Isoalantolactone Enhances the Antimicrobial Activity of Penicillin G against Staphylococcus aureus by Inactivating β-Lactamase during Protein Translation
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Chemicals
2.2. Enzyme Inhibition Assays
2.3. Real-Time RT-PCR Assay
2.4. Western Blot Assay
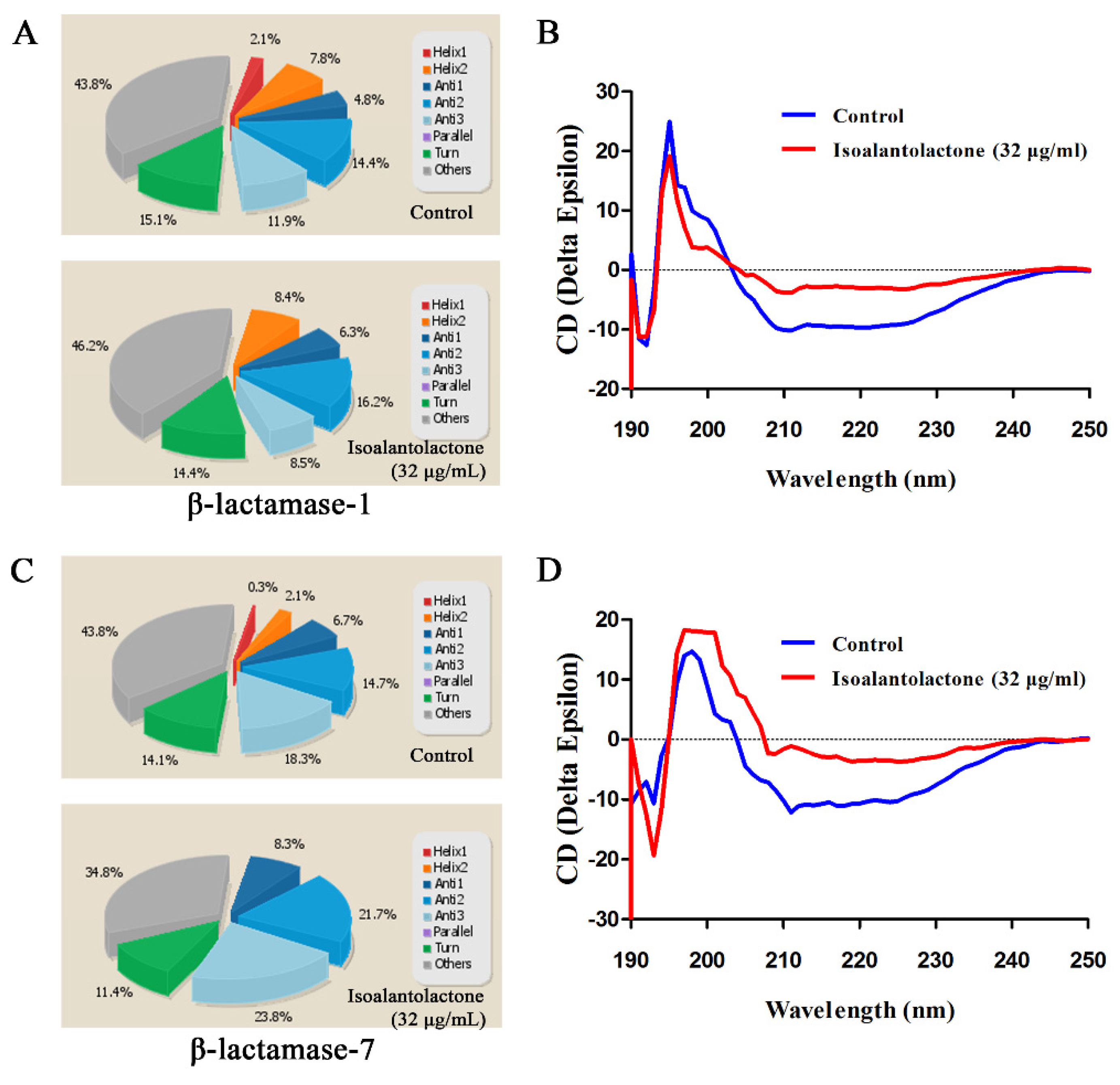
2.5. Secondary Structure Determination of β-Lactamases by CD
2.6. Susceptibility Testing
2.7. Growth Curves and Time-Killing Assays
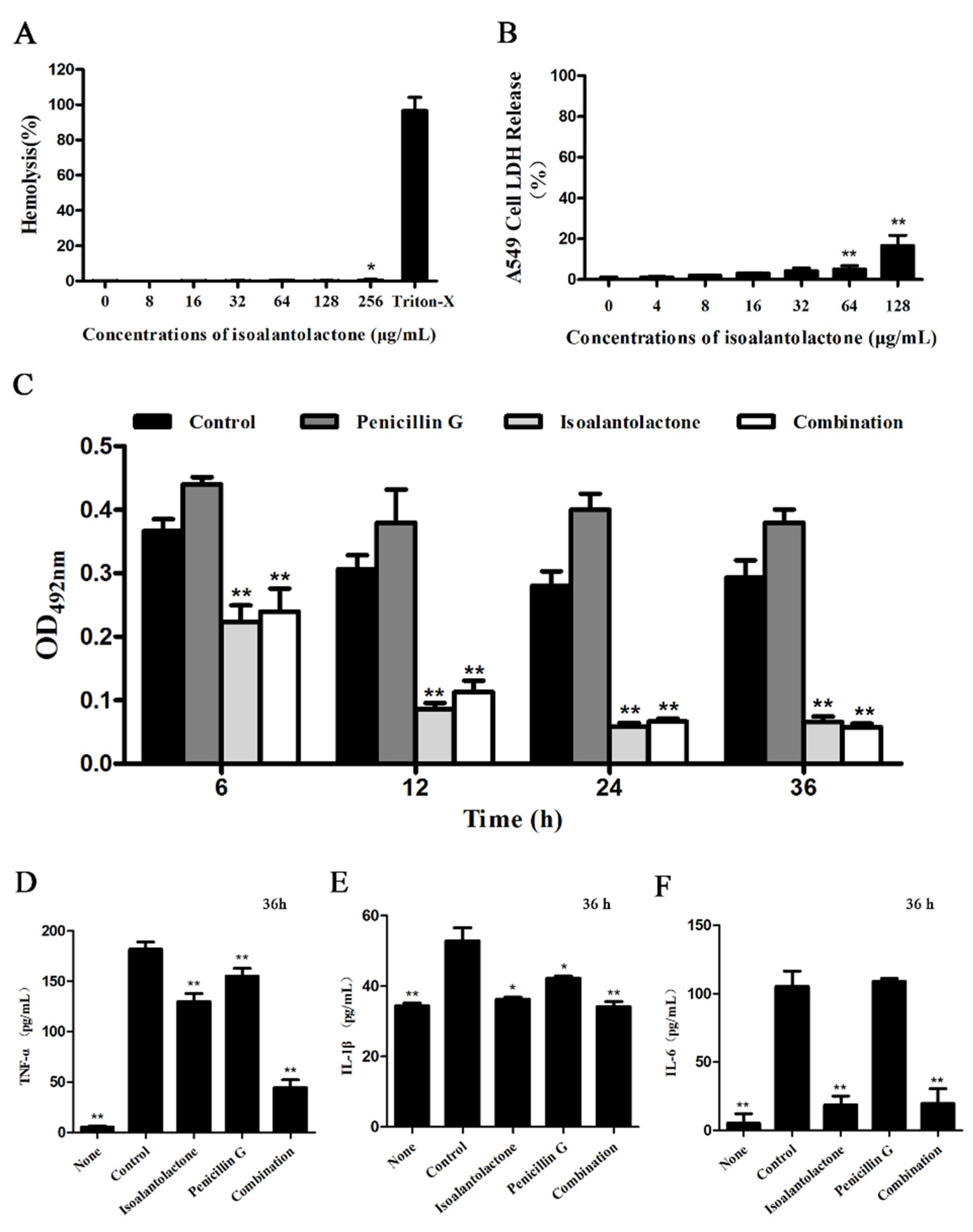
2.8. Cytotoxicity Assays
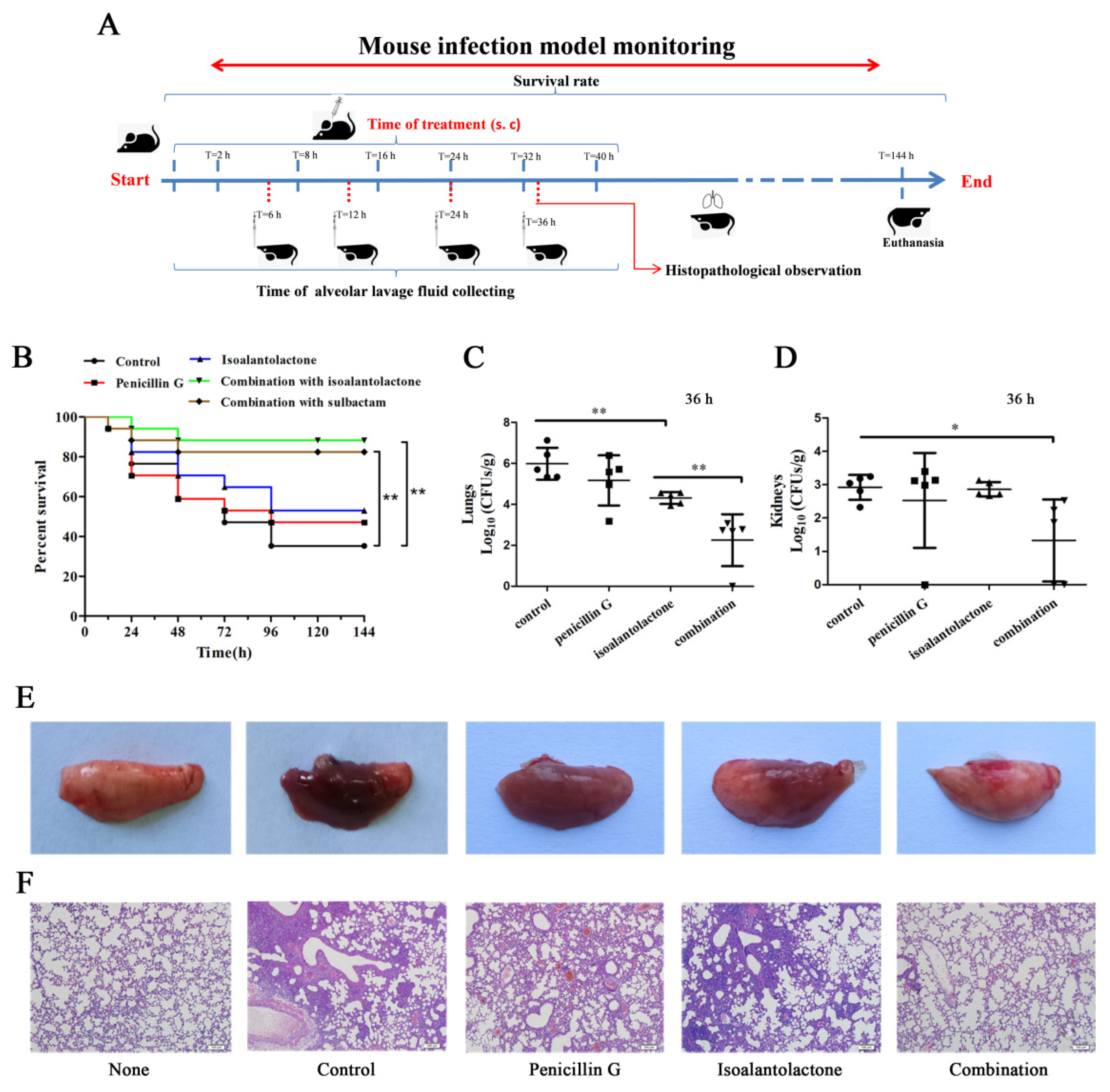
2.9. Mouse Model of Intranasal Lung Infection
2.10. Statistical Analysis
3. Results
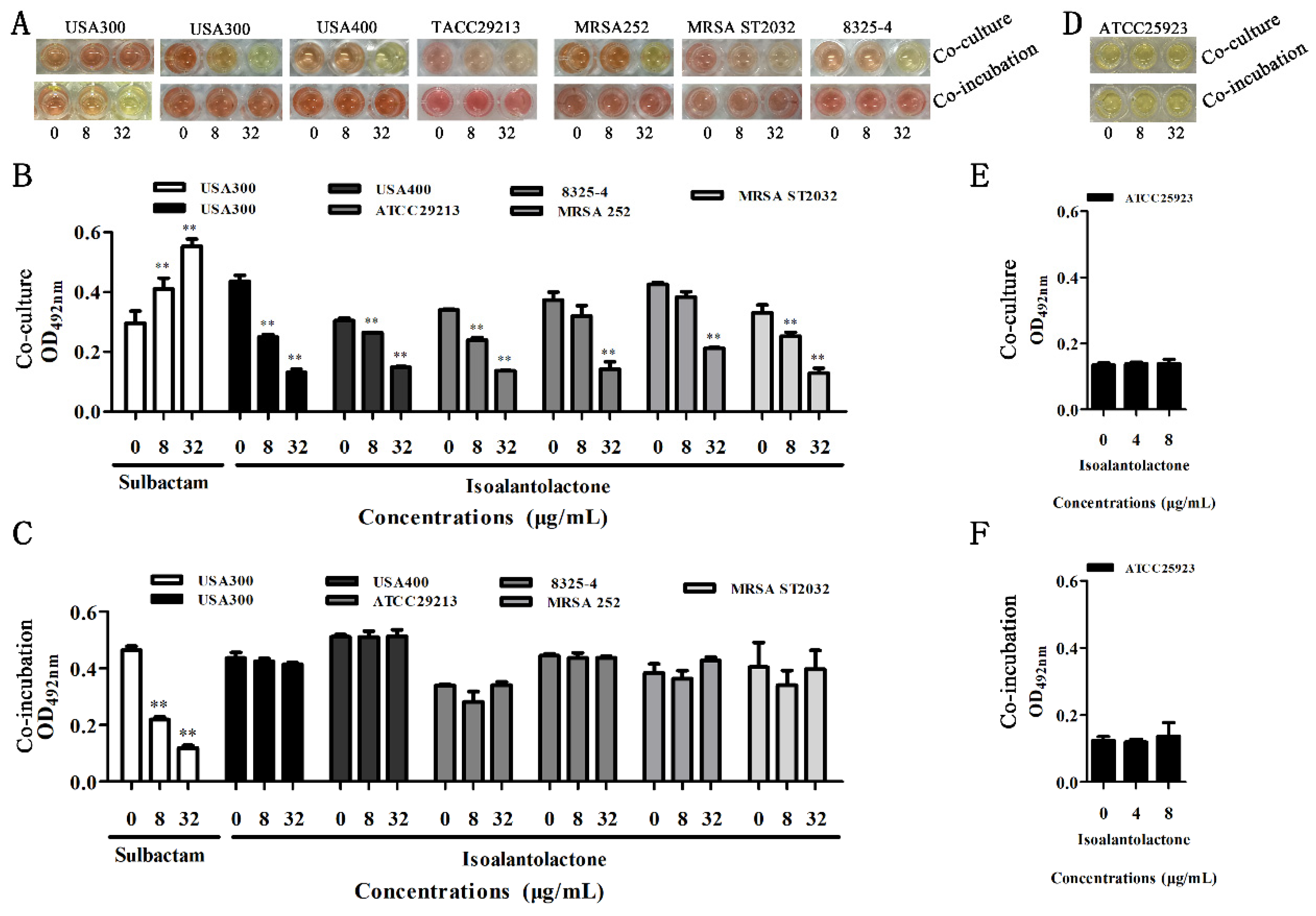
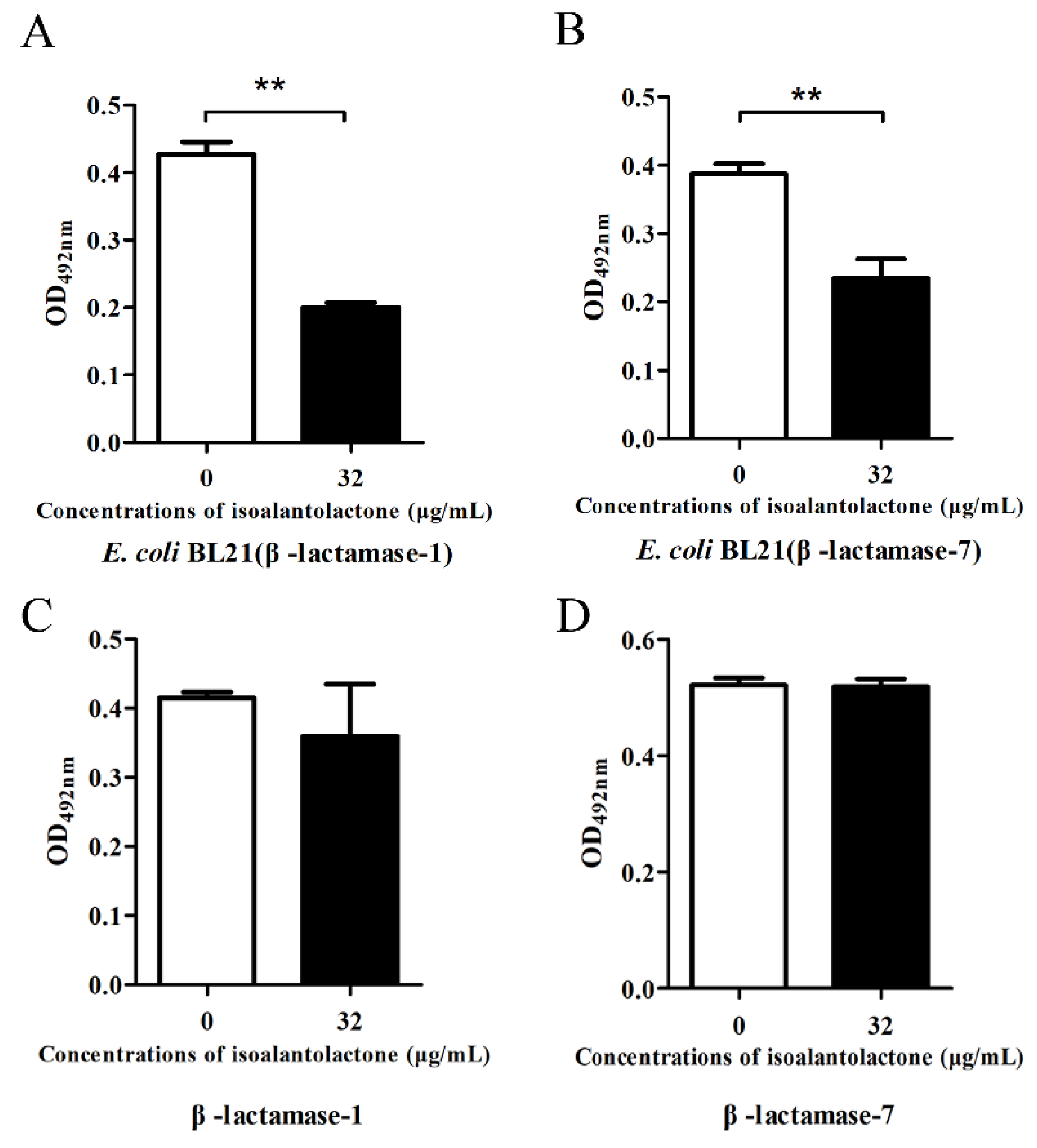
3.1. Effect of IAL on β-Lactamase Activity
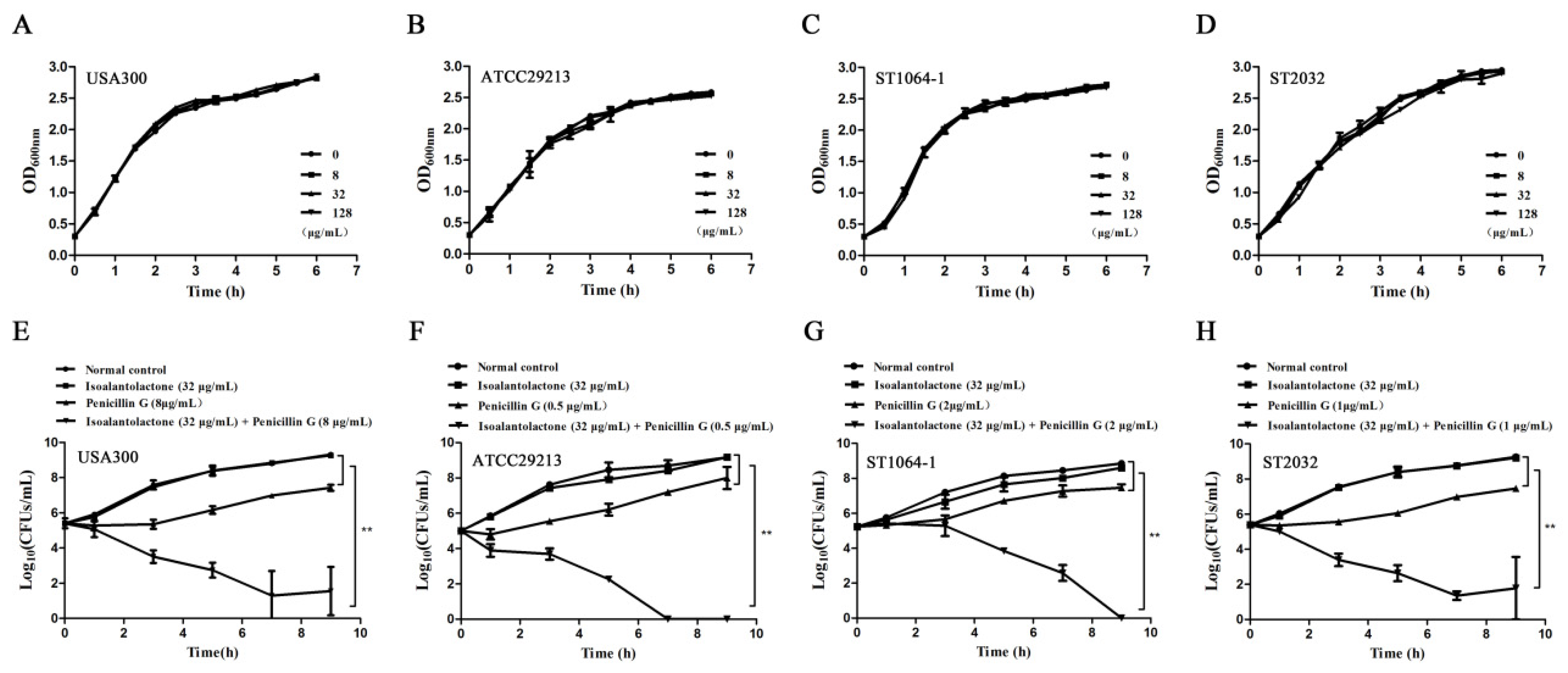
3.2. Effective in Vitro Antimicrobial Activity of IAL in Combination with Penicillin G against β-lactamase-Positive S. aureus
3.3. Effective Antimicrobial Activity of IAL in Combination with Penicillin G in S. aureus USA300-Infected Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rolinson, G.N. Celbenin—Resistant Staphylococci. Br. Med. J. 1961, 1, 113–114. [Google Scholar] [CrossRef]
- Wang, T.Z.; Kodiyanplakkal, R.P.L.; Calfee, D.P. Antimicrobial resistance in nephrology. Nat. Rev. Nephrol. 2019, 15, 463–481. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.A.; Eichenberger, E.M.; Shah, P.P.; Carugati, M.; Holland, T.L.; Fowler, V.G. Methicillin-resistant Staphylococcus aureus: An overview of basic and clinical research. Nat. Rev. Microbiol. 2019, 17, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Yee, R.; Cui, P.; Tian, L.; Zhang, S.; Shi, W.; Sullivan, D.; Zhu, B.; Zhang, W.; Zhang, Y. Identification of agents active against methicillin-resistant staphylococcus aureus usa300 from a clinical compound library. Pathogens 2017, 6, 44. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Antibiotic Resistance Threats in the United States; CDC: Atlanta, GA, USA, 2013.
