Cerebral HSV-1 Vasculitis as a Fatal Complication of Immunosuppression in Non-Hodgkin´s Lymphoma: A Case Report and Review of the Literature
Abstract
1. Introduction
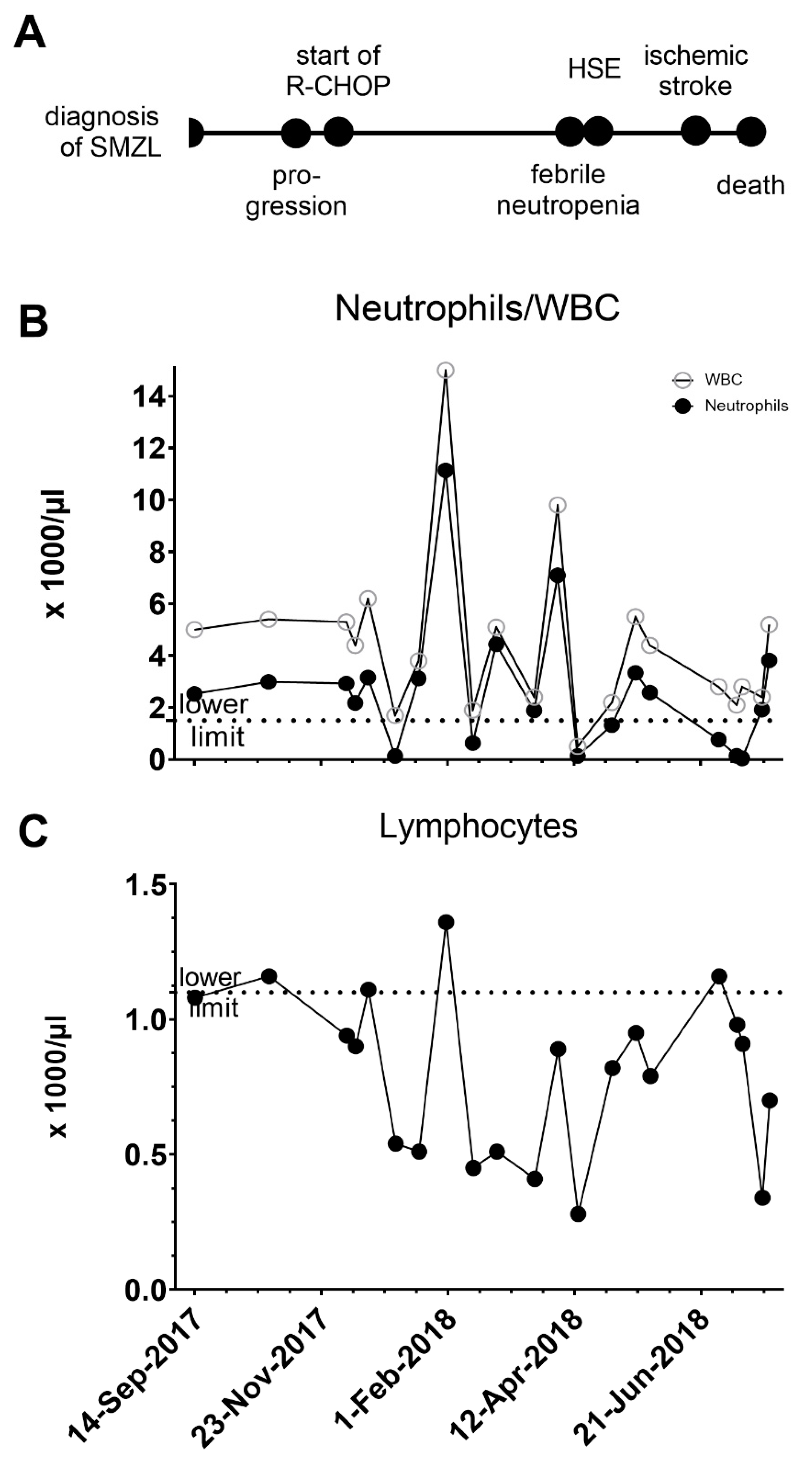
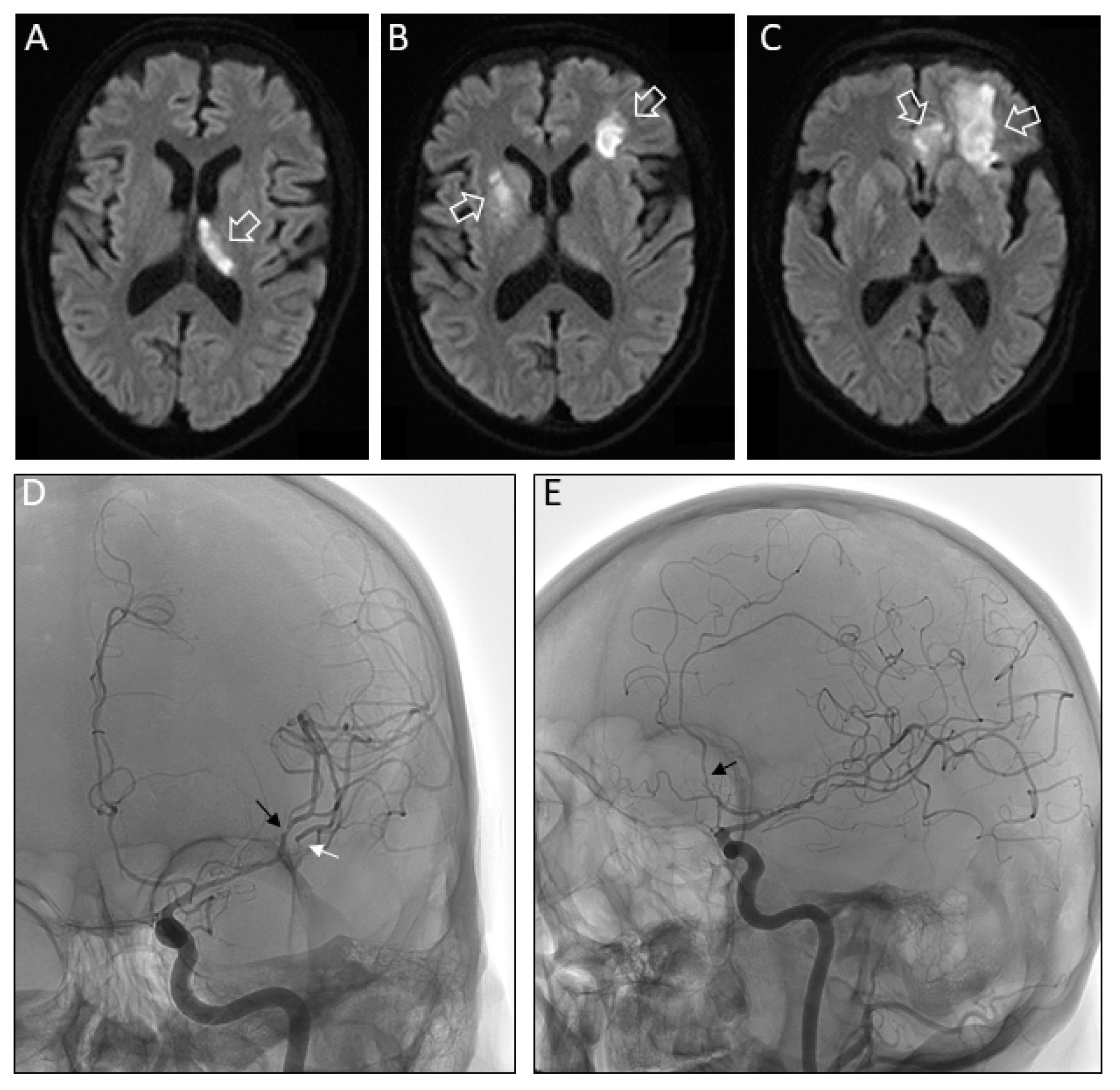
2. Case Study
3. Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jiang, M.; Bennani, N.N.; Feldman, A.L. Lymphoma classification update: B-cell non-Hodgkin lymphomas. Expert Rev. Hematol. 2017, 10, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Vose, J.M. Mantle cell lymphoma: 2017 update on diagnosis, risk-stratification, and clinical management. Am. J. Hematol. 2017, 92, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.S.D.; Tavares, R.S.; Farias, D.L.C. Splenic marginal zone lymphoma: A literature review of diagnostic and therapeutic challenges. Rev. Bras. Hematol. Hemoter. 2017, 39, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Piris, M.A.; Onaindia, A.; Mollejo, M. Splenic marginal zone lymphoma. Best Pract. Res. Clin. Haematol. 2017, 30, 56–64. [Google Scholar] [CrossRef]
- Bayraktar, S.; Stefanovic, A.; Montague, N.; Davis, J.; Murray, T.; Lossos, I.S. Central nervous system manifestations of marginal zone B-cell lymphoma. Ann. Hematol. 2010, 89, 1003–1009. [Google Scholar] [CrossRef]
- Weycker, D.; Chandler, D.; Barron, R.; Xu, H.; Wu, H.; Edelsberg, J.; Lyman, G.H. Risk of infection among patients with non-metastatic solid tumors or non-Hodgkin’s lymphoma receiving myelosuppressive chemotherapy and antimicrobial prophylaxis in US clinical practice. J. Oncol. Pharm. Pract. 2017, 23, 33–42. [Google Scholar] [CrossRef]
- Boucher, A.; Herrmann, J.L.; Morand, P.; Buzele, R.; Crabol, Y.; Stahl, J.P.; Mailles, A. Epidemiology of infectious encephalitis causes in 2016. Med. Mal. Infect. 2017, 47, 221–235. [Google Scholar] [CrossRef]
- Saito, M.; Kiyozaki, H.; Obitsu, T.; Imoto, H.; Taniyama, Y.; Takata, O.; Rikiyama, T. Herpes simplex virus-1 encephalitis induced by chemoradiotherapy and steroids in an esophageal cancer patient: A case report. BMC Cancer 2016, 16, 233. [Google Scholar] [CrossRef]
- Rommer, P.S.; Sellner, J. Repurposing multiple sclerosis drugs: A review of studies in neurological and psychiatric conditions. Drug Discov. Today 2019, 24, 1398–1404. [Google Scholar] [CrossRef]
- Perini, P.; Rinaldi, F.; Puthenparampil, M.; Marcon, M.; Perini, F.; Gallo, P. Herpes simplex virus encephalitis temporally associated with dimethyl fumarate-induced lymphopenia in a multiple sclerosis patient. Mult. Scler. Relat. Disord. 2018, 26, 68–70. [Google Scholar] [CrossRef]
