Characterization of Haemonchus contortus Excretory/Secretory Antigen (ES-15) and Its Modulatory Functions on Goat Immune Cells In Vitro
Abstract
1. Introduction
2. Results
2.1. Cloning of HcES-15 Gene
2.2. Construction and Identification of the Recombinant pET32a (+)-HcES-15 Plasmid
2.3. Sequence and Phylogenetic Analysis of HcES-15
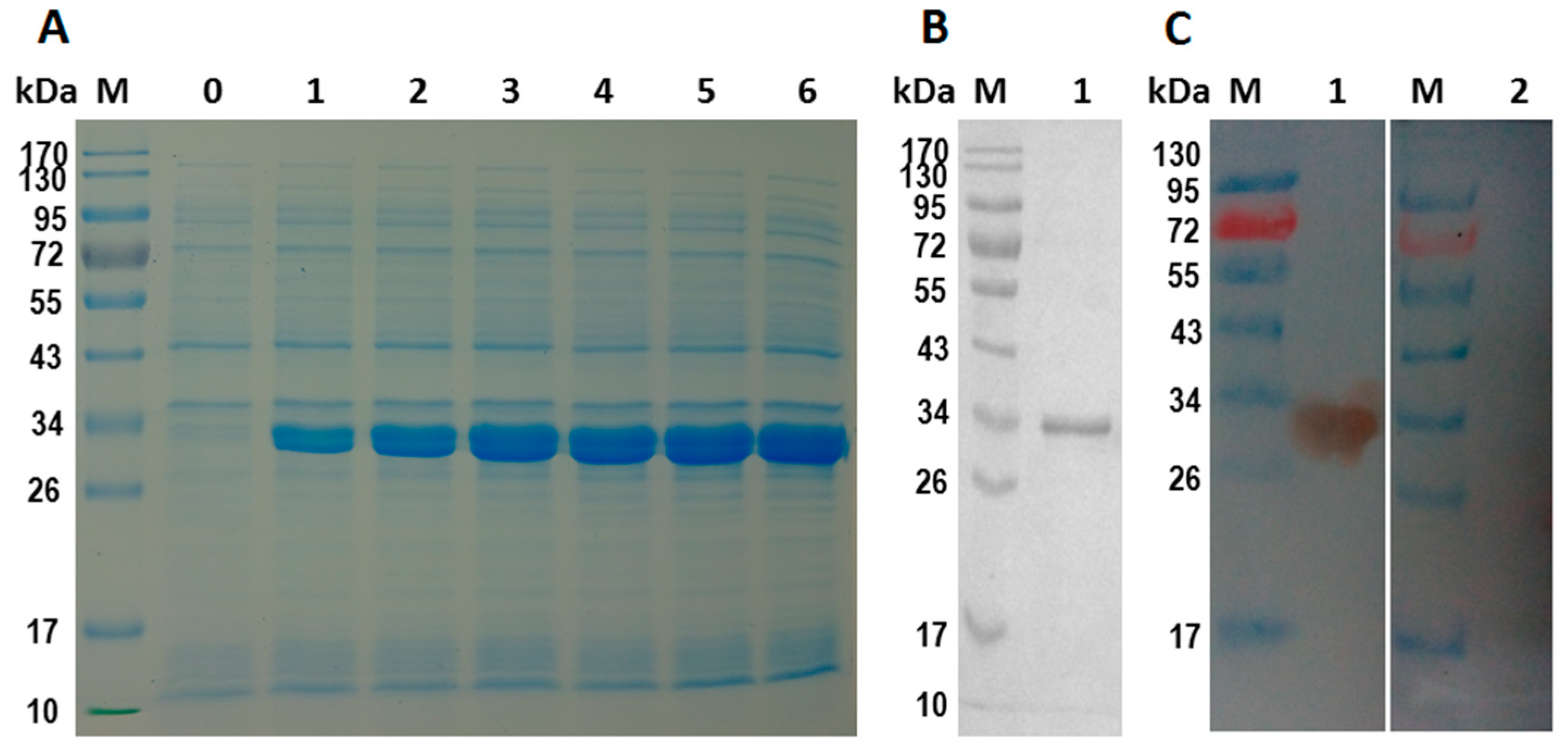
2.4. Expression and Purification of rHcES-15 Protein
2.5. Western-Blot Analysis of rHcES-15 Protein
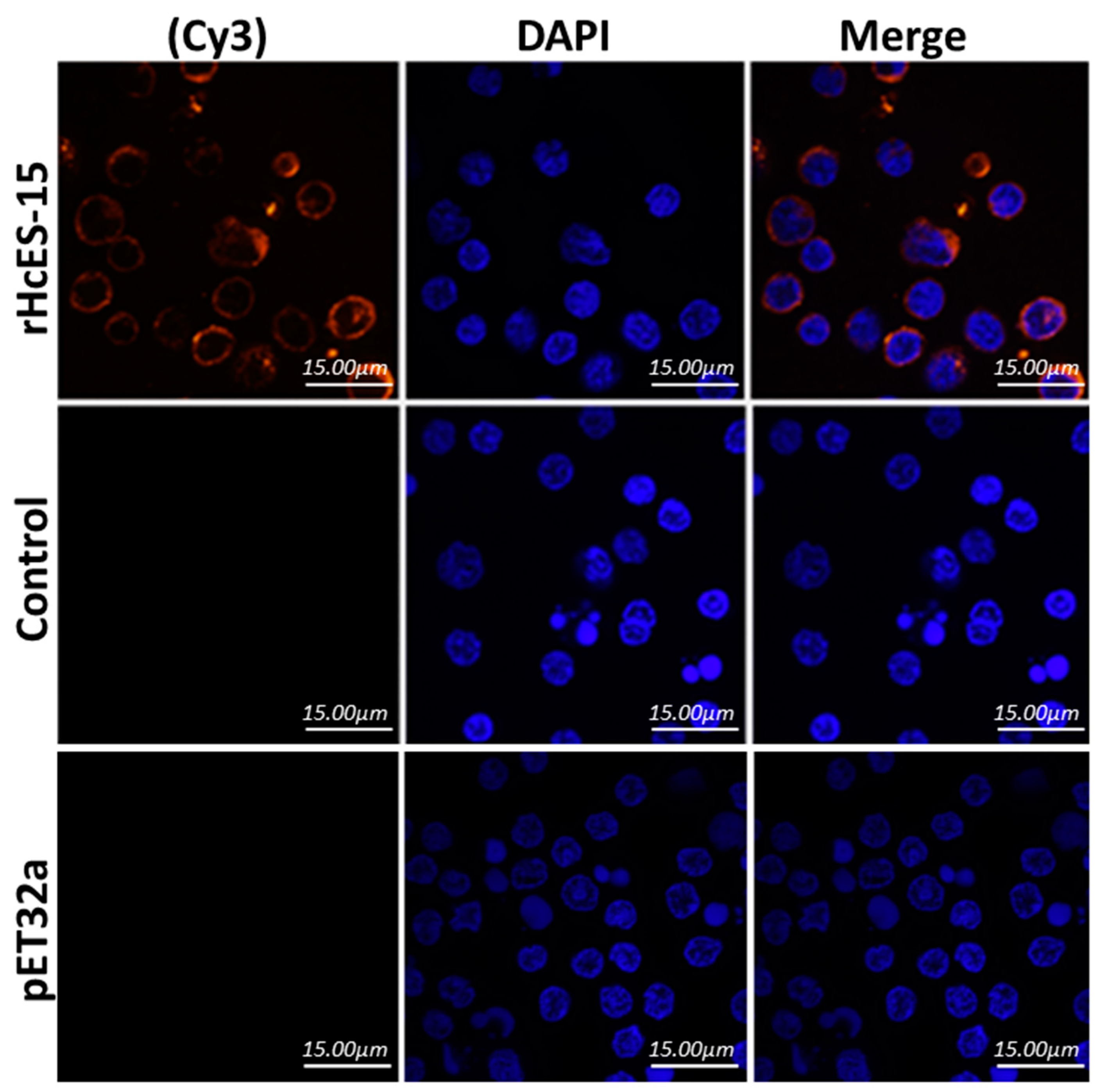
2.6. Binding of rHcES-15 to Goat PBMCs
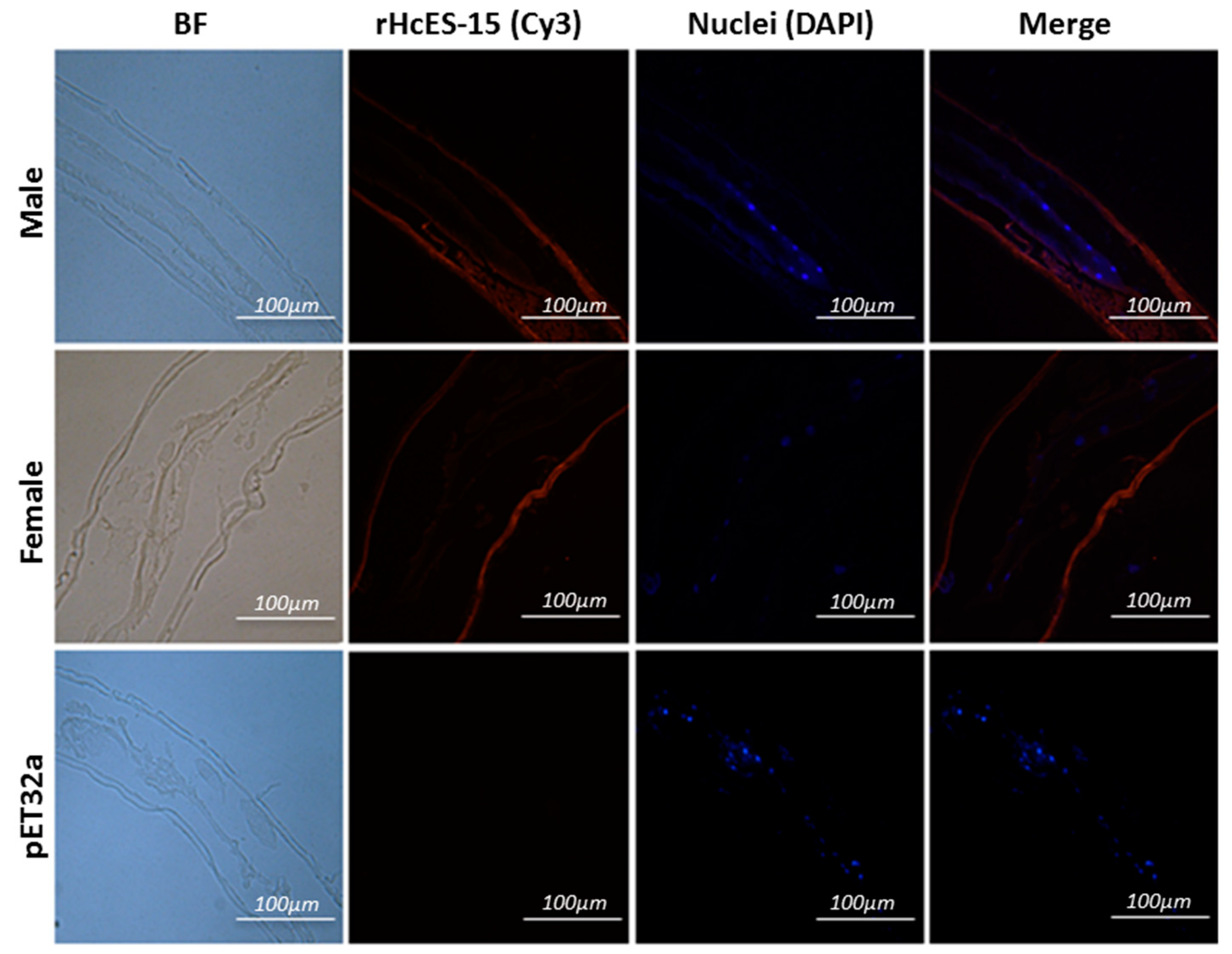
2.7. Expression of HcES-15 in Adult Worms of H. contortus
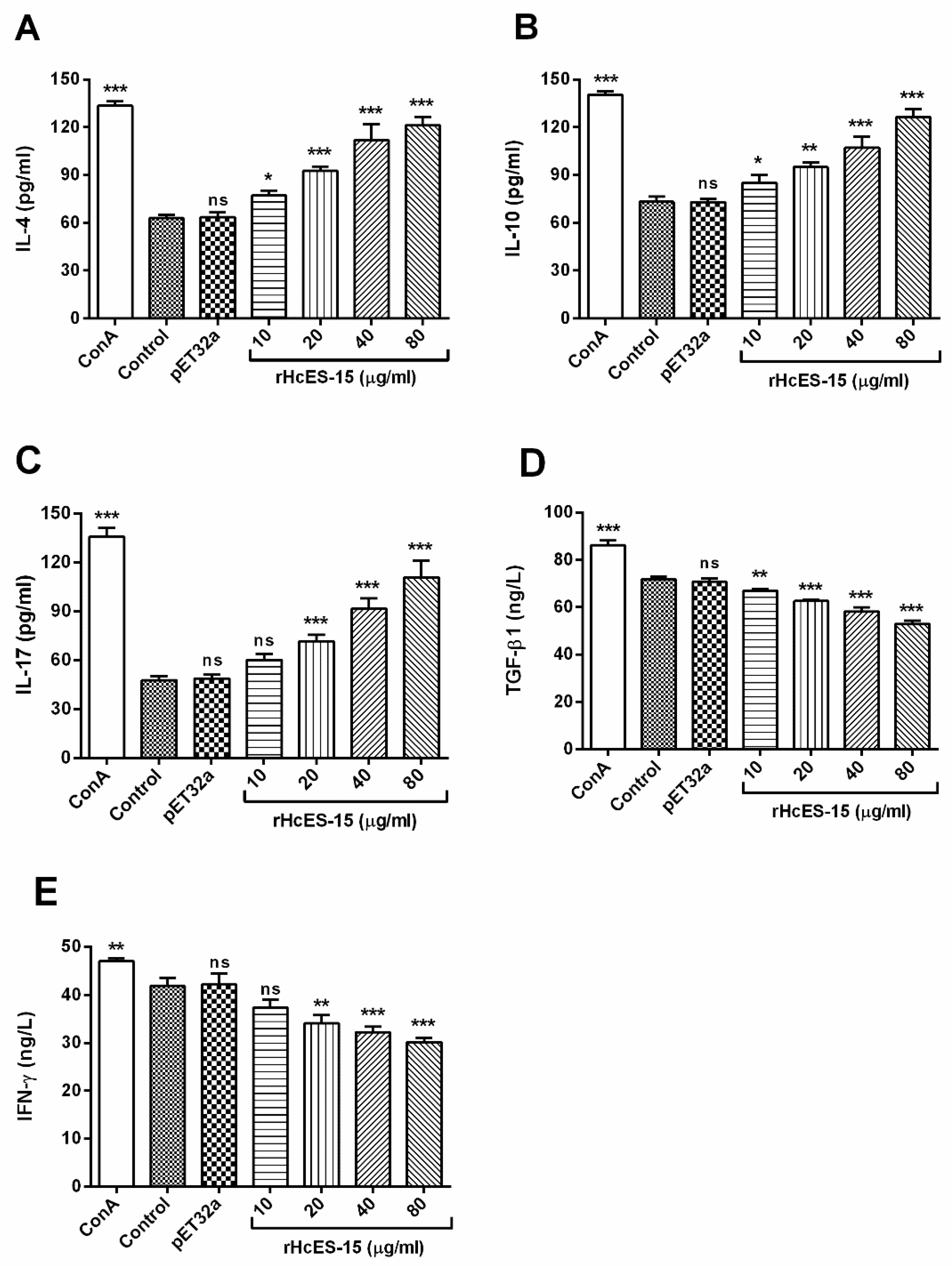
2.8. Detection of the Cytokine Levels by ELISA
2.9. rHcES-15 Effected the Proliferation of Goat PBMCs
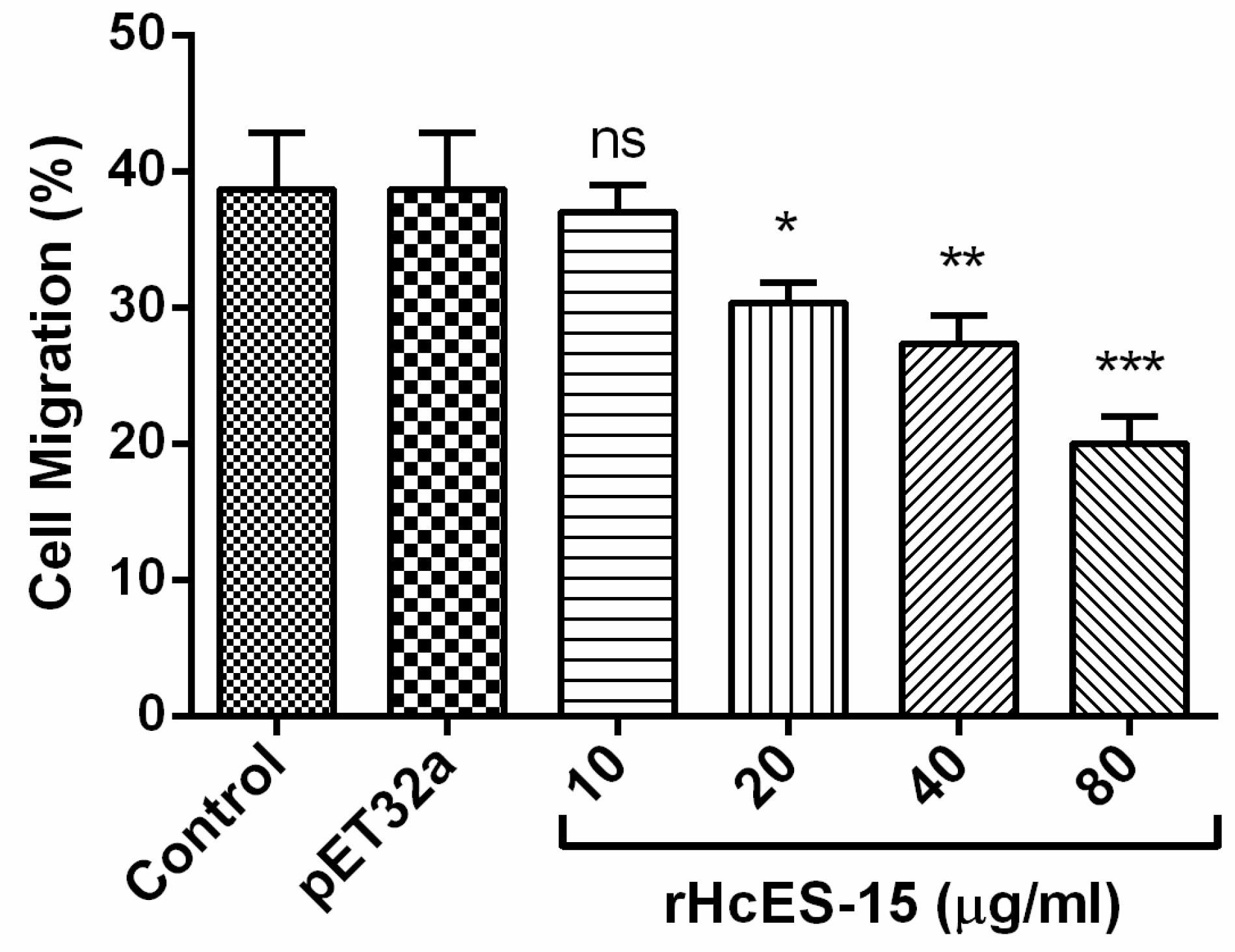
2.10. Cell Migration Assay
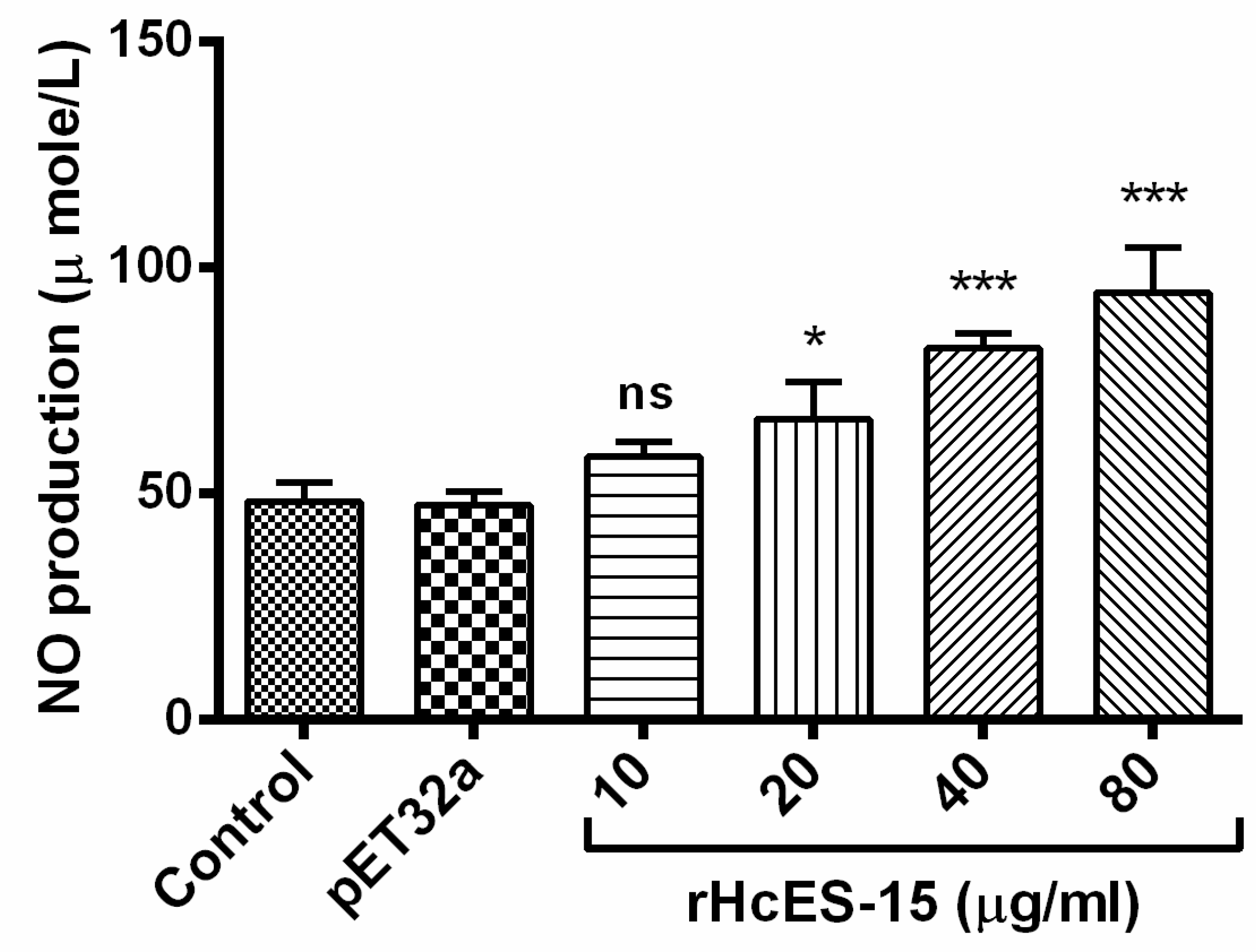
2.11. Nitric Oxide Production Assay
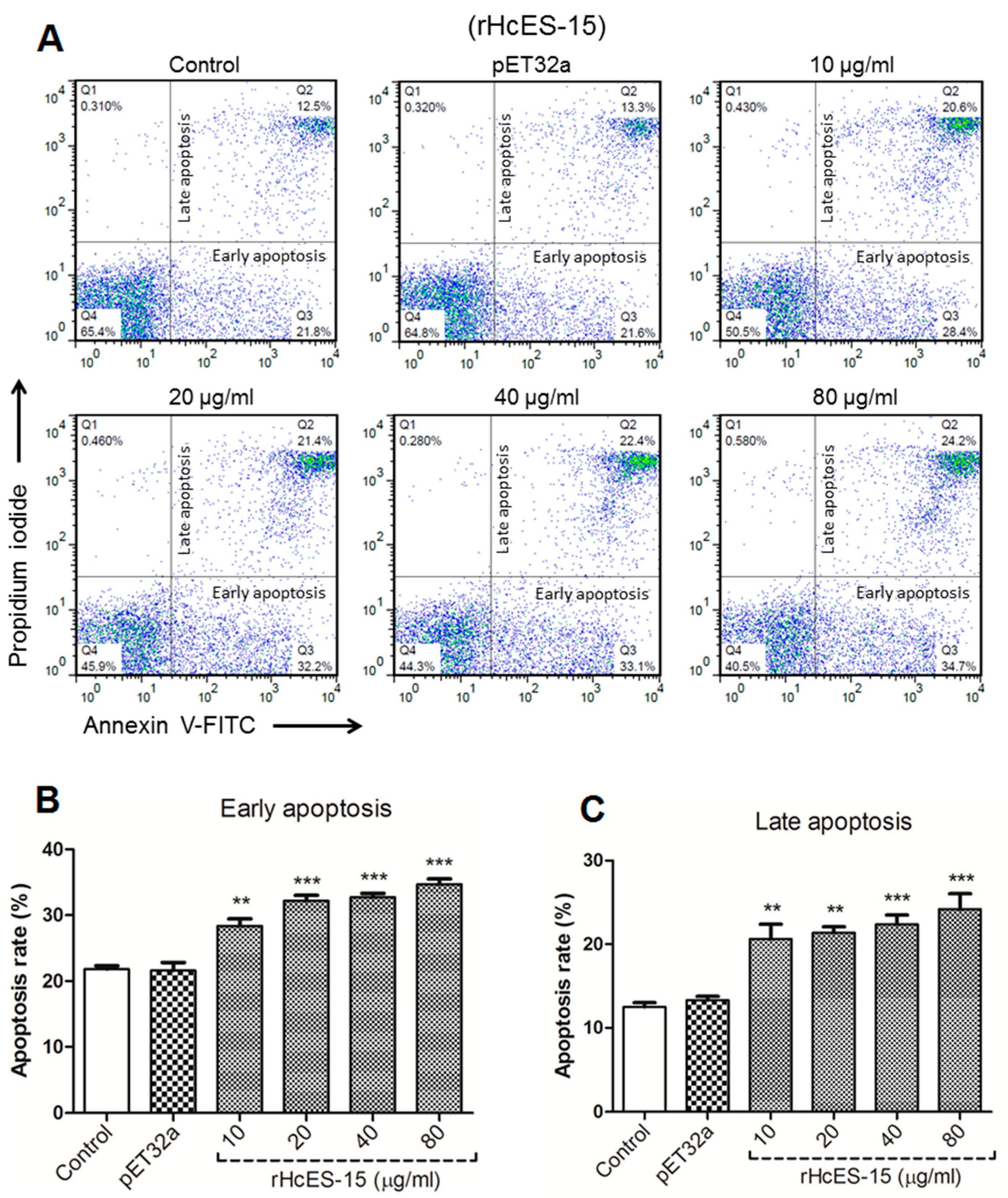
2.12. rHcES-15 Protein Enhance Apoptosis of Goat PBMCs
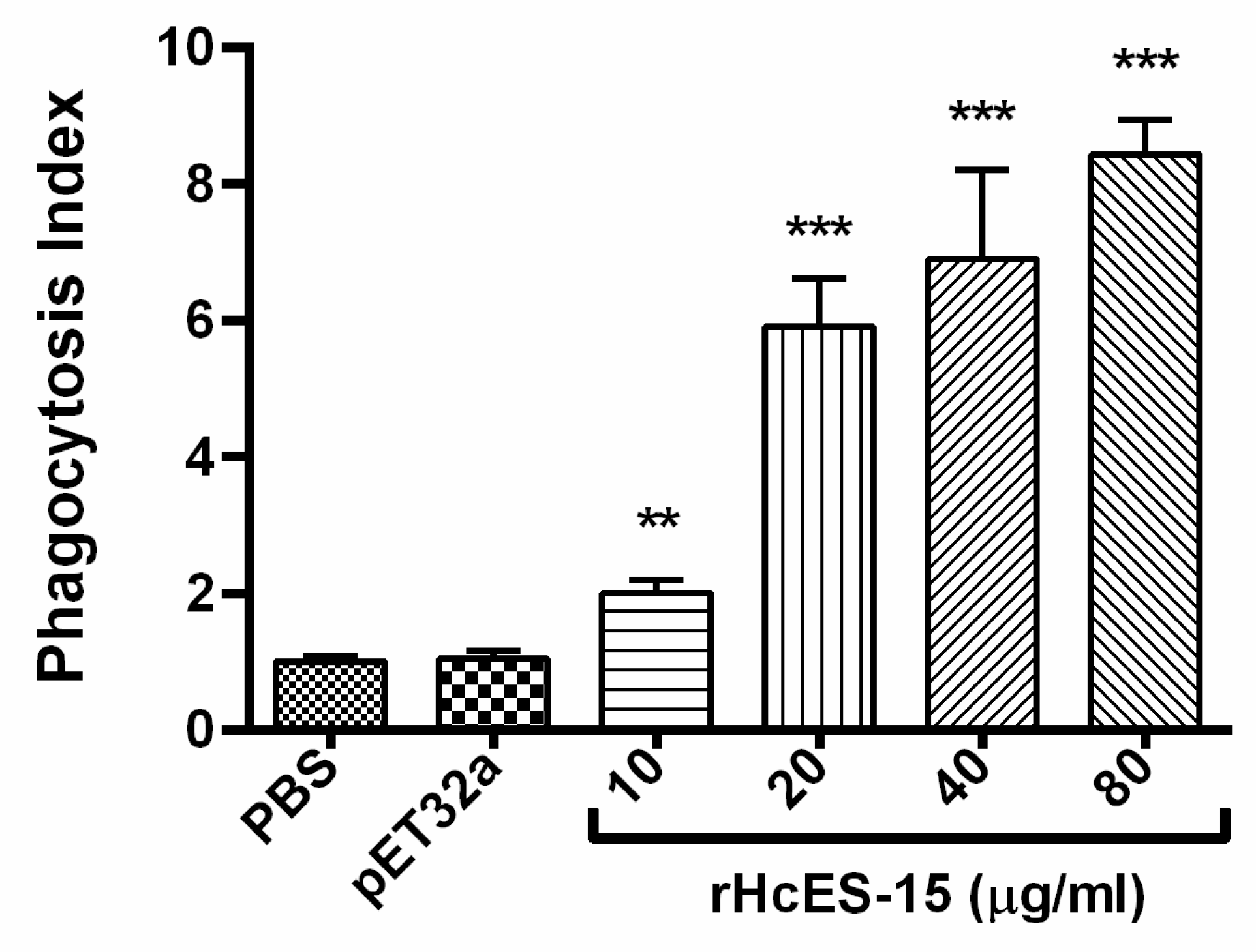
2.13. rHcES-15 Stimulated Phagocytosis of Goat Monocytes
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Animals and Parasites
4.3. Isolation of PBMCs and Monocytes
4.4. Synthesis of H. contortus cDNA
4.5. Molecular Cloning of HcES-15 Gene and Expression of rHcES-15 Protein
4.6. Alignments and Phylogenetic Analysis
4.7. Production of Antibodies
4.8. Immunoblot Analysis for the rHcES-15
4.9. Binding of rHcES-15 to Goat PBMCs
4.10. Localization of HcES-15 in Adult H. contortus Worms
4.11. Analysis of Cytokine Levels of PBMCs Treated with rHcES-15
4.12. Cell Migration Assay
4.13. Cell Proliferation Assay
4.14. Nitric Oxide Production Assay
4.15. Cell Apoptosis Assay
4.16. Cell Phagocytosis Activity
4.17. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Schallig, H.D.; Van Leeuwen, M.A.; Verstrepen, B.E.; Cornelissen, A.W. Molecular characterization and expression of two putative protective excretory secretory proteins of Haemonchus contortus1. Mol. Biochem. Parasitol. 1997, 88, 203–213. [Google Scholar] [CrossRef]
- Shalaby, H.A. Anthelmintics Resistance. How to Overcome it? Iran. J. Parasitol. 2013, 8, 18–32. [Google Scholar] [PubMed]
