Abstract
The present experimental study was conducted for the assessment of the efficacy of in vitro inhibition of myrrh oil on the propagation of Babesia bovis, B. divergens, B. bigemina, Theileria equi, and B. caballi and in vivo efficacy on B. microti in mice through fluorescence assay based on SYBR green I. The culture of B. divergens B. bovis and was used to evaluate the in vitro possible interaction between myrrh oil and other commercial compound, such as pyronaridine tetraphosphate (PYR), diminazene aceturate (DA), or luteolin. Nested-polymerase chain reaction protocol using primers of the small-subunit rRNA of B. microti was employed to detect any remnants of DNA for studied parasitic species either in blood or tissues. Results elucidated that; Myrrh oil significantly inhibit the growth at 1% of parasitic blood level for all bovine and equine piroplasm under the study. Parasitic regrowth was inhibited subsequently by viability test at 2 µg/mL for B. bigemina and B. bovis, and there was a significant improvement in the in vitro growth inhibition by myrrh oil when combined with DA, PYR, and luteolin. At the same time; mice treated with a combination of myrrh oil/DA showed a higher inhibition in emitted fluorescence signals than the group that challenged with 25 mg/kg of diminazene aceturate at 10 and 12 days post-infection. In conclusion, this study has recommended the myrrh oil to treat animal piroplasmosis, especially in combination with low doses of DA.
1. Introduction
Babesia and Theileria are tick-borne protozoal parasites which infect the erythrocytes of domesticated animals, and it is incriminated in producing significantly high economic losses in the livestock industry and animal trade all over the world [1]. The clinical picture of the disease is typically associated with hyperthermia, malaise, yellowish discoloration of all mucous membranes, especially conjunctiva, hemoglobin in the urine, and occasionally, the infected animal died [2]. B. divergens Babesia bovis, and Babesia bigemina were considered the main causative agents of babesiosis in cattle, that provoking a huge loss on the animal health and productivity [2]. Besides, B. divergens has public health significance and serious zoonotic importance in Europe [3]. On the other hand; Babesia caballi and Theileria equi considered the main causative agents in piroplasmosis of equines [4], but Unfortunately, bovine and equine Babesia infection have no suitable laboratory experimental animals to induce the in vivo studies for this parasites, and there are alternative that was conducted and established by scientists which are the mouse model infected with B. microti, which is rodent Babesia, and it was found that it is suitable for antibabesial drug evaluation against different Babesia spp., that infecting domestic and farm animals, since the inhibitory effect of the newly developed drug must be firstly evaluated in laboratory animals to determine the possible adverse effect of these hits before it is administered to animals under field condition [5]. Currently, the commercially available anti-piroplasm drugs have shown a toxic effect to the infected animal as imidocarb dipropionate as nausea, salivation, drowsiness, and recumbency, also other drugs as diminazene aceturate (DA) developed some degree of resistance from the treated parasite [1]. Accordingly, the discovery of alternatives that have more significant efficacy and safe anti-piroplasm agents is mandatory. In this attitude, using herbal therapies or compounds that extracted from the natural products might be an alternative strategy, such as the herb myrrh (Commiphora molmol), which has shown several clinical benefits, that is mainly attributed to its oil [6]. Myrrh oil has anti-inflammatory [7], analgesic [8], antischistosomal [9], fasciolicidal [10], antimoniezia [11], molluscicidal [12], acaricidal [13], mosquitocidal [14], antigiardial [15], anticoccidial [16] activities. However, the anti-piroplasm effect of myrrh oil was not yet experimentally conducted. Hence, this trial evaluated the potent inhibitory effect of myrrh oil as an anti-piroplasm candidate to inhibit the growth of bovine and equine piroplasmosis, and for Babesia microti in mice as an experimental model.
2. Results
2.1. In vitro Growth Inhibition Assay
The IC50s of myrrh oil revealed that the in vitro inhibitory effect of myrrh oil has a highly significant efficacy and susceptibility on equine piroplasm parasites than those observed in bovine Babesia parasites (Table 1), and the myrrh oil IC50 values to T. equi was similar to DA IC50 values (Table 1). 1 μg/mL myrrh oil significantly inhibits the in vitro growth of Babesia bigemina, Babesia bovis, B. caballi, and T. equi (P < 0.05) (Figure 1), but on the contrary, significantly inhibited B. divergens in vitro growth (P < 0.05) at 10 μg/mL (Figure 1). For regrowth assay; it was noted that Babesia divergens, T. equi and Babesia caballi were inhibited by viability test with 10 µg/mL (Table 2), and treatment of Babesia bovis and Babesia bigemina on the culture at 2 µg/mL myrrh oil prevented parasite regrowth (Table 2).

Table 1.
Evaluation of IC50 values of myrrh oil for bovine Babesia, equine Babesia, and Theileria parasites.
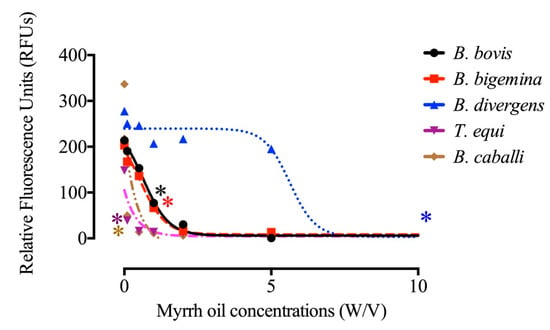
Figure 1.
Correlation between the log concentrations and relative fluorescence units (RFUs) of myrrh oil (µg/mL) on Babesia bigemina, Babesia bovis, B. divergens, B. caballi, and T. equi on the fourth day p.i. Every value is considered the mean of triplicate wells after subtraction of the background fluorescence for non-parasitized RBCs. Asterisks indicate a significant difference (ANOVA; * P < 0.05) between the myrrh oil-treated and the control cultures.

