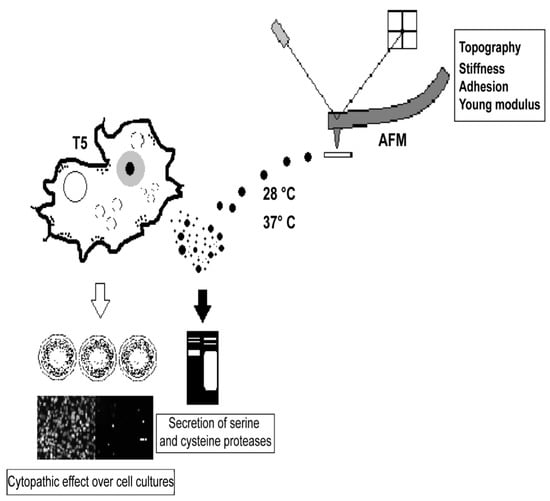
Isolation of Acanthamoeba T5 from Water: Characterization of Its Pathogenic Potential, Including the Production of Extracellular Vesicles
Abstract
1. Introduction
2. Results
2.1. Isolate Genotyping Reveals a Thermotolerant Acanthamoeba T5 Isolate
2.2. Virulence Factors of the Acanthamoeba T5 Isolate
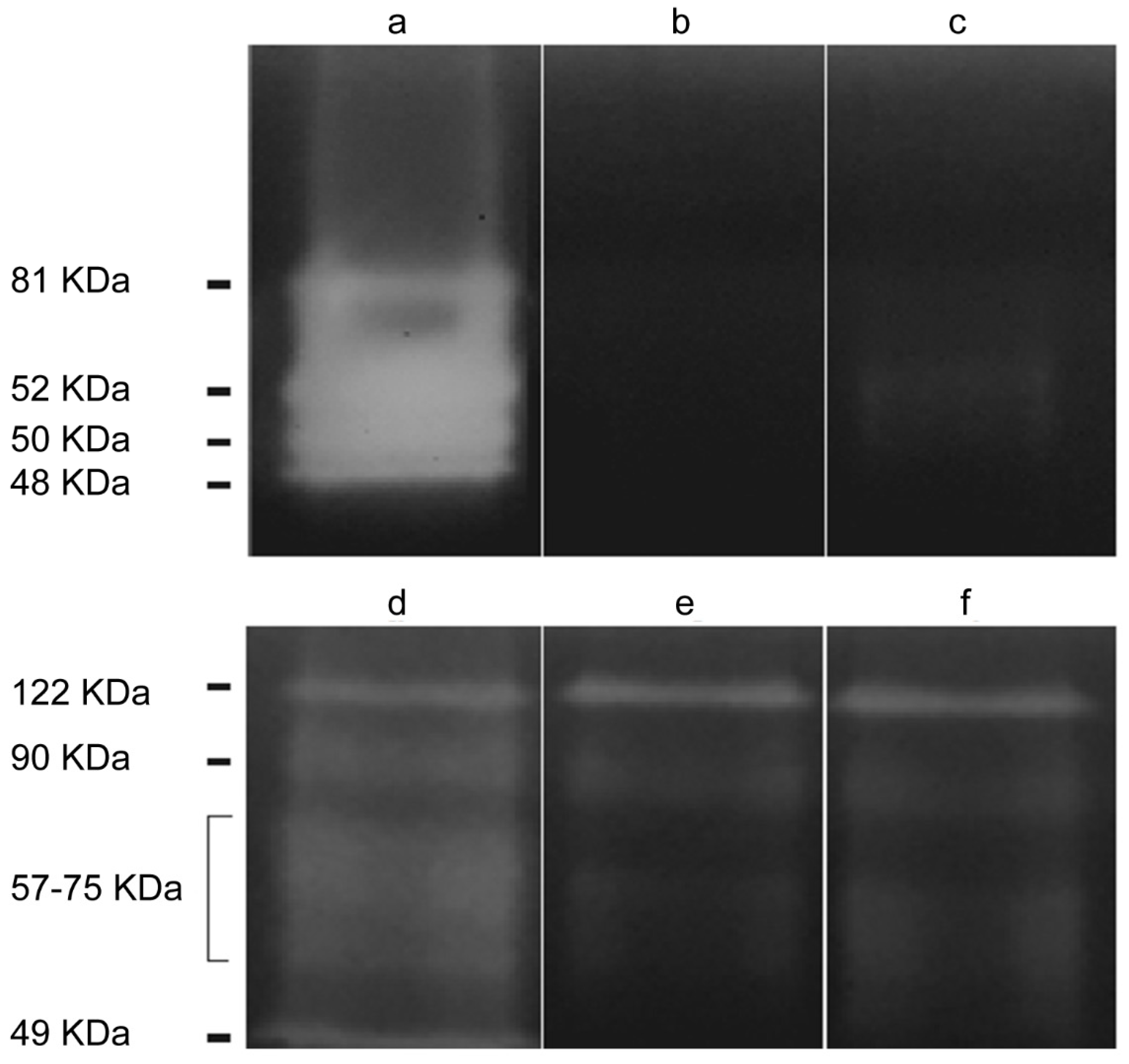
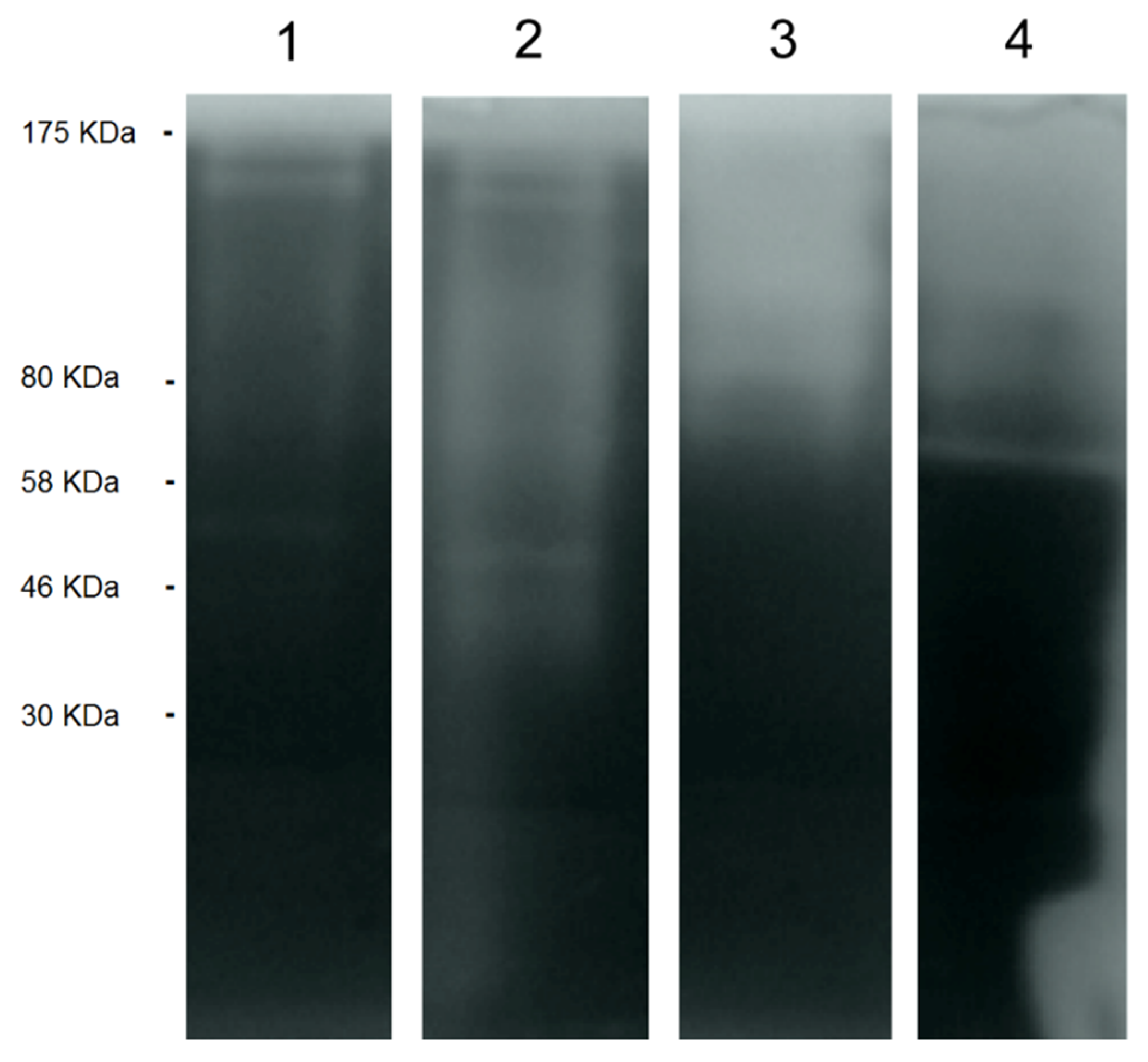
2.2.1. Acanthamoeba T5 Secretes Active Serine and Cysteine Proteases
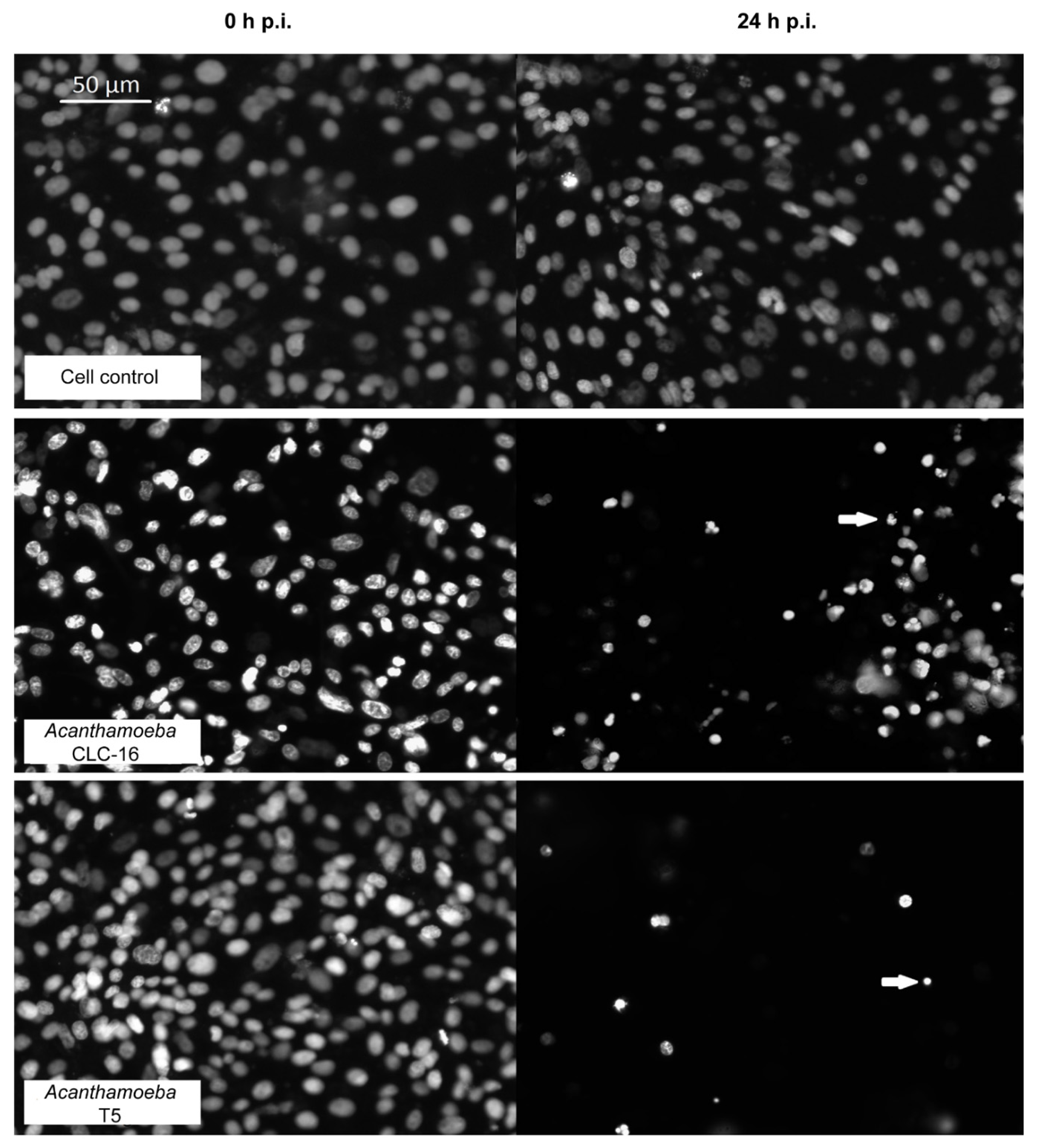
2.2.2. Cytopathic Effect of Acanthamoeba T5 over MDCK and Vero Cell Lines
2.3. Secretion of Extracellular Vesicles by Acanthamoeba T5
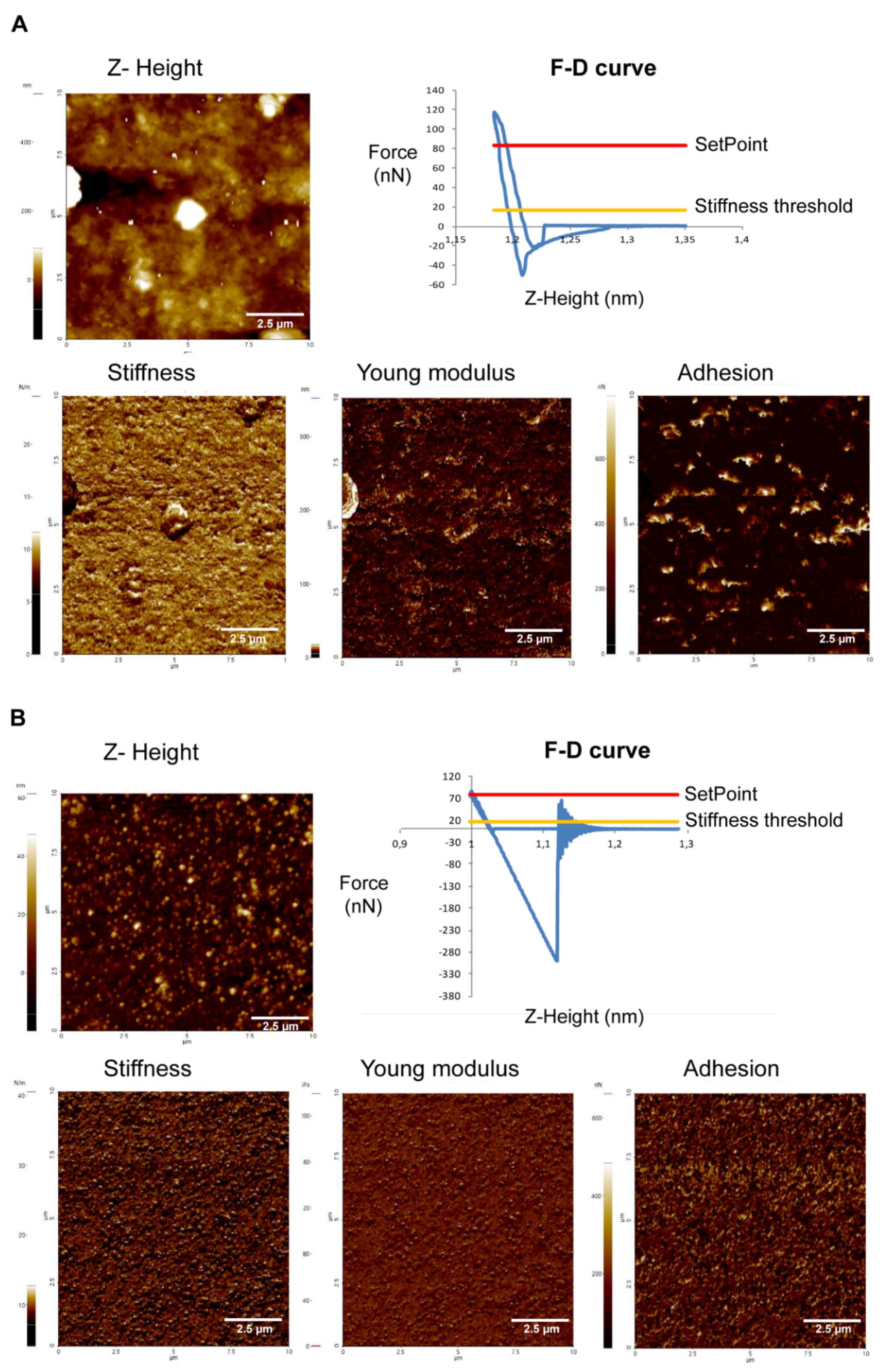
2.3.1. Acanthamoeba T5 Secrete Extracellular Vesicles in a Temperature-Dependent Way, with Different Sizes and Nanomechanical Properties
2.3.2. EVs of Acanthamoeba T5 Incubated at 37 °C have Higher Proteolytic Activity than Acanthamoeba T5 Incubated at 28 °C and Their Cargo Includes Serine and Cysteine Proteases
3. Discussion
4. Materials and Methods
4.1. Axenic Culture of Acanthamoeba
4.2. DNA Extraction and Isolate Genotyping
4.3. Osmotolerance and Thermotolerance Assays
4.4. Preparation of Acanthamoeba Conditioned Medium (ACM)
4.5. Determination and Characterization of Protease Secretion by Zymography
4.6. Evaluation of the in Vitro Effect of Acanthamoeba in Cell Cultures
4.7. Isolation and Purification of the Extracellular Vesicles of Acanthamoeba T5
4.8. Dynamic Light Scattering of the EVs of Acanthamoeba T5
4.9. Atomic Force Microscopy Imaging of the EVs of Acanthamoeba T5
4.10. Zymography of the EVs of Acanthamoeba T5
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fuerst, P.A. 18S ribosomal DNA typing and tracking of Acanthamoeba species isolates from corneal scrape specimens, contact lenses, lens cases, and home water supplies of Acanthamoeba keratitis patients in Hong Kong. J. Clin. Microbiol. 2002, 40, 1621–1625. [Google Scholar]
- Im, K.; Kim, D.S. Acanthamoebiasis in Korea: Two new cases with clinical cases review. Yonsei Med. J. 1988, 39, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Torno, M.S.; Babapour, R., Jr.; Gurevitch, A.; Witt, M.D. Cutaneous acanthamoebiasis in AIDS. J. Am. Acad. Dermatol. 2000, 42, 351–354. [Google Scholar] [CrossRef]