- Wertheim, H.F.; Melles, D.C.; Vos, M.C.; van Leeuwen, W.; van Belkum, A.; Verbrugh, H.A.; Nouwen, J.L. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect. Dis. 2005, 5, 751–762. [Google Scholar] [CrossRef]
- Appelbaum, P.C. The emergence of vancomycin-intermediate and vancomycin-resistant Staphylococcus aureus. Clin. Microbiol. Infect. 2006, 12, 16–23. [Google Scholar] [CrossRef]
- Vestergaard, M.; Frees, D.; Ingmer, H. Antibiotic Resistance and the MRSA Problem. Microbiol. Spectr. 2019, 7, GPP3-0057-2018. [Google Scholar] [CrossRef]
- Bush, K.; Bradford, P.A. β-Lactams and β-Lactamase Inhibitors: An Overview. Cold Spring Harbor Perspect. Med. 2016, 6, a025247. [Google Scholar] [CrossRef]
- Page, M.I. The reactivity of beta-lactams, the mechanism of catalysis and the inhibition of beta-lactamases. Curr. Pharm. Des. 1999, 5, 895. [Google Scholar]
- Lee, A.S.; de Lencastre, H.; Garau, J.; Kluytmans, J.; Malhotra-Kumar, S.; Peschel, A.; Harbarth, S. Methicillin-resistant Staphylococcus aureus. Nat. Rev. Dis. Prim. 2018, 4, 18033. [Google Scholar] [CrossRef]
- Quadri, L.E. Strategic paradigm shifts in the antimicrobial drug discovery process of the 21st century. Infect. Disord. Drug Targ. 2007, 7, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, A.B. Kinetics of Sulbactam Hydrolysis by beta-Lactamases, and Kinetics of beta-Lactamase Inhibition by Sulbactam. Antimicrob. Agent. Chemother. 2017, 61, e01612-17. [Google Scholar] [CrossRef] [PubMed]
- Barrett, T.C.; Mok, W.W.K.; Murawski, A.M.; Brynildsen, M.P. Enhanced antibiotic resistance development from fluoroquinolone persisters after a single exposure to antibiotic. Nat. Commun. 2019, 10, 1177. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.H.; Hu, Z.Q.; Hara, Y.; Shimamura, T. Inhibition of penicillinase by epigallocatechin gallate resulting in restoration of antibacterial activity of penicillin against penicillinase-producing Staphylococcus aureus. Antimicrob. Agent. Chemother. 2002, 46, 2266–2268. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.Y. TU You-you won Lasker Debakey clinical medical research award--for her outstanding achievements in studies on artemisinin. Chin. J. Integr. Tradit. Western Med. 2011, 31, 1301. [Google Scholar]