Table 2.
Myrrh oil evaluation for bovine Babesiosis, equine Babesia, and Theileria parasites by viability test results.
Several morphological changes in the parasites were observed after the treatment with myrrh oil in comparison with that recorded from the control cultures (Figure 2 and Figure 3), in which, parasites appeared degenerated from cultures of Babesia bovis (Figure 2B), Babesia bigemina (Figure 2D), Babesia caballi (Figure 3B), and Thieleria equi (Figure 3D) by light microscope. The pattern of parasitic growth in cultures using myrrh oil 250 µg/mL pretreated erythrocytes was similar to cultures used non-pretreated erythrocytes. Furthermore, erythrocyte morphology in pretreated cultures was similar to those the non-treated erythrocytes by light microscope (observations not listed).
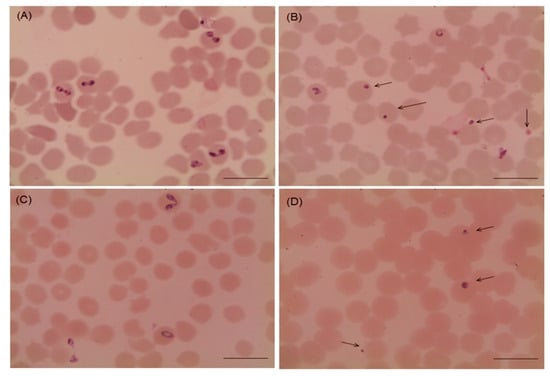
Figure 2.
Microscopy of Babesia bovis and Babesia bigemina handled with 10 µg/mL myrrh oil in cultures. (A) Babesia bovis control group, (B) myrrh oil-treated cultures group, (C) Babesia bigemina control, and (D) myrrh oil-treated cultures. The drug-treated cultures showed higher numbers of degenerated parasites indicated by arrows than the control cultures. Micrographs were taken on day 4 of treatment. Scale bars 10 μm.
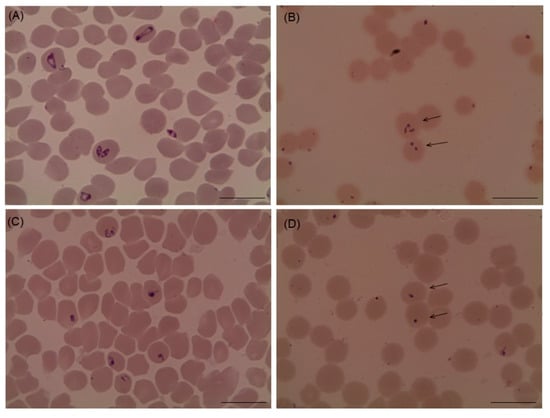
Figure 3.
microscopic examination of Babesia caballi and Theileria equi treated with 10 µg/mL myrrh oil cultures. (A) Babesia caballi control, (B) myrrh oil-treated cultures, (C) Theileria equi control, and (D) myrrh oil-treated cultures. The drug-treated cultures showed higher numbers of degenerated parasites indicated by arrows than the control cultures. Micrographs were taken on day 4 of treatment. Scale bars 10 μm.
2.2. Drug Combination Test
The significant in vitro inhibitory effect of myrrh oil combined with luteolin, DA, and pyronaridine tetraphosphate (PYR) on B. divergens and B. bovis was tested in this study, and results showed that, the inhibition rate of B. bovis was significantly high when treated with myrrh oil combined with DA, PYR, and luteolin at 0.50 IC50 myrrh oil and 0.12 IC50 DA, 0.12 IC50 myrrh oil and 0.50 IC50 PYR, and 0.25 IC50 myrrh oil and 0.50 IC50 luteolin (Table 3, Table 4, and Table 5). These findings confirmed the potential anti-B. bovis efficacy of myrrh oil, especially when administrated simultaneously in lower doses with other common antipiroplasma drugs, but on the contrary, combination of myrrh oil with other therapies did not enhance the in vitro inhibitory effect on B. divergens, and it was found that it is of no significant value (Table 3, Table 4, and Table 5).

Table 3.
Growth inhibition effect of myrrh oil combined with diminazene aceturate on B. divergens and B. bovis parasites.

Table 4.
Growth inhibition effect of myrrh oil combined with pyronaridine tetraphosphate on B. divergens and B. bovis parasites.

Table 5.
Growth inhibition effect of myrrh oil combined with luteolin on B. divergens and B. bovis parasites.
2.3. Chemotherapeutic Efficacy of Myrrh Oil on B. Microti Infection
The efficacy of in vivo inhibition of myrrh oil was detected against Babesia microti in a mouse model and explored that; myrrh oil alone exhibited inhibition (P < 0.05) in the emitted fluorescence signals from 12th–18th days p.i. in comparison with the positive control group. Peak fluorescence values were detected in the treated groups as 793.50, 1399, and 616.50 with 25 mg kg-1 diminazene aceturate, 200 mg kg-1 myrrh oil, also 100 mg kg-1 myrrh oil in combination with 12.5 mg kg−1 dimenazine aceturate at 10th days post infection, respectively, in contrary, positive control group, the average of maximum fluorescence values was 2161.11 at 12 days p.i. The intraperitoneal injections with 200 mg kg−1 for five successive days achieved 56.57% inhibition compared with 74.83% inhibition at 25 mg kg−1 DA at 12th day post infection. Of note, the inhibition in the fluorescence values were higher in mice manipulated with myrrh oil/diminazene aceturate combination than those obtained from mice treated with 25 mg kg−1 DA alone at 10 and 12 days p.i. Intraperitoneal injections of 100 mg kg-1 myrrh oil in combination with subcutaneous dose 12.5 mg kg−1 DA caused 61.08% and 77.80% inhibitory effect of growth at 10th and 12th days p.i., respectively compared with 49.91% and 74.83% inhibitions at 25 mg kg−1 DA at days 10 and 12 p.i., respectively (Figure 4).