- Walochnik, J.; Obwaller, A.; Aspöck, H. Correlations between morphological, molecular biological and physiological characteristics in clinical and nonclinical isolates of Acanthamoeba spp. Am. Soc. Microbiol. 2000, 66, 4408–4413. [Google Scholar] [CrossRef]
- Walochnik, J.; Aichelburg, A.; Assadian, O.; Steuer, A.; Visvesvara, G.; Vetter, N.; Aspöck, H. Granulomatous amoebic encephalitis caused by Acanthamoeba amoebae of genotype T2 in a human immunodeficiency virus-negative patient. Clin. J. Microbiol. 2008, 46, 338–340. [Google Scholar] [CrossRef]
- Di Cave, D.; Monno, R.; Bottalico, P.; Guerriero, S.; D’Amelio, S.; D’Orazi, C.; Berrilli, F. Acanthamoeba T4 and T15 genotypes associated with keratitis infections in Italy. Eur. J. Clin. Microbiol. Infect. Dis. 2008, 28, 607–612. [Google Scholar] [CrossRef]
- Lorenzo Morales, J.; Morcillo Laiz, R.; Martin Navarro, C.M.; López Vélez, R.; López Arencibia, A.; Arnalich Montiel, F.; Maciver, S.K.; Valladares, B.; Martínez Carretero, E. Acanthamoeba keratitis due to genotype T11 in a rigid gas permeable contact lens wearer in Spain. Contact Lens Anterior Eye 2011, 34, 83–86. [Google Scholar] [CrossRef]
- Omaña Molina, M.; Vanzzini Zago, V.; Hernández Martínez, D.; González Robles, A.; Salazar Villatoro, L.; Ramírez Flores, E.; Oregon Miranda, E.; Lorenzo Morales, J.; Martinez Palomo, A. Acanthamoeba genotypes T3 and T4 as causative agents of amoebic keratitis in Mexico. Parasitol. Res. 2016, 115, 873–878. [Google Scholar] [CrossRef]
- Spanakos, G.; Tzanetou, K.; Miltsakakis, D.; Patsoula, E.; Malamou Lada, E.; Vakalis, N.C. Genotyping of pathogenic Acanthamoebae isolated from clinical samples in Greece Report of a clinical isolate presenting T5 genotype. Parasitol. Int. 2006, 55, 147–149. [Google Scholar] [CrossRef]
- Ledee, D.R.; Iovieno, A.; Miller, D.; Mandal, N.; Diaz, M.; Fell, J.; Fini, M.E.; Alfonso, E.C. Molecular identification of T4 and T5 genotypes in isolates from Acanthamoeba keratitis patients. J. Clin. Microbiol. 2009, 47, 1458–1462. [Google Scholar] [CrossRef]