- Li, Z.; Qin, B.; Qi, X.; Mao, J.; Wu, D. Isoalantolactone induces apoptosis in human breast cancer cells via ROS-mediated mitochondrial pathway and downregulation of SIRT1. Arch. Pharm. Res. 2016, 39, 1441–1453. [Google Scholar] [CrossRef]
- Jin, C.; Zhang, G.; Zhang, Y.; Hua, P.; Song, G.; Sun, M.; Li, X.; Tong, T.; Li, B.; Zhang, X. Isoalantolactone induces intrinsic apoptosis through p53 signaling pathway in human lung squamous carcinoma cells. PLoS ONE 2017, 12, e0181731. [Google Scholar] [CrossRef]
- He, G.; Zhang, X.; Chen, Y.; Chen, J.; Li, L.; Xie, Y. Isoalantolactone inhibits LPS-induced inflammation via NF-kappaB inactivation in peritoneal macrophages and improves survival in sepsis. Biomed. Pharmacother. 2017, 90, 598–607. [Google Scholar] [CrossRef]
- Qiu, J.; Luo, M.; Wang, J.; Dong, J.; Li, H.; Leng, B.; Zhang, Q.; Dai, X.; Zhang, Y.; Niu, X.; et al. Isoalantolactone protects against Staphylococcus aureus pneumonia. Fems Microbiol. Lett. 2011, 324, 147–155. [Google Scholar] [CrossRef]
- Ding, Y.H.; Song, Y.D.; Wu, Y.X.; He, H.Q.; Yu, T.H.; Hu, Y.D.; Zhang, D.; Jiang, H.; Yu, K.; Li, X.; et al. Isoalantolactone suppresses LPS-induced inflammation by inhibiting TRAF6 ubiquitination and alleviates acute lung injury. Acta Pharmacol. Sin. 2019, 40, 64–74. [Google Scholar] [CrossRef]
- Bartlett, A.H.; Foster, T.J.; Hayashida, A.; Park, P.W. Alpha-toxin facilitates the generation of CXC chemokine gradients and stimulates neutrophil homing in Staphylococcus aureus pneumonia. J. Infect. Dis. 2008, 198, 1529–1535. [Google Scholar] [CrossRef] [PubMed]
- Liao, D.; Yang, S.; Wang, J.; Zhang, J.; Hong, B.; Wu, F.; Lei, X. Total Synthesis and Structural Reassignment of Aspergillomarasmine, A. Angew. Chem. 2016, 55, 4291–4295. [Google Scholar] [CrossRef] [PubMed]