- Yatsuda, A.P.; Krijgsveld, J.; Cornelissen, A.W.; Heck, A.J.; de Vries, E. Comprehensive analysis of the secreted proteins of the parasite Haemonchus contortus reveals extensive sequence variation and differential immune recognition. J. Biol. Chem. 2003, 278, 16941–16951. [Google Scholar] [CrossRef] [PubMed]
- Lightowlers, M.; Rickard, M. Excretory–secretory products of helminth parasites: Effects on host immune responses. Parasitology 1988, 96, S123–S166. [Google Scholar] [CrossRef]
- Yan, F.; Xu, L.; Liu, L.; Yan, R.; Song, X.; Li, X. Immunoproteomic analysis of whole proteins from male and female adult Haemonchus contortus. Vet. J. 2010, 185, 174–179. [Google Scholar] [CrossRef]
- Hawdon, J.M.; Jones, B.F.; Perregaux, M.A.; Hotez, P.J. Ancylostoma caninum: Metalloprotease release coincides with activation of infective larvae In Vitro. Exp. Parasitol. 1995, 80, 205–211. [Google Scholar] [CrossRef]
- Craig, H.; Wastling, J.M.; Knox, D.P. A preliminary proteomic survey of the In Vitro excretory/secretory products of fourth-stage larval and adult Teladorsagia circumcincta. Parasitology 2006, 132, 535–543. [Google Scholar] [CrossRef]
- Saverwyns, H.; Visser, A.; Nisbet, A.J.; Peelaers, I.; Gevaert, K.; Vercruysse, J.; Claerebout, E.; Geldhof, P. Identification and characterization of a novel specific secreted protein family for selected members of the subfamily Ostertagiinae (Nematoda). Parasitology 2008, 135, 63–70. [Google Scholar] [CrossRef]
- Yatsuda, A.P.; Eysker, M.; Vieira-Bressan, M.C.; De Vries, E. A family of activation associated secreted protein (ASP) homologues of Cooperia punctata. Res. Vet. Sci. 2002, 73, 297–306. [Google Scholar] [CrossRef]
- Jasmer, D.P.; McGuire, T.C. Protective immunity to a blood-feeding nematode (Haemonchus contortus) induced by parasite gut antigens. Infect. Immun. 1991, 59, 4412–4417. [Google Scholar] [CrossRef]
- Jasmer, D.P.; McGuire, T.C. Antigens with application toward immune control of blood-feeding parasitic nematodes. Br. Vet. J. 1996, 152, 251–268. [Google Scholar] [CrossRef]
- Smith, W.D.; Smith, S.K.; Murray, J.M. Protection studies with integral membrane fractions of Haemonchus contortus. Parasite Immunol. 1994, 16, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.S.; Munn, E.A.; Graham, M.; Tavernor, A.S.; Greenwood, C.A. Purification and evaluation of the integral membrane protein H11 as a protective antigen against Haemonchus contortus. Int. J. Parasitol. 1993, 23, 271–280. [Google Scholar] [CrossRef]
