London, UK, 1968; Volume 1, p. 998. [Google Scholar]
- Cortelazzo, A.L.; Vidal, B.C. Soybean seed proteins: Detection in situ and mobilization during germination. Rev. Bras. Bot. 1991, 14, 27–33. [Google Scholar]
- Jensen, W.A. Botanical Histochemistry: Principles and Practice; W.H. Freeman and Company: San Francisco, CA, USA, 1962; p. 408. [Google Scholar]
- Johansen, D.A. Plant Microtechnique; McGraw-Hill Book Company: New York, NY, USA, 1940; p. 523. [Google Scholar]
- Ruzin, S.E. Plant Microtechnique and Microscopy; Oxford University Press: New York, NY, USA, 1999. [Google Scholar]
- Marques, J.P.R.; Hoy, J.W.; Appezzato-da-Gloria, B.; Viveros, A.F.G.; Vieira, M.L.C.; Baisakh, N. Sugarcane cell wall-associated defense responses to infection by Sporisorium Scitamineum. Front. Plant Sci. 2018, 9, 698. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues Marques, J.P.; Bellato Spósito, M.; Amorim, L.; Sgarbiero Montanha, G.; Silva Junior, G.J.; Pereira de Carvalho, H.W.; Appezzato-da-Glória, B. Persistent Calyxes in Postbloom Fruit Drop: A Microscopy and Microanalysis Perspective. Pathogens 2020, 9, 251. https://doi.org/10.3390/pathogens9040251
Rodrigues Marques JP, Bellato Spósito M, Amorim L, Sgarbiero Montanha G, Silva Junior GJ, Pereira de Carvalho HW, Appezzato-da-Glória B. Persistent Calyxes in Postbloom Fruit Drop: A Microscopy and Microanalysis Perspective. Pathogens. 2020; 9(4):251. https://doi.org/10.3390/pathogens9040251
Chicago/Turabian StyleRodrigues Marques, João Paulo, Marcel Bellato Spósito, Lilian Amorim, Gabriel Sgarbiero Montanha, Geraldo José Silva Junior, Hudson Wallace Pereira de Carvalho, and Beatriz Appezzato-da-Glória. 2020. "Persistent Calyxes in Postbloom Fruit Drop: A Microscopy and Microanalysis Perspective" Pathogens 9, no. 4: 251. https://doi.org/10.3390/pathogens9040251
APA StyleRodrigues Marques, J. P., Bellato Spósito, M., Amorim, L., Sgarbiero Montanha, G., Silva Junior, G. J., Pereira de Carvalho, H. W., & Appezzato-da-Glória, B. (2020). Persistent Calyxes in Postbloom Fruit Drop: A Microscopy and Microanalysis Perspective. Pathogens, 9(4), 251. https://doi.org/10.3390/pathogens9040251