- Teng, Z.; Guo, Y.; Liu, X.; Zhang, J.; Niu, X.; Yu, Q.; Deng, X.; Wang, J. Theaflavin-3,3 -digallate increases the antibacterial activity of beta-lactam antibiotics by inhibiting metallo-beta-lactamase activity. J. Cell. Mol. Med. 2019, 23, 6955–6964. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Feng, H.; Lu, J.; Xiang, H.; Wang, D.; Dong, J.; Wang, J.; Wang, X.; Liu, J.; Deng, X. Eugenol reduces the expression of virulence-related exoproteins in Staphylococcus aureus. Appl. Environ. Microbiol. 2010, 76, 5846–5851. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, J.; Guo, Y.; Liu, X.; Liu, S.; Niu, X.; Wang, Y.; Deng, X. Discovery of a potential MCR-1 inhibitor that reverses polymyxin activity against clinical mcr-1-positive Enterobacteriaceae. J. Infect. 2019, 78, 364–372. [Google Scholar] [CrossRef]
- Shen, X.; Niu, X.; Li, G.; Deng, X.; Wang, J. Amentoflavone Ameliorates Streptococcus suis-Induced Infection In Vitro and In Vivo. Appl. Environ. Microbiol. 2018, 84, e01804-18. [Google Scholar] [CrossRef]
- Micsonai, A.; Wien, F.; Kernya, L.; Lee, Y.H.; Goto, Y.; Réfrégiers, M.; Kardos, J. Accurate secondary structure prediction and fold recognition for circular dichroism spectroscopy. Proc. Natl. Acad. Sci. USA 2015, 112, E3095–E3103. [Google Scholar] [CrossRef]
- Xu, R.; Polk, R.E.; Stencel, L.; Lowe, D.K.; Guharoy, R.; Duggal, R.W.; Wiest, M.; Putney, K.S.; Flint, N.B.; Flint, N.B. Antibiogram compliance in University HealthSystem Consortium participating hospitals with Clinical and Laboratory Standards Institute guidelines. Am. J. Health-Syst. Pharm. AJHP 2012, 69, 598–606. [Google Scholar] [CrossRef]
- Li, J.; Dong, J.; Qiu, J.Z.; Wang, J.F.; Luo, M.J.; Li, H.E.; Leng, B.-F.; Ren, W.-Z.; Deng, X.M. Peppermint oil decreases the production of virulence-associated exoproteins by Staphylococcus aureus. Molecules 2011, 16, 1642–1654. [Google Scholar] [CrossRef]
- Bou, P.L.G. β-lactamase inhibitors: The story so far. Curr. Med. Chem. 2009, 16, 3740–3765. [Google Scholar]
- Sandanayaka, V.P.; Com, S.; Prashad, A.S. Resistance to beta-lactam antibiotics: Structure and mechanism based design of beta-lactamase inhibitors. Curr. Med. Chem. 2002, 9, 1145–1165. [Google Scholar] [CrossRef] [PubMed]