- Pfender, N.; Jelcic, I.; Linnebank, M.; Schwarz, U.; Martin, R. Reactivation of herpesvirus under fingolimod: A case of severe herpes simplex encephalitis. Neurology 2015, 84, 2377–2378. [Google Scholar] [CrossRef] [PubMed]
- Tan, I.L.; McArthur, J.C.; Venkatesan, A.; Nath, A. Atypical manifestations and poor outcome of herpes simplex encephalitis in the immunocompromised. Neurology 2012, 79, 2125–2132. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Donner, M.; Colan, D. Fatal herpes encephalitis in a patient with small cell lung cancer following prophylactic cranial radiation—A case report with review of literature. Anticancer Res. 2013, 33, 3263–3268. [Google Scholar] [PubMed]
- Mantero, V.; Cossu, M.; Rigamonti, A.; Fiumani, A.; Basilico, P.; Tassi, L.; Salmaggi, A. HSV-1 encephalitis relapse after epilepsy surgery: A case report and review of the literature. J. Neurovirol. 2019. [Google Scholar] [CrossRef]
- Venkatesan, A.; Geocadin, R.G. Diagnosis and management of acute encephalitis: A practical approach. Neurol. Clin. Pract. 2014, 4, 206–215. [Google Scholar] [CrossRef]
- Jakob, N.J.; Lenhard, T.; Schnitzler, P.; Rohde, S.; Ringleb, P.A.; Steiner, T.; Wildemann, B. Herpes simplex virus encephalitis despite normal cell count in the cerebrospinal fluid. Crit. Care Med. 2012, 40, 1304–1308. [Google Scholar] [CrossRef]
- Gregory, S.A.; Case, D.C., Jr.; Bosserman, L.; Litwak, D.L.; Berry, W.R.; Kalman, L.A.; Belt, R.J.; Saven, A. Fourteen-day CHOP supported with granulocyte colony-stimulating factor in patients with aggressive non-Hodgkin’s lymphoma: Results of a phase II study. Clin. Lymphoma 2003, 4, 93–98. [Google Scholar] [CrossRef]
- Ito, K.; Okamoto, M.; Inaguma, Y.; Okamoto, A.; Ando, M.; Ando, Y.; Tsuge, M.; Tomono, A.; Kakumae, Y.; Hayashi, T.; et al. Influence of R-CHOP Therapy on Immune System Restoration in Patients with B-Cell Lymphoma. Oncology 2016, 91, 302–310. [Google Scholar] [CrossRef]
- Hauer, L.; Pikija, S.; Schulte, E.C.; Sztriha, L.K.; Nardone, R.; Sellner, J. Cerebrovascular manifestations of herpes simplex virus infection of the central nervous system: A systematic review. J. Neuroinflamm. 2019, 16, 19. [Google Scholar] [CrossRef]
- Modi, S.; Mahajan, A.; Dharaiya, D.; Varelas, P.; Mitsias, P. Burden of herpes simplex virus encephalitis in the United States. J. Neurol. 2017, 264, 1204–1208. [Google Scholar] [CrossRef]