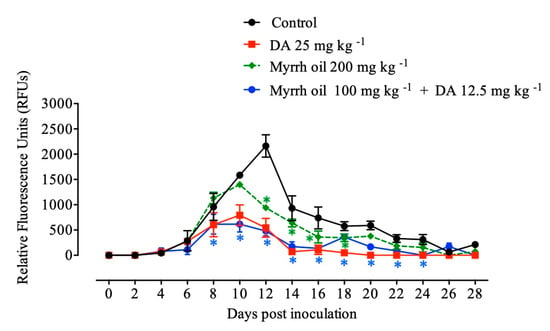
Figure 4.
Inhibition of growth by myrrh oil, diminazene aceturate (DA), and both drugs combined on Babesia microti. Every value is considered the mean ± standard deviation of five mice per experimental group. Asterisks indicate significant differences (ANOVA; * P < 0.05) between the myrrh oil–treated and control groups.
Mice that treated with a combination of myrrh oil with a low dose of Diminazene aceturate normalized the assessed hematological variables in similar to groups treated with 25 mg kg−1 DA (Figure 5). Such findings revealed the promising antibabesial efficacy of myrrh oil/DA combination therapy.
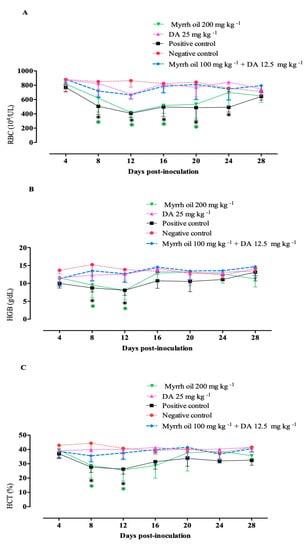
Figure 5.
Hematological variables in B. microti- infected mice subjected to myrrh oil. (A) RBCs. (B) Hemoglobin (HGB). (C) Hematocrit (HCT). Each value is the mean ± standard deviation of five mice per experimental group. Asterisks indicate a significant difference (ANOVA; * P < 0.05) between the treated or infected mice and the uninfected mice.
On day 28 p.i. a nested PCR protocol of B. microti using small subunit rRNA (ss-rRNA) gene to detect any remnant of the parasites in the blood samples and tissues of experimental mice. Unfortunately, positive results obtained—indicating that the parasite gene was found in the blood and all examined tissues from treated groups of mice with Diminazene aceturate alone or combined with myrrh oil (Figure 6).
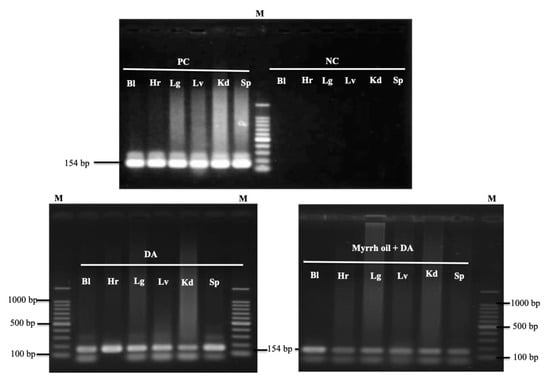
Figure 6.
PCR of the ss-rRNA gene in blood and different organs of B. microti–infected mice treated with ethanol (positive control), 25 mg kg−1 diminazene aceturate (DA), and DA (12.5 mg kg−1) combined with myrrh oil (100 mg kg−1). PC, positive control; NC, negative control; Bl, blood; Hr, heart; Lg, lung; Lv, liver; Kd, kidney; Sp, spleen, M indicates a 100 bp DNA ladder.
3. Discussion
In the current study, the inhibitory effect of myrrh oil against the growth of B. bovis, B. bigemina, B. divergens, T. equi and B. caballi in vitro and against B. microti in vivo was evaluated. IC50 values of myrrh oil for Babesia species and T. equi were very low compared with the IC50 value for P. falciparum (100 µg/mL) [17]. IC50 values of myrrh oil for Babesia species and T. equi were nearly similar to epoxomicin [19]. Interestingly, IC50 values of myrrh oil for Babesia species and T. equi were lower than those of quinuronium sulfate [20], imidocarb dipropionate [21], heparin [22], curdlan sulfate [23], triclosan [24], (-)-epigallocatechin-3-gallate [25], nerolidol [26], ciprofloxacin [27], fusidic acid [28], miltefosine [29], allicin [30], N-acetyl-L-cysteine [31], and thymoquinone [32]. Additionally, the IC50 value of myrrh oil for B. divergens was lower than those of fluoroquinolones, including enrofloxacin, enoxacin, trovafloxacin, norfloxacin, and ofloxacin [33]. IC50 values of myrrh oil for piroplasm parasites were very low compared with the drug’s IC50s for mammalian cells, such as MCF-7 (19.8 µg/mL), HepG2 (39.2 µg/mL), Hela (34.3 µg/mL), HS-1 (22.7 µg/mL), and A459 (41.4 µg/mL) [18], highlighting the high selectivity index of myrrh oil. Furthermore, in the current study, the very high concentration of myrrh oil did not affect the bovine or equine RBCs. Such findings confirm the non-toxic effect of myrrh oil. The issue that indicates the safety of myrrh oil on the in vitro studies level and recommends the further use of this promising anti-piroplasm candidate in the in vivo studies.