- Page, M.G. β-Lactamase inhibitors. Drug Res. Updates 2000, 3, 109–125. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, Y.; Niu, X.; Wang, T.; Li, J.; Liu, Z.; Wang, J.; Tang, S.; Wang, Y.; Deng, X. Magnolol restores the activity of meropenem against NDM-1-producing Escherichia coli by inhibiting the activity of metallo-beta-lactamase. Cell Death Discov. 2018, 4, 28. [Google Scholar] [CrossRef] [PubMed]
- Cain, M.R. Design and Synthesis of Metallo-Β-Lactamase Inhibitors; University of Leeds: Leeds, UK, 2015. [Google Scholar]
- Diep, B.A.; Gill, S.R.; Chang, R.F.; Phan, T.H.; Chen, J.H.; Davidson, M.G.; Lin, F.; Lin, J.; Carleton, H.A.; Mongodin, E.F.; et al. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet 2006, 367, 731–739. [Google Scholar] [CrossRef]
- Kuroda, M.; Ohta, T.; Uchiyama, I.; Baba, T.; Yuzawa, H.; Kobayashi, I.; Cui, L.; Oguchi, A.; Aoki, K.; Nagai, Y.; et al. Whole genome sequencing of meticillin-resistant Staphylococcus aureus. Lancet 2001, 357, 1225–1240. [Google Scholar] [CrossRef]
- Brodersen, R. Stability of penicillin G in aqueous solution as a function of hydrogen ion concentration and temperature. Acta Pharmacologica Et Toxicologica 1947, 3, 345–363. [Google Scholar] [CrossRef]
- Michnik, A.; Michalik, K.; Marcoin, W. Influence of magnesium glutamate on stability of penicillin G aqueous solution. Int. J. Pharm. 2004, 273, 149–158. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Nnankya, M.; Matthews, G. Malaria Control: Better Health, Better Future 2016-Eradicate Malaria for Good. Outlooks Pest Manag. 2016, 27, 129–132. [Google Scholar] [CrossRef]

| Gene Names | Primer Names | Oligonucleotide Primer Sequence (5′–3′) |
|---|---|---|
| Metallo-beta-lactamase | β-lactamase-1-F | ccgggatccatgagccgcttgatacgcatgagtg |
| β-lactamase-1-R | ccgctcgagttatattgtatatattggcgttgg | |
| RT-β-lactamase-1-F | gatgttgaatcgtttaacgtgtcacatgatgc | |
| RT-β-lactamase-1-R | ggataacgacacattctcaacatatcgacgtc | |
| Possible beta-lactamase | β-lactamase-2-F | ccgggatccatgatcattgatcctatttgtga |
| β-lactamase-2-R | ccgctcgagttatgtctttactatgaacag | |
| Possible bifunctional beta-lactamase/ rhodanese domain protein | β-lactamase-3-F | cgcggatccatgttttttaaacagttttacga |