- Smith, S.K.; Smith, W.D. Immunisation of sheep with an integral membrane glycoprotein complex of Haemonchus contortus and with its major polypeptide components. Res. Vet. Sci. 1996, 60. [Google Scholar] [CrossRef]
- Schallig, H.D.; van Leeuwen, M.A.; Cornelissen, A.W. Protective immunity induced by vaccination with two Haemonchus contortus excretory secretory proteins in sheep. Parasite Immunol. 1997, 19, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Miller, H. The protective mucosal response against gastrointestinal nematodes in ruminants and laboratory animals. Vet. Immunol. Immunopathol. 1984, 6, 167–259. [Google Scholar] [CrossRef]
- Sher, A.; Coffman, R. Regulation of immunity to parasites by T cells and T cell-derived cytokines. Annu. Rev. Immunol. 1992, 10, 385–409. [Google Scholar] [CrossRef]
- Gill, H.; Watson, D.; Brandon, M. Monoclonal antibody to CD4+ T cells abrogates genetic resistance to Haemonchus contortus in sheep. Immunol. 1993, 78, 43. [Google Scholar]
- Gadahi, J.A.; Yongqian, B.; Ehsan, M.; Zhang, Z.C.; Wang, S.; Yan, R.F.; Song, X.K.; Xu, L.X.; Li, X.R. Haemonchus contortus excretory and secretory proteins (HcESPs) suppress functions of goat PBMCs In Vitro. Oncotarget 2016, 7, 35670–35679. [Google Scholar] [CrossRef]
- Gadahi, J.A.; Li, B.; Ehsan, M.; Wang, S.; Zhang, Z.; Wang, Y.; Hasan, M.W.; Yan, R.; Song, X.; Xu, L. Recombinant Haemonchus contortus 24 kDa excretory/secretory protein (rHcES-24) modulate the immune functions of goat PBMCs In Vitro. Oncotarget 2016, 7, 83926–83937. [Google Scholar] [CrossRef]
- Sun, Y.; Yan, R.; Muleke, C.I.; Zhao, G.; Xu, l.; Li, X. Recombinant Galectins of Haemonchus contortus Parasite Induces Apoptosis in the Peripheral Blood Lymphocytes of Goat. Int. J. Pept. Res. Ther. 2007, 13, 387–392. [Google Scholar] [CrossRef]
- Gadahi, J.A.; Wang, S.; Bo, G.; Ehsan, M.; Yan, R.; Song, X.; Xu, L.; Li, X. Proteomic Analysis of the Excretory and Secretory Proteins of Haemonchus contortus (HcESP) Binding to Goat PBMCs In Vivo Revealed Stage-Specific Binding Profiles. PLoS ONE 2016, 11, 0159796. [Google Scholar] [CrossRef] [PubMed]