Some herbs were evaluated against babesiosis and theileriosis in vitro, such as green tea, black tea, hibiscus, cinnamon, and peppermint [34], and flavonoids isolated from the flowers of Pulsatilla flavescens [35].
In our study, in combination with DA, luteolin and PYR, the in vitro inhibitory effect of myrrh oil was increased against B. bovis growth. These findings are analogous to allicin/Da [30] and TQ/DA in vitro inhibitory actions on B. bovis [32]. The effect of myrrh oil on in vitro inhibition if used together with DA, PYR or luteolin, B. divergens was not improved. In vitro B. divergens suppression of development, on the other hand, in blend with DA, luteolin, and PYR were increased by treatment with a fluoroquinolone (enoxacin, enrofloxacin, and trovafloxacin) [33]. Such non-improvement in the inhibitory effects of myrrh oil on B. divergens, when used in combination with the selected antibabesial drugs, might be attributed to the fact that parasite species, strain, and size affect the in vitro inhibitory activity of the drug combination on the Babesia growth [30,36,37,38].
The apparent effect on Babesia and Theileria, as an in vitro inhibitor, has led us to test its in vivo inhibitory effect on B. microti. Results showed that lower doses of the mixture of myrrh oil/DA are in general more active than a high DA dose alone.
Inhibition of B. Microti development caused by a mixture of 100 mg kg−1 myrrh oil and 12.5 mg kg−1 DA is higher than 70 percent inhibition rate for clindamycin/ quinine mixture [34], 56.35 and 53.25 percent inhibition rate for the 85 mg kg−1 PYR joined with 10 mg kg-1 DA [35], 67 percent hang-up rate for the 50 mg kg−1 enoxacin and 10 mg kg−1 DA, and 62.5 percent inhibition rates for 50 mg kg–1 oral dose of TQ and 10 mg kg -1 subcutaneous dose of DA [32].
The nested PCR assay was performed to assess the capacity of the administered combination therapy to further remove parasite DNA from the organs of the animal. PCR magnification observed by the B. microti ss-rRNA gene in the blood and organs of mice cured with myrrh oil/DA combination. Likewise, the PYR/DA combination did not completely eliminate the infection from different organs of B. micro-infected animals on the thirtieth day post-infection [35].
Conversely, combination therapy consists of enoxacin/DA removed B. microti infection from the lungs of treated mice, but not from other organs (spleen, heart, and kidney) on day 20 p.i. [33]. This nucleic acid residue of the parasite that has been found in the blood and organs of the treated mice in our sample highlights the failure of each DA alone and DA/myrrh oil combination to totally remove parasite DNA from the animal body on 28th-day p.i., may subsequently be reinfected.
Future research is, therefore, desired to determine the in vivo inhibiting results of a safe anti-piroplasm agent, myrrh oil when used in combination with DA, low doses of imidocarb dipropionate, or recently developed anti-piroplasm drugs, such as clofazimine [36], PYR, or luteolin [16,37] for longer durations of infection.
Myrrh oil contains many active compounds, such as sesquiterpenoids, including furanodiene, 1(l0)Z,4Z-furanodiene-6-one(1), 2-methoxyfuranodiene, 2-acetoxyfuranodiene, 4,5-dihydrofuranodtene-6-one, 2-methoxyfuranoguaia-9-ene-8-one, isofuranogermacrene (1), lindestrene (2), furanoeudesma-l,3-diene (3), furanodiene (4), and nerolidol [18,39,40]. The antibabesial effect of myrrh oil may be attributed to one or more constituents, such as terpen nerolidol that was reported as a potent antibabesial agent [26,41].
Although the present study has shown an improvement in the in vitro inhibitory effect of myrrh oil when used in combination with DA, PYR, and luteolin on B. bovis growth, synergetic or antagonistic relationships between these combination therapies have not yet been assessed against B. microti growth on the rats. Additional experiments are, therefore, required to decide the possible inhibitory results of these combinations on B. microti development in the mouse model. Extra, future research is necessary to elucidate the mechanism for improving the inhibitory effect of myrrh oil when used in association with DA, PYR, or luteolin. This study evaluated the inhibitory effect of myrrh oil as whole oil, and neglected the purification of the oil compounds. Subsequently, future studies are required to purify the components of myrrh oil and test their inhibitory effects against the growth of Babesia parasites separately. Although the present study evaluated the in vivo inhibitory effects of myrrh oil when used in combination with DA, the inhibitory effect of DA when administrated as monotherapy at a dose rate of 12.5 mg kg−1 was not evaluated. Thus, further studies are warranted to evaluate the inhibitory effect if DA (12.5 mg kg−1) alone to rule out the possibility that DA at this dose may cause similar efficacy as the combination therapy.
4. Materials and Methods
4.1. Chemicals
SYBR Green I (SGI) nucleic acid stain (Lonza, Rockland, USA; 10,000x) was processed at −20 °C and warm-up prior to using. A lyse buffer involving Tris (130 mM; pH 7.5), EDTA (10 mM), saponin (0.016per cent; W/V), and TritonX-100 (1.6%; V/V) was ready previously and stockpiled at 4 °C. Myrrh oil has been obtained from Sigma-Aldrich (Saint Louis, USA). 100 mg/mL stock solutions in Ethanol (Eth) were ready and stockpiled at −30 °C before use. DA (Ganaseg, Ciba-Geigy Japan Ltd., Tokyo, Japan) is a widely put to use antibabesial drug used as a confident regulator drug. A functioning store solution of 10 mM of DA or luteolin, and pyronaridine tetraphosphate (PYR) (Sigma-Aldrich, Japan) has been dissolved in double-distilled water (DDW) and stockpiled at −30 °C until needed for usage.