| β-lactamase-3-R | ccgctcgagttattttaatgattctggaaaatc | |
| Possible beta-lactamase / penicillin binding protein | β-lactamase-4-F | cgcggatccatgaaatttaataaagtaaaactagt |
| β-lactamase-4-R | ccgctcgagttattgaacaataacacccttcg | |
| AtsA/ElaC family / metallo-beta- lactamase | β-lactamase-5-F | cgcggatccatggaagttacattttttggaac |
| β-lactamase-5-R | ccgctcgagttagattttaaaactatcaaaatct | |
| Metallo-beta-lactamase | β-lactamase-6-F | cgcggatccatgaaacaattacatccaaatga |
| β-lactamase-6-R | ccgctcgagttatttattgtttgattctttttgtt | |
| Beta-lactamase | β-lactamase-7-F | cgcggatccatgagtttaataaagaaaaagaataaag |
| β-lactamase-7-R | ccgctcgagttaaatttcagaaattactggaataat | |
| RT-β-lactamase-7-F | cacacggacatgagcacgcgattggtgc | |
| RT-β-lactamase-7-R | tatgaagtgtgaatacaaacacctaaac | |
| Metallo-beta-lactamase | β-lactamase-8-F | cgcggatccatgaggatttcaagcttaactt |
| β-lactamase-8-R | ccgctcgagttaaccgtgtaaaaatggattt | |
| RT-β-lactamase-8-F | cctggtgaatgtccaggtgtgtgtaac | |
| RT-β-lactamase-8-R | gatatagttgatcgattcgatgtcccgg | |
| Possible metallo-beta-lactamase | β-lactamase-9-F | cgcggatccatgactaatcaatttaaaaata |
| β-lactamase-9-R | ccgctcgagttagctattctcgccctcacgt | |
| Possible metallo-beta-lactamase | β-lactamase-10-F | cgcggatccatgaagttatcatttcatggt |
| β-lactamase-10-R | ccgctcgagttaaaactgaacagattcacctgg | |
| RT-β-lactamase-10-F | cagacctgtatcaccagtatgataaattg | |
| RT-β-lactamase-10-R | ggctgactatctttcttcatatcacggtg | |
| S. aureus 16S ribosomal RNA | RT-16S rRNA-F | tgggatttgcttgacctcgcgg |
| RT-16S rRNA-R | gggggacaaagtgacaggtggt |
| Species | Source | β-lactamase Confirm | MIC (μg/mL) | Antibiotics | MIC (μg/mL) | FIC Index | |
|---|---|---|---|---|---|---|---|
| IAL | Alone | Combination | |||||
| Penicillin G | 53.33 ± 18.48 | 5.33 ± 2.31 | 0.19 ± 0.00 | ||||
| S. aureus USA300 | American Type Culture Collection (ATCC)BAA-1717 | + | ≥512 | Meropenem | 1.33 ± 0.58 | 0.27 ± 0.07 | 0.25 ± 0.00 |
| Cefalotin | 2.67 ± 1.15 | 0.50 ± 0.00 | 0.27 ± 0.07 | ||||
| S. aureus USA400 | American Type Culture Collection (ATCC) | + | ≥512 | Penicillin G | 3.33 ± 1.15 | 0.50 ± 0.00 | 0.23 ± 0.07 |