- van Zyl, L.M.; Andrews, N.; Chebade, M.; Sadlon, T.A.; Badenoch, R.P. Acanthamoeba lenticulata keratitis in a hard contact lens wearer. Clin. Exp. Ophthalmol. 2013, 41, 810–812. [Google Scholar] [CrossRef] [PubMed]
- Barete, S.; Combes, A.; De Jonckeere, J.F.; Darty, A.; Varnous, S.; Martinez, V.; Ptacek, S.G.; Caumes, E.; Capron, F.; Francès, C.; et al. Fatal disseminated Acanthamoeba lenticulata acanthamebiasis in a heart transplant patient. Emerg. Infect. Dis. 2007, 13, 736–738. [Google Scholar] [CrossRef] [PubMed]
- Lackner, P.; Beer, R.; Broessner, G.; Helbok, R.; Pfausler, B.; Brenneis, C.; Auer, H.; Walochnik, J.; Schmutzhard, E. Acute granulomatous Acanthamoeba encephalitis in an immunocompetent patient. Neurocri. Care 2010, 12, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Iovieno, A.; Oechsler, R.A.; Ledee, D.R.; Miller, D.; Alfonso, E.C. Drug resistant severe Acanthamoeba keratitis caused by rare T5 Acanthamoeba genotype. Eye Contact Lens 2010, 36, 183–184. [Google Scholar] [CrossRef]
- Barbeau, J.; Buhler, T. Biofilms augment the number of free-living amoebae in dental unit waterlines. Res. Microbiol. 2001, 152, 753–760. [Google Scholar] [CrossRef]
- Dendana, F.; Sellami, H.; Jarraya, F.; Sellami, A.; Makni, F.; Cheikhrouhou, F.; Hachicha, J.; Ayadi, A. Free-living amoebae (FLA): Detection, morphological and molecular identification of Acanthamoeba genus in the hydraulic system of an haemodialysis unit in Tunisia. Parasite 2008, 15, 137–142. [Google Scholar] [CrossRef][Green Version]
- Trabelsi, H.; Sellami, A.; Dendana, F.; Sellami, H.; Cheikh Rouhou, F.; Makni, F.; Ben, D.S.; Ayadi, A. Free-living amoebae (FLA): Morphological and molecular identification of Acanthamoeba in dental unit water. Parasite 2010, 17, 67–70. [Google Scholar] [CrossRef]
- Retana Moreira, L.; Abrahams Sandí, E.; Cabello Vílchez, A.M.; Reyes Batlle, M.; Valladares, B.; Martínez, C.E.; Piñero, E.J.; Lorenzo Morales, J. Isolation and molecular characterization of Acanthamoeba and Balamuthia mandrillaris from combination shower units in Costa Rica. Parasitol. Res. 2014, 113, 4117–4122. [Google Scholar] [CrossRef]
- Retana Moreira, L.; Abrahams Sandí, E.; Castro Artavia, E.; Fernández Sánchez, A.; Castro Castillo, A.; Reyes Batlle, M.; Lorenzo Morales, J. Isolation and molecular characterization of Acanthamoeba strains from dental units in Costa Rica. J. Eukar. Microbiol. 2015, 62, 733–736. [Google Scholar] [CrossRef]