- Hollender, A.; Kvaloy, S.; Nome, O.; Skovlund, E.; Lote, K.; Holte, H. Central nervous system involvement following diagnosis of non-Hodgkin’s lymphoma: A risk model. Ann. Oncol. 2002, 13, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Bokstein, F.; Lossos, A.; Lossos, I.S.; Siegal, T. Central nervous system relapse of systemic non-Hodgkin’s lymphoma: Results of treatment based on high-dose methotrexate combination chemotherapy. Leuk. Lymphoma 2002, 43, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Li, J.; Lin, J.; Liu, D.; Yu, L.; Wang, Q. High-Grade Transformation in a Splenic Marginal Zone Lymphoma with a Cerebral Manifestation. Am. J. Case Rep. 2017, 18, 611–616. [Google Scholar] [CrossRef][Green Version]
- Thoennissen, N.H.; Keyvani, K.; Voelker, H.U.; Bremer, J.; Krug, U.; Muller-Tidow, C.; Koch, P.; Muller-Hermelink, H.K.; Berdel, W.E. Splenic marginal zone lymphoma: Transformation to diffuse large B-cell lymphoma with isolated cerebral manifestation. J. Clin. Oncol. 2008, 26, 4509–4511. [Google Scholar] [CrossRef] [PubMed]
- Fadugba, O.; Nguyen, T.T.; McGill, J.; Wagner-Johnston, N. De novo large B-cell lymphoma in a parenchymal brain lesion: Evidence of clonal evolution from splenic marginal zone lymphoma. J. Clin. Oncol. 2011, 29, e234–e236. [Google Scholar] [CrossRef] [PubMed]
- Wedgwood, A.; Medeiros, L.J.; Romaguera, J.E. CD20+ nodal marginal zone B-cell lymphoma with CD20-recurrence as an intracranial dural-based mass. Leuk. Lymphoma 2006, 47, 2253–2256. [Google Scholar] [CrossRef]
- Wada, Y.; Nishimura, Y.; Hashimoto, K. A patient with splenic marginal zone lymphoma presenting with spastic paraplegia as the initial symptom. Case Rep. Neurol. 2011, 3, 39–44. [Google Scholar] [CrossRef]
- Gotlib, V.; Singareddy, S.; Gergis, U.; Vakios, J.; Guevara, E.; Chadburn, A.; Yavorkovsky, L.L.; Patel, A.; Butt, A.; Nayak, A. Leptomeningeal involvement in a patient with splenic lymphoma with villous lymphocytes. Leuk. Lymphoma 2002, 43, 1337–1340. [Google Scholar] [CrossRef]
- Bruna, J.; Martinez-Yelamos, S.; Alonso, E.; Romagosa, V.; Arruga, J.; Arruga, J.; Domingo, A.; Rojas-Marcos, I.; Petit, J.; Rubio, F. Meningeal lymphomatosis as the first manifestation of splenic marginal zone lymphoma. Int. J. Hematol. 2005, 82, 63–65. [Google Scholar] [CrossRef]