- Schallig, H.D.; Van Leeuwen, M.A.; Hendrikx, W.M. Immune responses of Texel sheep to excretory/secretory products of adult Haemonchus contortus. Parasitology 1994, 108, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Schallig, H.D.F.H.; Van Leeuwen, M.A.W.; Hendrikx, W.M.L. Isotype-specific serum antibody responses of sheep to Haemonchus contortus antigens. Vet. Parasitol. 1995, 56, 149–162. [Google Scholar] [CrossRef]
- Savin, K.W.; Dopheide, T.A.A.; Frenkel, M.J.; Wagland, B.M.; Grant, W.N.; Ward, C.W. Characterization, cloning and host-protective activity of a 30-kilodalton glycoprotein secreted by the parasitic stages of Trichostrongylus colubriformis. Mol. Biochem. Parasitol. 1990, 41, 167–176. [Google Scholar] [CrossRef]
- Huang, H.; Li, Y.; Qi, X. Cytokine signaling in the differentiation of innate effector cells. Jak-Stat 2013, 2, e23531. [Google Scholar] [CrossRef]
- Kikodze, N.; Pantsulaia, I.; Rekhviashvili, K.; Iobadze, M.; Dzhakhutashvili, N.; Pantsulaia, N.; Kukuladze, N.; Bikashvili, N.; Metreveli, D.; Chikovani, T. Cytokines and T regulatory cells in the pathogenesis of type 1 diabetes. Georgian. Med. News. 2013, 222, 29–35. [Google Scholar]
- Shakya, K.P.; Miller, J.E.; Horohov, D.W. A Th2 type of immune response is associated with increased resistance to Haemonchus contortus in naturally infected Gulf Coast Native lambs. Vet. Parasitol. 2009, 163, 57–66. [Google Scholar] [CrossRef]
- Uchikawa, R.; Matsuda, S.; Arizono, N. Suppression of gamma interferon transcription and production by nematode excretory-secretory antigen during polyclonal stimulation of rat lymph node T cells. Infect. Immun. 2000, 68, 6233–6239. [Google Scholar] [CrossRef]
- Gadahi, J.A.; Ehsan, M.; Wang, S.; Zhang, Z.; Yan, R.; Song, X.; Xu, L.; Li, X. Recombinant protein of Haemonchus contortus small GTPase ADP-ribosylation factor 1 (HcARF1) modulate the cell mediated immune response In Vitro. Oncotarget 2017, 8, 112211–112221. [Google Scholar] [CrossRef]
- Taylor, A.; Verhagen, J.; Blaser, K.; Akdis, M.; Akdis, C.A. Mechanisms of immune suppression by interleukin-10 and transforming growth factor-beta: The role of T regulatory cells. Immunology 2006, 117, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Grencis, R.K.; Humphreys, N.E.; Bancroft, A.J. Immunity to gastrointestinal nematodes: Mechanisms and myths. Immunol. Rev. 2014, 260, 183–205. [Google Scholar] [CrossRef] [PubMed]