- van der Pol, E.; Boing, A.N.; Harrison, P.; Sturk, A.; Nieuwland, R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol. Rev. 2012, 64, 676–705. [Google Scholar] [CrossRef]
- Deolindo, P.; Evans Osses, I.; Ramirez, M.I. Microvesicles and exosomes as vehicles between protozoan and host cell communication. Biochem. Soc. Trans. 2013, 41, 252–257. [Google Scholar] [CrossRef]
- Marcilla, A.; Martin Jaular, L.; Trelis, M.; de Menezes Neto, A.; Osuna, A.; Bernal, D.; Fernandez Becerra, C.; Almeida, I.C.; del Portillo, H.A. Extracellular vesicles in parasitic diseases. J. Extracel. Ves. 2014, 3, 25040. [Google Scholar] [CrossRef] [PubMed]
- Trocoli Torrecilhas, A.C.; Tonelli, R.R.; Pavanelli, W.R.; da Silva, J.S.; Schumacher, R.I.; de Souza, W.; Silva, N.C.; de Almeida Abrahamsohn, I.; Colli, W.; Manso Alves, M.J. Trypanosoma cruzi: Parasite shed vesicles increase heart parasitism and generate an intense inflammatory response. Microbes Infect. 2009, 11, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Cestari, I.; Ansa Addo, E.; Deolindo, P.; Inal, J.M.; Ramirez, M.I. Trypanosoma cruzi immune evasion mediated by host cell derived microvesicles. J. Immunol. 2012, 188, 1942–1952. [Google Scholar] [CrossRef]
- Retana Moreira, L.; Rodríguez Serrano, F.; Osuna, A. Extracellular vesicles of Trypanosoma cruzi tissue-culture cell-derived trypomastigotes: Induction of physiological changes in nonparasitized culture cells. PLoS Negl. Trop. Dis. 2019, 13, e0007163. [Google Scholar] [CrossRef]
- Silverman, J.M.; Reiner, N.E. Exosomes and other microvesicles in infection biology: Organelles with unanticipated phenotypes. Cell. Microbiol. 2011, 13, 1–9. [Google Scholar] [CrossRef]
- Atayde, V.D.; da Silva Lira Filho, A.; Chaparro, V.; Zimmermann, A.; Martel, C.; Jaramillo, M.; Olivier, M. Exploitation of the Leishmania exosomal pathway by Leishmania RNA virus 1. Nat. Microbiol. 2019, 4, 714–723. [Google Scholar] [CrossRef]
- Aline, F.; Bout, D.; Amigorena, S.; Roingeard, P.; Dimier Poisson, I. Toxoplasma gondii antigen-pulsed-dendritic cell-derived exosomes induce a protective immune response against T. gondii infection. Infect. Immun. 2004, 72, 4127–4137. [Google Scholar] [CrossRef]