4.2. In Vitro Growth Inhibition Assay and Viability Test
Myrrh oil was tested for its chemotherapeutic activity against B. bovis (Texas strain) [16], B. bigemina (Argentina strain) [23], B. caballi [26], and T. equi (United States Department of Agriculture) [4]. Parasites have been cultured in bovine or equine red blood cells using a continuous microaerophilic stationary phase culture system [16,23]. The inhibitory results of myrrh oil on the growth of Babesia/Theileria was checked by fluorescence assay using SGI stain [23,42]. Double 96-well plates (Nunc, Roskilde, Denmark) were used for cultivating bovine Babesia and equine Babesia/Theileria pRBCs using medium alone or with defined concentrations—0.1, 0.5, 1.0, 2, 5, or 10 µg/mL for myrrh oil. The concentrations used have been based on a preliminary study. Positive control cultures earned 0.1, 0.5, 1.0, 2, 5, and 10 µg/mL of DA. Of negative experimental controller, non-drug cultures and cultures containing only Eth (0.0025 per cent of myrrh oil) and DDW (0.02 per cent for DA) were prepared. Bovine Babesia and equine Babesia/Theileria parasites pRBCs were cultured at 1% parasitemia in 96-well plates using 2.5 percent HCT for B. bovis and B. bigemina and 5% HCT for further Babesia and Theileria parasites. The pRBCs were cultured for four days in triplicate wells for each concentration of the drug. On the fourth day of cultivation, the IC50 values were calculated by adding lyse buffer containing 2× SGI to every drug dilution on the first 96-well plate. While, for the second plate, 1.5 µL (for parasites cultivated with 5 per cent HCT) and 0.75 µL (for parasites cultivated with 2.5 per cent HCT) of each of the control and drug-treated infected RBCs were mixed with 3.5 µL of parasite-free RBCs and 1.75 µL for parasites cultivated with 5 per cent and 2.5 per cent HCT, respectively on the fourth day of cultivation [22]. Afterwards, bovine and equine packed red blood cells were overhung in a new medium with no drug. The plates were then incubated at 37 °C for the next 4 days without the medium being replaced. The first or second plate then kept warm for 6 h in a dark dwelling at 25 °C, and fluorescence appraisals were calculated via a fluorescence plate reader (Fluoroskan Ascent, Thermo Electron Informatics, Philadelphia, PA, USA) at 485 nm and 518 nm wavelengths, respectively. Gain values have been fixed at 100. That research has been threefold replicated.
4.3. Myrrh Oil in Combination with Other Antibabesial Drugs in Vitro
The combination of myrrh oil therapies with commonly used anti-piroplasm drugs, DA, and the recently developed anti-piroplasm drugs, luteolin or PYR [16] was examined in vitro cultures of B. bovis and B. divergens (the parasites that showed the highest IC50 for myrrh oil). Myrrh oil/DA (M1, M2, M3, M4, M5, and M6), luteolin or PYR mixtures (M1, M2, M3, M4, M5, M6, M7, M8, and M9) were ready as mentioned before [16] with certain reforms. Combinations were founded on the measured IC50 values extracted from the in vitro fluorescence assay. Drug-free cultures have been used for experimental monitoring. Cultures containing only DA, luteolin or PYR IC50s of the parasite were used as positive drug monitors. Three separate trials were conducted, consisting of three-fold drug combination experiments over a 4-day period using 2.5 per cent and 5 per cent HCT for B. bovis and B. divergens, respectively. The fluorescence values were determined on the fourth day of cultivation, by tallying lytic buffer to every drug combination as mentioned above.
4.4. Determination of Morphological Changes and the Toxic Effect of Myrrh Oil on Host Erythrocytes in Vitro
Morphological fluctuations in drug-exposed Babesia and T. equi parasites were seen using a microscope [23,27]. The experiment was conducted at a parasitemia of 1% for all parasites. The growth inhibition assay was completed in 96-well plates at 10% HCT (20 µL of red blood cell inoculum and 200 µL of the correct medium), and the parasites were treated with 10 µg/mL of myrrh oil. The plates were cultivated for four consecutive days, as mentioned above. Variations in the morphology of drug-exposed Babesia species have been determined by the use of light microscopy in a Giemsa-stained thin erythrocyte smear coating and contrasted with the controller.
The poisonousness of myrrh oil to host erythrocytes was assessed by way of mentioned formerly [25]. Bovine and equine erythrocytes were incubated either with either medium alone or medium with a very high concentration of myrrh oil (250 μg/mL) for 3 h at 37 °C. RBCs were at that point cleaned three turns with drug-free media and driven for 72 hours. Parasite development in pretreated erythrocytes was witnessed and matched to control untreated cells. Experiments were performed in triple wells for each drug concentration for all parasite species and in three isolated trials.
4.5. Chemotherapeutic Efficacy of Myrrh Oil on the Growth of B. Microti in Mice
All in vivo experimental protocols in this study were approved by the Animal Care and Use Committee, Obihiro University of Agriculture and Veterinary Medicine (Approval No. 27-65). All experiments were conducted in accordance with the Fundamental Guidelines for Proper Conduct of Animal Experiment and Related Activities in Academic Research Institutions under the jurisdiction of the Ministry of Education, Culture, Sports, Science and Technology, Japan.