- Schopf, L.R.; Hoffmann, K.F.; Cheever, A.W.; Urban, J.F.; Jr Wynn, T.A. IL-10 is critical for host resistance and survival during gastrointestinal helminth infection. J. Immunol. 2002, 168, 2383–2392. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Gao, H.; Qian, Y. Th17 differentiation and their pro-inflammation function. Adv. Exp. Med. Biol. 2014, 841, 99–151. [Google Scholar] [PubMed]
- Gadahi, J.A.; Ehsan, M.; Wang, S.; Zhang, Z.; Wang, Y.; Yan, R.; Song, X.; Xu, L.; Li, X. Recombinant protein of Haemonchus contortus 14-3-3 isoform 2 (rHcftt-2) decreased the production of IL-4 and suppressed the proliferation of goat PBMCs In Vitro. Exp. Parasitol. 2016, 171, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Morissette, R.; Schoenhoff, F.; Xu, Z.; Shilane, D.A.; Griswold, B.F.; Chen, W.; Yang, J.; Zhu, J.; Fert-Bober, J.; Sloper, L.; et al. Transforming Growth Factor-β and Inflammation in Vascular (Type IV) Ehlers–Danlos Syndrome. Circ. Cardiovasc. Genet. 2014, 7, 80–88. [Google Scholar]
- Li, M.O.; Wan, Y.Y.; Sanjabi, S.; Robertson, A.K.; Flavell, R.A. Transforming growth factor-beta regulation of immune responses. Ann. Rev. Immunol. 2006, 24, 99–146. [Google Scholar] [CrossRef]
- Lebman, D.A.; Edmiston, J.S. The role of TGF-beta in growth, differentiation, and maturation of B lymphocytes. Microbes Infect. 1999, 1, 1297–1304. [Google Scholar] [CrossRef]
- Sanjabi, S.; Oh, S.A.; Li, M.O. Regulation of the Immune Response by TGF-beta: From Conception to Autoimmunity and Infection. Cold Spring Harb Perspect Biol. 2017, 9, 6. [Google Scholar] [CrossRef]
- Ghoreschi, K.; Laurence, A.; Yang, X.P.; Tato, C.M.; McGeachy, M.J.; Konkel, J.E.; Ramos, H.L.; Wei, L.; Davidson, T.S.; Bouladoux, N.; et al. Generation of pathogenic TH17 cells in the absence of TGF-β signalling. Nature 2010, 467, 967–971. [Google Scholar] [CrossRef]
- Turner, D.G.; Wildblood, L.A.; Inglis, N.F.; Jones, D.G. Characterization of a galectin-like activity from the parasitic nematode, Haemonchus contortus, which modulates ovine eosinophil migration In Vitro. Vet. Immunol. Immunopathol. 2008, 122, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Brunet, L.R. Nitric oxide in parasitic infections. Int. Immunopharmacol. 2001, 1, 1457–1467. [Google Scholar] [CrossRef]