- Beauvillain, C.; Juste, M.O.; Dion, S.; Pierre, J.; Dimier Poisson, I. Exosomes are an effective vaccine against congenital toxoplasmosis in mice. Vaccine 2009, 27, 1750–1757. [Google Scholar] [CrossRef]
- del Cacho, E.; Gallego, M.; Lee, S.H.; Lillehoj, H.S.; Quilez, J.; Lillehoj, E.P.; Sánchez Acedo, C. Induction of protective immunity against Eimeria tenella infection using antigen-loaded dendritic cells (DC) and DC-derived exosomes. Vaccine 2011, 29, 3818–3825. [Google Scholar] [CrossRef]
- Hu, G.; Gong, A.Y.; Roth, A.L.; Huang, B.Q.; Ward, H.D.; Zhu, G.; Hanson, N.D.; Chen, X.M. Release of luminal exosomes contributes to TLR4 mediated epithelial antimicrobial defense. PLoS Pathog. 2013, 9, e1003261. [Google Scholar] [CrossRef] [PubMed]
- Twu, O.; de Miguel, N.; Lustig, G.; Stevens, G.C.; Vashisht, A.A.; Wohlschlegel, J.A.; Johnson, P.J. Trichomonas vaginalis exosomes deliver cargo to host cells and mediate host: Parasite interactions. PLoS Pathog. 2013, 9, e1003482. [Google Scholar] [CrossRef] [PubMed]
- Evans Osses, I.; Mojoli, A.; Monguió Tortajada, M.; Marcilla, A.; Aran, V.; Amorim, M.; Inal, J.; Borràsm, F.E.; Ramirez, M. Microvesicles released from Giardia intestinalis disturb host-pathogen response in vitro. Eur. J. Cell Biol. 2017, 96, 131–142. [Google Scholar] [CrossRef] [PubMed]
- de Souza Gonçalves, D.; da Silva Ferreira, M.; Guimarães, A.J. Extracellular vesicles from the protozoa Acanthamoeba castellanii: Their role in pathogenesis, environmental adaptation and potential applications. Bioengineering 2019, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.C.; Tsai, C.Y.; Huang, J.M.; Wu, S.R.; Chu, L.J.; Huang, K.Y. Quantitative proteomic analysis and functional characterization of Acanthamoeba castellanii exosome-like vesicles. Parasit. Vectors 2019, 12, 467. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, R.; Khan, N.A. Biology and pathogenesis of Acanthamoeba. Parasit. Vectors 2012, 5, 1–6. [Google Scholar] [CrossRef]
- Khan, N.A.; Jarroll, E.L.; Panjwani, N.; Cao, Z.; Paget, T.A. Proteases as markers for differentiation of pathogenic and nonpathogenic species of Acanthamoeba. J. Clin. Microbiol. 2000, 38, 2858–2861. [Google Scholar] [CrossRef]