| S. aureus ATCC29213 | American Type Culture Collection (ATCC) | + | ≥512 | Penicillin G | 2.00 ± 0.00 | 0.25 ± 0.00 | 0.19 ± 0.00 |
| S. aureus 252 | American Type Culture Collection (ATCC) | + | ≥512 | Penicillin G | 32.00 ± 0.00 | 10.67 ± 4.62 | 0.38 ± 0.17 |
| S. aureus 8325-4 | presented by Professor Timothy J. Foster | + | >512 | Penicillin G | 0.50 ± 0.00 | 0.063 ± 0.00 | 0.19 ± 0.00 |
| S. aureus ATCC25923 | American Type Culture Collection (ATCC) | — | 21.33 ± 9.24 | Penicillin G | 0.008 ± 0.000 | 0.008 ± 0.000 | 1.21 ± 0.07 |
| MRSA ST1010 | Obtained from the First Hospital of Jilin University in Jilin, China | + | ≥512 | Penicillin G | 42.67 ± 18.48 | 8.00 ± 0.00 | 0.27 ± 0.07 |
| MRSA ST1015-1 | Obtained from porcine samples collected in Shandong, China | + | ≥512 | Penicillin G | 64.00 ± 0.00 | 16.00 ± 0.00 | 0.31 ± 0.00 |
| MRSA ST1015-2 | Obtained from porcine samples collected in Shandong, China | + | ≥512 | Penicillin G | 64.00 ± 0.00 | 13.33 ± 4.62 | 0.27 ± 0.07 |
| MRSA ST1016-1 | Obtained from porcine samples collected in Shandong, China | + | ≥512 | Penicillin G | 16.00 ± 0.00 | 4.00 ± 0.00 | 0.31 ± 0.00 |
| MRSA ST1053 | Obtained from porcine samples collected in Shandong, China | + | ≥512 | Penicillin G | 13.33 ± 4.62 | 2.33 ± 1.53 | 0.23 ± 0.07 |
| MRSA ST1060 | Obtained from porcine samples collected in Shandong, China | + | ≥512 | Penicillin G | 32.00 ± 0.00 | 8.00 ± 0.00 | 0.31 ± 0.00 |
| MRSA ST1061-1 | Obtained from porcine samples collected in Shandong, China | + | ≥512 | Penicillin G | 21.33 ± 9.24 | 5.33 ± 2.31 | 0.31 ± 0.00 |
| MRSA ST1061-2 | Obtained from porcine samples collected in Shandong, China | + | ≥512 | Penicillin G | 8.00 ± 0.00 | 0.83 ± 0.29 | 0.17 ± 0.04 |
| MRSA ST1064-1 | Obtained from porcine samples collected in Shandong, China | + | ≥512 | Penicillin G | 21.33 ± 9.24 | 1.83 ± 1.89 | 0.14 ± 0.05 |
| MRSA ST1064-2 | Obtained from porcine samples collected in Shandong, China | + | ≥512 | Penicillin G | 34.67 ± 28.10 | 8.67 ± 7.02 | 0.31 ± 0.00 |
| MRSA ST1067-1 | Obtained from porcine samples collected in Shandong, China | + | ≥512 | Penicillin G | 42.67 ± 18.48 | 5.33 ± 2.31 | 0.19 ± 0.00 |