In vivo inhibition of myrrh oil test for B. microti (Munich strain) [32] was performed twice in BALB/c mice aged eight weeks old (purchased from CLEA Japan, Tokyo, Japan) using the previously described method [38]. Every mouse was housed under strict pathogen-free conditions. Twenty-five female BALB/c mice were equally divided into five groups. All mice were injected intraperitoneally with 1 × 107 B. microti-infected RBCs except for mice in the first community, persisted uninfected and used as the negative monitor. Once the infected mice revealed around 1% parasitemia, mice in the investigational groups were given regular injections for five days. Myrrh oil was dissolved in Eth (50% v/v), and DA was dissolved in DDW (12.5%), and then weakened in PBS or DDW prior to injection. Mice in the second group were received intraperitoneal doses of Eth in PBS (0.006 per cent) and were used equally inactive governor. DA has been given in subcutaneous doses to the mice in the third group at a rate of 25 mg kg−1. Myrrh oil was administered intraperitoneally either only at a dose rate of 200 mg kg−1 or in conjunction with a subcutaneous dose of DA at a dosage level of 100 mg kg−1 myrrh oil and 12.5 mg kg−1 DA for mice in the fourth and fifth groups, respectively. The inhibitory effects of the drugs administered on the growth of B. microti were tracked each 48 h until 28 days after infection or the ending of parasitemia using fluorescence spectrophotometer [38]. After completion of the experiment, all the mice were humanely euthanized using inhalational agent, chloroform as a primary method of euthanasia, and cervical dislocation (physical euthanasia) was performed to all mice.
4.6. The Potential of Myrrh Oil in the Recovery from the Anemia Accompanying Babesia
HCT estimates, hemoglobin (HGB) levels, RBC counts, were used to determine the ability of myrrh oil to recover the B. microti- infected mice with induced anemia. Ten microliter blood samples were possessed from all mice each 96 h and hematological variables were monitored using Celltac α MEK-6450 automatic hematology analyzer (Nihon Kohden Corporation, Tokyo, Japan).
4.7. PCR Detection of B. Microti in Mice
Blood and tissue parasite DNA in (heart, lung, liver, kidney and spleen) samples possessed from mice cured with myrrh oil/DA mixture, DA alone, and Eth (affirmative control) were identified using nested PCR tests directing the B. microti small subunit rRNA (ss-rRNA) gene on day 28 after infection (p.i.). A NucleoSpin tissue kit (Macherey-Nagel, Düren, Germany) and a QIAamp DNA Blood Mini Kit (Qiagen, Tokyo, Japan) were used for tissues and blood DNA extraction. PCR cycling was accomplished, as mentioned earlier by Rizk et al. [33,38].
4.8. Statistical Analysis
GraphPad Prism 5th edition from GraphPad Software Inc. (CA, USA) was directed to assess the substantial alterations concerning the groups tested using a one-way ANOVA method. The value of P < 0.05 was deemed of analytical importance. Statistically noteworthy variances between the drug-exposed and affirmative-controller clusters have been used as an indicator of the recurrence of the parasite in a viability test [42].
5. Conclusions
In conclusion, the potential anti-piroplasm effects of myrrh oil have been demonstrated in vitro and in vivo in this research. Noteworthy, myrrh oil once used in conjunction with a low dose of DA, myrrh oil has a higher inhibitory effect on B. microti development following treatment with the standard dose (25 mg kg−1) of the widely used anti-piroplasm drug, DA. The consequences of this study propose that myrrh oil may be useful for the therapy of animal piroplasmosis, specifically when used in conjunction with a low dose of DA. The issue that might help to avoid the opposition to DA.
Author Contributions
Conceived and planned the experiments: M.A., S.A.E.-S.E.-S., M.A.R., I.I.; Conducted the experiments: M.A., S.A.E.-S.E.-S., M.A.R.; Analyzed the data: M.A.R., M.A.O., M.M.A.-D., M.S.A.-A.; Provided reagents/materials/analysis tools: M.A.R., M.M.A.-D., I.I.; Wrote the manuscript: M.A., S.A.E.-S.E.-S., M.M.A.-D., M.A.R., A.R.A.A. All authors revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by the Ministry of Education, Culture, Sports, Science and Technology of Japan. The funding bodies had no role in the scheme of the research and assemblage, analysis and interpretation of data, and in writing the manuscript.
Acknowledgments
The researchers would like to great thank Prof. Naoaki Yokoyama, National Research Center for Protozoan Diseases, Obihiro University of Agriculture and Veterinary Medicine, Inada-cho, Obihiro, Hokkaido, Japan for his scientific support and discussion.
Conflicts of Interest
The authors do not declare a conflict of interest.