- Khan, N.A. Acanthamoeba: biology and increasing importance in human health. FEMS Microbiol. Rev. 2006, 30, 564–595. [Google Scholar] [CrossRef]
- Omaña Molina, M.; González Robles, A.; Salazar Villatoro, L.I.; Lorenzo Morales, J.; Cristóbal Ramos, A.R.; Hernández Ramírez, V.I.; Talamás Rohana, P.; Mendez Cruz, A.R.; Martínez Palomo, A. Reevaluating the role of Acanthamoeba proteases in tissue invasion: observation of cytopathogenic mechanisms on MDCK cell monolayers and hamster corneal cells. Biomed. Res. Int. 2013, 461329. [Google Scholar]
- Alsam, S.; Sissons, J.; Jayasekera, S.; Khan, N.A. Extracellular proteases of Acanthamoeba castellanii (encephalitis isolate belonging to T1 genotype) contribute to increased permeability in an in vitro model of the human blood-brain barrier. J. Infect. 2005, 51, 150–156. [Google Scholar] [CrossRef]
- Clarke, D.W.; Niederkorn, J.Y. The pathophysiology of Acanthamoeba keratitis. Trends Parasitol. 2006, 22, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Ramírez Rico, G.; Martínez Castillo, M.; de la Garza, M.; Shibayama, M.; Serrano-Luna, J.J. Acanthamoebacastellanii Proteases are capable of degrading iron-binding proteins as a possible mechanism of pathogenicity. J. Eukar. Microbiol. 2015, 62, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Bayer Santos, E.; Lima, F.M.; Ruiz, J.C.; Almeida, I.C.; da Silveira, J.F. Characterization of the small RNA content of Trypanosoma cruzi extracellular vesicles. Mol. Biochem. Parasitol. 2014, 193, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Garcia Silva, M.R.; Cura das Neves, R.F.; Cabrera Cabrera, F.; Sanguinetti, J.; Medeiros, L.C.; Robello, C.; Naya, H.; Fernandez Calero, T.; Souto Padron, T.; de Souza, W.; et al. Extracellular vesicles shed by Trypanosoma cruzi are linked to small RNA pathways, life cycle regulation, and susceptibility to infection of mammalian cells. Parasitol. Res. 2014, 113, 285–304. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, M.I.; Deolindo, P.; de Messias Reason, I.J.; Arigi, E.A.; Choi, H.; Almeida, I.C.; Evans Osses, I. Dynamic flux of microvesicles modulate parasite-host cell interaction of Trypanosoma cruzi in eukaryotic cells. Cell. Microbiol. 2016, 19, e12672. [Google Scholar] [CrossRef] [PubMed]