- Yamazaki, K.; Shimizu, S.; Negami, T.; Sawada, K.; Nanasawa, H.; Ohta, M.; Konda, S.; Okuda, K. Leukemic meningitis in a patient with splenic lymphoma with villous lymphocytes (SLVL). Meningitis as a possible initial manifestation of SLVL. Cancer 1994, 74, 61–65. [Google Scholar] [CrossRef]
- Angelopoulou, M.K.; Vassilakopoulos, T.P.; Konstantinou, E.; Boutsikas, G.; Asimakopoulos, J.V.; Triantafyllou, E.F.; Petevi, K.; Kanellopoulos, A.; Moschogiannis, M.; Pangalis, G.A.; et al. Central nervous system involvement in primary bone marrow or splenic marginal zone lymphoma: Report of two cases and review of the literature. Hematol. Oncol. 2019, 37, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Weidauer, S.; Wagner, M.; Enkirch, S.J.; Hattingen, E. CNS Infections in Immunoincompetent Patients: Neuroradiological and Clinical Features. Clin. Neuroradiol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Rommer, P.S.; Milo, R.; Han, M.H.; Satyanarayan, S.; Sellner, J.; Hauer, L.; Illes, Z.; Warnke, C.; Laurent, S.; Weber, M.S.; et al. Immunological Aspects of Approved MS Therapeutics. Front. Immunol. 2019, 10, 1564. [Google Scholar] [CrossRef] [PubMed]
- Haggiag, S.; Prosperini, L.; Galgani, S.; Pozzilli, C.; Pinnetti, C. Extratemporal herpes encephalitis during natalizumab treatment: A case report. Mult. Scler. Relat. Disord. 2016, 10, 134–136. [Google Scholar] [CrossRef]
- Jaijakul, S.; Salazar, L.; Wootton, S.H.; Aguilera, E.; Hasbun, R. The clinical significance of neutrophilic pleocytosis in cerebrospinal fluid in patients with viral central nervous system infections. Int. J. Infect. Dis. 2017, 59, 77–81. [Google Scholar] [CrossRef]
- Tumpey, T.M.; Chen, S.H.; Oakes, J.E.; Lausch, R.N. Neutrophil-mediated suppression of virus replication after herpes simplex virus type 1 infection of the murine cornea. J. Virol. 1996, 70, 898–904. [Google Scholar] [CrossRef]
- Amin, I.; Younas, S.; Afzal, S.; Shahid, M.; Idrees, M. Herpes Simplex Virus Type 1 and Host Antiviral Immune Responses: An Update. Viral Immunol. 2019, 32, 424–429. [Google Scholar] [CrossRef]
- Solomon, T.; Michael, B.D.; Smith, P.E.; Sanderson, F.; Davies, N.W.; Hart, I.J.; Holland, M.; Easton, A.; Buckley, C.; Kneen, R.; et al. Management of suspected viral encephalitis in adults—Association of British Neurologists and British Infection Association National Guidelines. J. Infect. 2012, 64, 347–373. [Google Scholar] [CrossRef]