| MRSA ST1068-1 | Obtained from porcine samples collected in Shandong, China | + | ≥512 | Penicillin G | 32.00 ± 0.00 | 4.00 ± 3.46 | 0.19 ± 0.11 |
| MRSA ST2022 | Obtained from porcine samples collected in Shandong, China | + | ≥512 | Penicillin G | 16.00 ± 13.86 | 2.00 ± 1.73 | 0.19 ± 0.00 |
| MRSA ST2064 | Obtained from porcine samples collected in Shandong, China | + | ≥512 | Penicillin G | 106.7 ± 36.95 | 4.00 ± 0.00 | 0.10 ± 0.02 |
| MRSA ST2032 | Obtained from porcine samples collected in Shandong, China | + | ≥512 | Penicillin G | 6.67 ± 2.31 | 0.67 ± 0.29 | 0.19 ± 0.06 |
| Sulbactam | |||||||
| S. aureus USA300 | American Type Culture Collection (ATCC)BAA-1717 | + | 128.00 ± 0.00 | Penicillin G | 53.33 ± 18.48 | 3.33 ± 0.33 | 0.313 ± 0.00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Guo, Y.; Wen, Z.; Ci, X.; Xia, L.; Wang, Y.; Deng, X.; Wang, J. Isoalantolactone Enhances the Antimicrobial Activity of Penicillin G against Staphylococcus aureus by Inactivating β-Lactamase during Protein Translation. Pathogens 2020, 9, 161. https://doi.org/10.3390/pathogens9030161
Zhou Y, Guo Y, Wen Z, Ci X, Xia L, Wang Y, Deng X, Wang J. Isoalantolactone Enhances the Antimicrobial Activity of Penicillin G against Staphylococcus aureus by Inactivating β-Lactamase during Protein Translation. Pathogens. 2020; 9(3):161. https://doi.org/10.3390/pathogens9030161
Chicago/Turabian StyleZhou, Yonglin, Yan Guo, Zhongmei Wen, Xinxin Ci, Lining Xia, Yanling Wang, Xuming Deng, and Jianfeng Wang. 2020. "Isoalantolactone Enhances the Antimicrobial Activity of Penicillin G against Staphylococcus aureus by Inactivating β-Lactamase during Protein Translation" Pathogens 9, no. 3: 161. https://doi.org/10.3390/pathogens9030161
APA StyleZhou, Y., Guo, Y., Wen, Z., Ci, X., Xia, L., Wang, Y., Deng, X., & Wang, J. (2020). Isoalantolactone Enhances the Antimicrobial Activity of Penicillin G against Staphylococcus aureus by Inactivating β-Lactamase during Protein Translation. Pathogens, 9(3), 161. https://doi.org/10.3390/pathogens9030161