- Mantel, P.Y.; Hoang, A.N.; Goldowitz, I.; Potashnikova, D.; Hamza, B.; Vorobjev, I.; Ghiran, I.; Toner, M.; Irimia, D.; Ivanov, A.R.; et al. Malaria-infected erythrocyte-derived microvesicles mediate cellular communication within the parasite population and with the host immune system. Cell Host Microbe 2013, 13, 521–534. [Google Scholar] [CrossRef]
- Fernandez Calero, T.; Garcia Silva, R.; Pena, A.; Robello, C.; Persson, H.; Rovira, C.; Naya, H.; Cayota, A. Profiling of small RNA cargo of extracellular vesicles shed by Trypanosoma cruzi reveals a specific extracellular signature. Mol. Biochem. Parasitol. 2015, 199, 19–28. [Google Scholar] [CrossRef]
- Tatischeff, I.; Lavialle, F.; Pigaglio-Deshayes, S.; Pechoux Longin, C.; Chinsky, L.; Alfsen, A. Dictyostelium extracellular vesicles containing hoechst 33342 transfer the dye into the nuclei of living cells: A fluorescence study. J. Fluoresc. 2008, 18, 319–328. [Google Scholar] [CrossRef]
- Du, Q.; Kawabe, Y.; Schilde, C.; Chen, Z.H.; Schaap, P. The evolution of aggregative multicellularity and cell-cell communication in the Dictyostelia. J. Mol. Biol. 2005, 427, 3722–3733. [Google Scholar] [CrossRef]
- Sharma, S.; Rasool, H.I.; Palanisamy, V.; Mathisen, C.; Schmidt, M.; Wong, D.T.; Gimzewski, J.K. Structural-mechanical characterization of nanoparticles-exosomes in human saliva, using correlative AFM, FESEM and force spectroscopy. ACS Nano 2010, 4, 1921–1926. [Google Scholar] [CrossRef]
- Xiao, L.; Tang, M.; Lia, Q.; Zhou, A. Non-invasive detection of biomechanical and biochemical responses of human lung cells to short time chemotherapy exposure using AFM and confocal Raman spectroscopy. Anal. Methods 2013, 5, 874–879. [Google Scholar] [CrossRef]
- Whitehead, B.; Wu, L.P.; Aslan, H.; Dong, M.; Dyrskjøt, L.; Ostenfeld, M.S.; Moghimi, S.M.; Howard, K.A. Tumour exosomes display differential mechanical and complement activation properties dependent on malignant state: Implications in endothelial leakiness. J. Extracell. Ves. 2015, 4, 29685. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Available online: https://www.cdc.gov/parasites/naegleria/prevention.html (accessed on 13 November 2019).