- Miljkovic, D.; Cvetkovic, I.; Vuckovic, O.; Stosic-Grujicic, S.; Mostarica, S.M.; Trajkovic, V. The role of interleukin-17 in inducible nitric oxide synthase-mediated nitric oxide production in endothelial cells. Cell. Mol. Life. Sci. 2003, 60, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, M.-U.-H.; Memon, M.A.; Jamil, T.; Naqvi, S.Z.; Aimulajiang, K.; Gadahi, J.A.; Xu, L.; Song, X.; Li, X.; Yan, R. Galectin Domain Containing Protein from Haemonchus contortus Modulates the Immune Functions of Goat PBMCs and Regulates CD4+ T-Helper Cells In Vitro. Biomolecules 2020, 10, 116. [Google Scholar] [CrossRef]
- Barbosa, A.P.; Campos, D.M.; Semerene, A.R.; Teixeira, A.R.; Santana, J.M. Lagochilascaris minor third-stage larvae secrete metalloproteases with specificity for fibrinogen and native collagen. Microbes. Infect. 2006, 8, 2725–2732. [Google Scholar] [CrossRef]
- Benjathummarak, S.; Kumsiri, R.; Nuamtanong, S.; Kalambaheti, T.; Waikagul, J.; Viseshakul, N.; Maneerat, Y. Third-stage Gnathostoma spinigerum larva excretory secretory antigens modulate function of Fc gamma receptor I-mediated monocytes in peripheral blood mononuclear cell culture. Trop. Med. Health. 2016, 44, 5. [Google Scholar] [CrossRef]
- Chen, L.; Rao, K.V.; He, Y.X. Ramaswamy K: Skin-stage schistosomula of Schistosoma mansoni produce an apoptosis-inducing factor that can cause apoptosis of T cells. J. Biol. Chem. 2002, 277, 34329–34335. [Google Scholar] [CrossRef]
- James, E.R.; Green, D.R. Manipulation of apoptosis in the host-parasite interaction. Trends. Parasitol. 2004, 20, 280–287. [Google Scholar] [CrossRef]
- Babu, S.; Blauvelt, C.P.; Nutman, T.B. Filarial parasites induce NK cell activation, type 1 and type 2 cytokine secretion, and subsequent apoptotic cell death. J. Immunol. 2007, 179, 2445–2456. [Google Scholar] [CrossRef]
- Chen, H.Y.; Weng, I.C.; Li, C.S.; Wan, L.; Liu, F.T. Examination of Galectins in Phagocytosis. Methods Mol. Biol. 2015, 1207, 201–213. [Google Scholar]