References
- Rizk, M.A.; El-Sayed, S.A.E.-S.; El-Khodery, S.; Yokoyama, N.; Igarashi, I. Discovering the in vitro potent inhibitors against Babesia and Theileria parasites by repurposing the Malaria Box: A review. Vet. Parasitol. 2019, 274, 108895. [Google Scholar] [CrossRef] [PubMed]
- Uilenberg, G. Babesia—A historical overview. Vet. Parasitol. 2006, 138, 3–10. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, S.A.E.-S.; Rizk, M.A.; Terkawi, M.A.; Yokoyama, N.; Igarashi, I. Molecular identification and antigenic characterization of Babesia divergens erythrocyte binding protein (BdEBP) as a potential vaccine candidate. Parasitol. Int. 2017, 66, 721–726. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, S.A.E.-S.; Rizk, M.A.; Terkawi, M.A.; Mousa, A.; Elsayed, G.; Fouda, M.; Yokoyama, N.; Igarashi, I. Cocktail of Theileria equi antigens for detecting infection in equines. Asian Pac. J. Trop. Biomed. 2015, 5, 977–981. [Google Scholar] [CrossRef]
- Vannier, E.; Gewurz, B.E.; Krause, P.J. Human babesiosis. Infect. Dis. Clin. N. Am. 2008, 22, 469–488. [Google Scholar] [CrossRef]
- Bakari, G.G.; Max, R.A.; Mdegela, R.H.; Phiri, E.C.; Mtambo, M.M. Effect of resinous extract from Commiphora swynnertonii (Burrt) on experimental coccidial infection in chickens. Trop. Anim. Health Prod. 2013, 45, 455–459. [Google Scholar] [CrossRef]
- Singh, R.; Joshi, V.; Gambhir, S. Anti-inflammatory activity of some traditional medicinal plants. Anc. Sci. Life 1998, 18, 160. [Google Scholar]
- Dolara, P.; Luceri, C.; Ghelardini, C.; Monserrat, C.; Aiolli, S.; Luceri, F.; Lodovici, M.; Menichetti, S.; Romanelli, M.N. Analgesic effects of myrrh. Nature 1996, 379, 29. [Google Scholar] [CrossRef]
- Massoud, A.; Ibrahim, S. Light microscopic study of the effect of new antischistosmal drug (myrrh extract) on the liver of mice. J. Egypt. Soc. Parasitol. 2005, 35, 971–988. [Google Scholar]
- Soliman, O.; El-Arman, M.; Abdul-Samie, E.; El-Nemr, H.; Massoud, A. Evaluation of myrrh (Mirazid) therapy in fascioliasis and intestinal schistosomiasis in children: Immunological and parasitological study. J. Egypt. Soc. Parasitol. 2004, 34, 941–966. [Google Scholar]
- Haridy, F.; Dawoud, H.; Morsy, T. Efficacy of Commiphora molmol (Mirazid) against sheep naturally infected with monieziasis expansa in Al-Santa Center, Gharbia Governorate, Egypt. J. Egypt. Soc. Parasitol. 2004, 34, 775–782. [Google Scholar]
- Al-Mathal, E.; Fouad, M. Myrrh (Commiphora molmol) in treatment of human and sheep dicrocoeliasis dendriticum in Saudi Arabia. J. Egypt. Soc. Parasitol. 2004, 34, 713–720. [Google Scholar] [PubMed]
- Massoud, A.; Kutkat, M.; Abdel, S.S.; El-Khateeb, R.M.; Labib, I.M. Acaricidal efficacy of Myrrh (Commiphora molmol) on the fowl tick Argas persicus (Acari: Argasidae). J. Egypt. Soc. Parasitol. 2005, 35, 667–686. [Google Scholar] [PubMed]
- Massoud, A.; Labib, I.M.; Rady, M. Biochemical changes of Culex pipiens larvae treated with oil and oleo-resin extracts of Myrrh Commiphora molmol. J. Egypt. Soc. Parasitol. 2001, 31, 517–529. [Google Scholar] [PubMed]
- Fathy, F. Effect of mirazid (Commiphora molmol) on experimental giardiasis. J. Egypt. Soc. Parasitol. 2011, 41, 155–177. [Google Scholar]
- Rizk, M.A.; El-Sayed, S.A.E.-S.; Terkawi, M.A.; Youssef, M.A.; El Said, E.S.E.S.; Elsayed, G.; El-Khodery, S.; El-Ashker, M.; Elsify, A.; Omar, M. Optimization of a fluorescence-based assay for large-scale drug screening against Babesia and Theileria parasites. PLoS ONE 2015, 10, e0125276. [Google Scholar] [CrossRef] [PubMed]
- Fujisaki, R.; Kamei, K.; Yamamura, M.; Nishiya, H.; Inouye, S.; Takahashi, M.; Abe, S. In vitro and in vivo anti-plasmodial activity of essential oils, including hinokitiol. Southeast Asian J. Trop. Med. Public Health 2012, 43, 270–279. [Google Scholar]
- Chen, Y.; Zhou, C.; Ge, Z.; Liu, Y.; Liu, Y.; Feng, W.; Li, S.; Chen, G.; Wei, T. Composition and potential anticancer activities of essential oils obtained from myrrh and frankincense. Oncol. Lett. 2013, 6, 1140–1146. [Google Scholar] [CrossRef]
- AbouLaila, M.; Nakamura, K.; Govind, Y.; Yokoyama, N.; Igarashi, I. Evaluation of the in vitro growth-inhibitory effect of epoxomicin on Babesia parasites. Vet. Parasitol. 2010, 167, 19–27. [Google Scholar] [CrossRef]
- Brockelman, C.R.; Tan-ariya, P. Development of an in vitro microtest to assess drug susceptibility of Babesia bovis and Babesia bigemina. J. Parasitol. 1991, 77, 994–997. [Google Scholar] [CrossRef]
- Rodriguez, R.; Trees, A. In vitro responsiveness of Babesia bovis to imidocarb dipropionate and the selection of a drug-adapted line. Vet. Parasitol. 1996, 62, 35–41. [Google Scholar] [CrossRef]
- Bork, S.; Yokoyama, N.; Ikehara, Y.; Kumar, S.; Sugimoto, C.; Igarashi, I. Growth-inhibitory effect of heparin on Babesia parasites. Antimicrob. Agents Chemother. 2004, 48, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, I.; Njonge, F.K.; Kaneko, Y.; Nakamura, Y. Babesia bigemina: In Vitro and in VivoEffects of Curdlan Sulfate on Growth of Parasites. Exp. Parasitol. 1998, 90, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Bork, S.; Yokoyama, N.; Matsuo, T.; Claveria, F.G.; Fujisaki, K.; Igarashi, I. Growth inhibitory effect of triclosan on equine and bovine Babesia parasites. Am. J. Trop. Med. Hyg. 2003, 68, 334–340. [Google Scholar] [CrossRef]