- Karrasch, M.; Liermann, K.; Betz, B.B.; Wagner, S.; Scholl, S.; Dahms, C.; Sauerbrei, A.; Kunze, A. Rapid acquisition of acyclovir resistance in an immunodeficient patient with herpes simplex encephalitis. J. Neurol. Sci. 2018, 384, 89–90. [Google Scholar] [CrossRef]
- Carod Artal, F.J. Clinical management of infectious cerebral vasculitides. Expert Rev. Neurother. 2016, 16, 205–221. [Google Scholar] [CrossRef]
- Bradshaw, M.J.; Venkatesan, A. Herpes Simplex Virus-1 Encephalitis in Adults: Pathophysiology, Diagnosis, and Management. Neurotherapeutics 2016, 13, 493–508. [Google Scholar] [CrossRef] [PubMed]
- Kamiya-Matsuoka, C.; Paker, A.M.; Chi, L.; Youssef, A.; Tummala, S.; Loghin, M.E. Posterior reversible encephalopathy syndrome in cancer patients: A single institution retrospective study. J. Neurooncol. 2016, 128, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, A.; Klein, R. Posterior reversible encephalopathy syndrome (PRES) after combination chemotherapy for lymphoma. Am. J. Ther. 2014, 21, e137–e142. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Kasparian, S.; Iyer, S.; Pingali, S.R. Cryptococcal meningoencephalitis in patients with mantle cell lymphoma on ibrutinib. Ecancermedicalscience 2018, 12, 836. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nardone, R.; Carnicelli, L.; Brigo, F.; Pikija, S.; Hauer, L.; Sellner, J. Cerebral HSV-1 Vasculitis as a Fatal Complication of Immunosuppression in Non-Hodgkin´s Lymphoma: A Case Report and Review of the Literature. Pathogens 2020, 9, 193. https://doi.org/10.3390/pathogens9030193
Nardone R, Carnicelli L, Brigo F, Pikija S, Hauer L, Sellner J. Cerebral HSV-1 Vasculitis as a Fatal Complication of Immunosuppression in Non-Hodgkin´s Lymphoma: A Case Report and Review of the Literature. Pathogens. 2020; 9(3):193. https://doi.org/10.3390/pathogens9030193
Chicago/Turabian StyleNardone, Raffaele, Luca Carnicelli, Francesco Brigo, Slaven Pikija, Larissa Hauer, and Johann Sellner. 2020. "Cerebral HSV-1 Vasculitis as a Fatal Complication of Immunosuppression in Non-Hodgkin´s Lymphoma: A Case Report and Review of the Literature" Pathogens 9, no. 3: 193. https://doi.org/10.3390/pathogens9030193
APA StyleNardone, R., Carnicelli, L., Brigo, F., Pikija, S., Hauer, L., & Sellner, J. (2020). Cerebral HSV-1 Vasculitis as a Fatal Complication of Immunosuppression in Non-Hodgkin´s Lymphoma: A Case Report and Review of the Literature. Pathogens, 9(3), 193. https://doi.org/10.3390/pathogens9030193