- Wang, W.; Wang, S.; Zhang, H.; Yuan, C.; Yan, R.; Song, X.; Xu, L.; Li, X. Galectin Hco-gal-m from Haemonchus contortus modulates goat monocytes and T cell function in different patterns. Parasites Vectors 2014, 7, 342. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, I.C.; Mavrangelos, C.; Fung, K.; Ayhan, M.; Levichkin, I.; Johnston, A.; Zola, H.; Hoogenraad, N.J. Characterisation of the protein composition of peripheral blood mononuclear cell microsomes by SDS-PAGE and mass spectrometry. J. Immunol. Methods 2005, 305, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Ehsan, M.; Gao, W.; Gadahi, J.A.; Lu, M.; Liu, X.; Wang, Y.; Yan, R.; Xu, L.; Song, X.; Li, X. Arginine kinase from Haemonchus contortus decreased the proliferation and increased the apoptosis of goat PBMCs In Vitro. Parasites Vectors 2017, 10, 311. [Google Scholar] [CrossRef]
- Ehsan, M.; Wang, W.; Gadahi, J.A.; Hasan, M.W.; Lu, M.; Wang, Y.; Liu, X.; Haseeb, M.; Yan, R.; Xu, L.; et al. The Serine/Threonine-Protein Phosphatase 1 From Haemonchus contortus Is Actively Involved in Suppressive Regulatory Roles on Immune Functions of Goat Peripheral Blood Mononuclear Cells. Front. Immunol. 2018, 9, 1627. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, C.; Wang, L.; Lu, M.; Wang, Y.; Wen, Y.; Yan, R.; Xu, L.; Song, X.; Li, X. Transmembrane protein 147 (TMEM147): Another partner protein of Haemonchus contortus galectin on the goat peripheral blood mononuclear cells (PBMC). Parasites Vectors 2016, 9, 355. [Google Scholar] [CrossRef][Green Version]
- Lu, M.; Tian, X.; Yang, X.; Yuan, C.; Ehsan, M.; Liu, X.; Yan, R.; Xu, L.; Song, X.; Li, X. The N- and C-terminal carbohydrate recognition domains of Haemonchus contortus galectin bind to distinct receptors of goat PBMC and contribute differently to its immunomodulatory functions in host-parasite interactions. Parasites Vectors 2017, 10, 409. [Google Scholar] [CrossRef]

| Sequence Type | Nucleotides/Proteins Description | Identity (%) | Accession Number (GenBank) |
|---|---|---|---|
| Nucleic Acid | H. contortus 15 kDa excretory/secretory protein | 96 | AY821552.1 |
| H. contortus 15 kDa excretory/secretory protein | 95 | U64792.1 | |
| H. contortus isolate Bareilly p15 | 95 | JF826244.1 | |
| Haemonchus placei genome assembly | 96 | LM596575.1 | |
| 96 | LM589008.1 | ||
| 94 | LM588224.1 | ||
| 95 | LM585135.1 | ||
| 95 | LM591864.1 | ||
| 91 | LM587365.1 | ||
| 86 | LM594683.1 | ||
| Amino Acid | 15 kDa excretory/secretory protein (H. contortus) | 100 | AAV84000.1 |
| p15 (H. contortus) | 97 | CDJ85218.1 | |