- Aboulaila, M.; Yokoyama, N.; Igarashi, I. Inhibitory effects of (-)-epigallocatechin-3-gallate from green tea on the growth of Babesia parasites. Parasitology 2010, 137, 785–791. [Google Scholar] [CrossRef]
- AbouLaila, M.; Sivakumar, T.; Yokoyama, N.; Igarashi, I. Inhibitory effect of terpene nerolidol on the growth of Babesia parasites. Parasitol. Int. 2010, 59, 278–282. [Google Scholar] [CrossRef]
- AbouLaila, M.; Munkhjargal, T.; Sivakumar, T.; Ueno, A.; Nakano, Y.; Yokoyama, M.; Yoshinari, T.; Nagano, D.; Katayama, K.; El-Bahy, N. Apicoplast-targeting antibacterials inhibit the growth of Babesia parasites. Antimicrob. Agents Chemother. 2012, 56, 3196–3206. [Google Scholar] [CrossRef]
- Salama, A.A.; AbouLaila, M.; Moussa, A.A.; Nayel, M.A.; El-Sify, A.; Terkawi, M.A.; Hassan, H.Y.; Yokoyama, N.; Igarashi, I. Evaluation of in vitro and in vivo inhibitory effects of fusidic acid on Babesia and Theileria parasites. Vet. Parasitol. 2013, 191, 1–10. [Google Scholar] [CrossRef]
- AbouLaila, M.; Batadoj, D.; Salama, A.; Munkhjargal, T.; Ichikawa-Seki, M.; Terkawi, M.A.; Yokoyama, N.; Igarashi, I. Evaluation of the inhibitory effects of miltefosine on the growth of Babesia and Theileria parasites. Vet. Parasitol. 2014, 204, 104–110. [Google Scholar] [CrossRef]
- Salama, A.A.; AbouLaila, M.; Terkawi, M.A.; Mousa, A.; El-Sify, A.; Allaam, M.; Zaghawa, A.; Yokoyama, N.; Igarashi, I. Inhibitory effect of allicin on the growth of Babesia and Theileria equi parasites. Parasitol. Res. 2014, 113, 275–283. [Google Scholar] [CrossRef]
- Rizk, M.A.; El-Sayed, S.A.E.-S.; AbouLaila, M.; Yokoyama, N.; Igarashi, I. Evaluation of the inhibitory effect of N-acetyl-L-cysteine on Babesia and Theileria parasites. Exp. Parasitol. 2017, 179, 43–48. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, S.A.E.-S.; Rizk, M.A.; Yokoyama, N.; Igarashi, I. Evaluation of the in vitro and in vivo inhibitory effect of thymoquinone on piroplasm parasites. Parasites Vectors 2019, 12, 37. [Google Scholar] [CrossRef] [PubMed]
- Rizk, M.A.; AbouLaila, M.; El-Sayed, S.A.E.-S.; Guswanto, A.; Yokoyama, N.; Igarashi, I. Inhibitory effects of fluoroquinolone antibiotics on Babesia divergens and Babesia microti, blood parasites of veterinary and zoonotic importance. Infect. Drug Resist. 2018, 11, 1605. [Google Scholar] [CrossRef] [PubMed]
- Aboulaila, M.R.; Rizk, M.A.; El-Sayed, S.; Yoko-Yama, N.; Igarashi, I. In vitro antiparasitic effects of six beverages on the growth of Babesia and Theileria parasites. Ann. Complement. Altern. Med. 2018, 3, 3–8. [Google Scholar]
- Ganchimeg, D.; Batbold, B.; Murata, T.; Davaapurev, B.-O.; Munkhjargal, T.; Tuvshintulga, B.; Suganuma, K.; Igarashi, I.; Buyankhishig, B.; Sasaki, K. Flavonoids isolated from the flowers of Pulsatilla flavescens and their anti-piroplasm activity. J. Nat. Med. 2019, 73, 633–640. [Google Scholar] [CrossRef]
- Guswanto, A.; Nugraha, A.B.; Tuvshintulga, B.; Tayebwa, D.S.; Rizk, M.A.; Batiha, G.E.-S.; Gantuya, S.; Sivakumar, T.; Yokoyama, N.; Igarashi, I. 17-DMAG inhibits the multiplication of several Babesia species and Theileria equi on in vitro cultures, and Babesia microti in mice. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 104–111. [Google Scholar] [CrossRef]
- Nugraha, A.B.; Tuvshintulga, B.; Guswanto, A.; Tayebwa, D.S.; Rizk, M.A.; Gantuya, S.; Batiha, G.E.-S.; Beshbishy, A.M.; Sivakumar, T.; Yokoyama, N. Screening the Medicines for Malaria Venture Pathogen Box against piroplasm parasites. Int. J. Parasitol. Drugs Drug Resist. 2019, 10, 84–90. [Google Scholar] [CrossRef]
- Rizk, M.A.; El-Sayed, S.A.E.-S.; AbouLaila, M.; Eltaysh, R.; Yokoyama, N.; Igarashi, I. Performance and consistency of a fluorescence-based high-throughput screening assay for use in Babesia drug screening in mice. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Brieskorn, C.H.; Noble, P. Drei neue furanogermacrene aus myrrhe. Tetrahedron Lett. 1980, 21, 1511–1514. [Google Scholar] [CrossRef]
- Maradufu, A.; Warthen Jr, J.D. Furanosesquiterpenoids from Commiphora myrrh oil. Plant Sci. 1988, 57, 181–184. [Google Scholar] [CrossRef]
- Rizk, M.A.; Ji, S.; Liu, M.; El-Sayed, S.A.E.-S.; Li, Y.; Byamukama, B.; Ringo, A.E.; Xuan, X.; Igarashi, I. Closing the empty anti-Babesia gibsoni drug pipeline in vitro using fluorescence-based high throughput screening assay. Parasitol. Int. 2020, 75, 102054. [Google Scholar] [CrossRef] [PubMed]
- Rizk, M.A.; El-Sayed, S.A.E.-S.; AbouLaila, M.; Tuvshintulga, B.; Yokoyama, N.; Igarashi, I. Large-scale drug screening against Babesia divergens parasite using a fluorescence-based high-throughput screening assay. Vet. Parasitol. 2016, 227, 93–97. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).