| 15 kDa excretory/secretory protein (H. contortus) | 92 | CDJ84355.1 | |
| p15 (H. contortus) | 92 | CDJ85217.1 | |
| 15 kDa excretory/secretory protein (H. contortus) | 89 | O18518.1 | |
| p15 (H. contortus) | 88 | AEG76953.1 | |
| 15 kDa excretory/secretory protein (H. contortus) | 81 | CDJ84626.1 | |
| p15 (H. contortus) | 83 | CDJ84625.1 | |
| 15 kDa excretory/secretory protein (H. contortus) | 85 | CDJ84539.1 | |
| p15 (H. contortus) | 95 | CDJ84538.1 | |
| p15 (H. contortus) | 88 | CDJ85219.1 | |
| 30 kDa antigenic glycoprotein (T. colubriformis) | 38 | O97391.1 | |
| Unnamed protein product (H. contortus) | 25 | CDJ84854.1 | |
| Unnamed protein product (H. contortus) | 30 | CDJ81999.1 |
| Name | Sequence (5′ – 3′) | GenBank Accession Number | Restriction Sites |
|---|---|---|---|
| HcES-15 (Forward) | AAAGGATCCATGTTCTTCGC TTTTGC | AY821552.1 | BamH I |
| HcES-15 (Reverse) | CTGGAATTCTCAGTTGGGGGTATTGT | EcoR I |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ehsan, M.; Gadahi, J.A.; Hasan, M.W.; Haseeb, M.; Ali, H.; Yan, R.; Xu, L.; Song, X.; Zhu, X.-Q.; Li, X. Characterization of Haemonchus contortus Excretory/Secretory Antigen (ES-15) and Its Modulatory Functions on Goat Immune Cells In Vitro. Pathogens 2020, 9, 162. https://doi.org/10.3390/pathogens9030162
Ehsan M, Gadahi JA, Hasan MW, Haseeb M, Ali H, Yan R, Xu L, Song X, Zhu X-Q, Li X. Characterization of Haemonchus contortus Excretory/Secretory Antigen (ES-15) and Its Modulatory Functions on Goat Immune Cells In Vitro. Pathogens. 2020; 9(3):162. https://doi.org/10.3390/pathogens9030162
Chicago/Turabian StyleEhsan, Muhammad, Javaid Ali Gadahi, Muhammad Waqqas Hasan, Muhammad Haseeb, Haider Ali, Ruofeng Yan, Lixin Xu, Xiaokai Song, Xing-Quan Zhu, and Xiangrui Li. 2020. "Characterization of Haemonchus contortus Excretory/Secretory Antigen (ES-15) and Its Modulatory Functions on Goat Immune Cells In Vitro" Pathogens 9, no. 3: 162. https://doi.org/10.3390/pathogens9030162
APA StyleEhsan, M., Gadahi, J. A., Hasan, M. W., Haseeb, M., Ali, H., Yan, R., Xu, L., Song, X., Zhu, X.-Q., & Li, X. (2020). Characterization of Haemonchus contortus Excretory/Secretory Antigen (ES-15) and Its Modulatory Functions on Goat Immune Cells In Vitro. Pathogens, 9(3), 162. https://doi.org/10.3390/pathogens9030162