- Thomas, J.M.; Ashbolt, N.J. Do Free-Living Amoebae in Treated Drinking Water Systems Present an Emerging Health Risk? Environ. Sci. Technol. 2011, 45, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Kilvington, S.; Gray, T.; Dart, J.; Morlet, N.; Beeching, J.R.; Frazer, D.G.; Matheson, M. Acanthamoeba keratitis: The role of domestic tap water contamination in the United Kingdom. IOVS 2004, 45, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.J.; Yu, H.S. The role of domestic tap water in Acanthamoeba contamination in contact lens storage cases in Korea. Korean J. Parasitol. 2005, 43, 47–50. [Google Scholar] [CrossRef]
- Boost, M.; Cho, P.; Lai, S.; Sun, W.M. Detection of Acanthamoeba in tap water and contact lens cases using polymerase chain reaction. Optom. Vis. Sci. 2008, 85, 526–530. [Google Scholar] [CrossRef]
- Fallah, E.; Jafarpour, Z.; Mahami-Oskouei, M.; Haghighi, A.; Niyyati, M.; Spotin, A.; Khezri, A. Molecular Characterization of Acanthamoeba isolates from surface resting waters in northwest Iran. Iran J. Parasitol. 2017, 12, 355–363. [Google Scholar]
- Maschio, V.J.; Chies, F.; Carlesso, A.M.; Carvalho, A.; Rosa, S.P.; Van Der Sand, S.T.; Rott, M.B. Acanthamoeba T4, T5 and T11 isolated from mineral water bottles in southern Brazil. Curr. Microbiol. 2015, 70, 6–9. [Google Scholar] [CrossRef]
- Sente, C.; Erume, J.; Naigaga, I.; Magambo, P.K.; Ochwo, S.; Mulindwa, J.; Namara, B.G.; Kato, C.D.; Sebyatika, G.; Muwonge, K.; et al. Occurrence and genetic characterisation of Acanthamoeba spp. from environmental and domestic water sources in Queen Elizabeth Protected Area, Uganda. Parasit. Vectors 2016, 9, 127. [Google Scholar] [CrossRef]
- De Jonckheere, J.F.; Michel, R. Species identification and virulence of Acanthamoeba strains from human nasal mucosa. Parasitol. Res. 1988, 74, 314–316. [Google Scholar] [CrossRef]
- Castro Artavia, E.; Retana Moreira, L.; Lorenzo-Morales, J.; Abrahams Sandí, E. Potentially pathogenic Acanthamoeba genotype T4 isolated from dental units and emergency combination showers. Mem. Inst. Oswaldo Cruz 2017, 112, 817–821. [Google Scholar] [CrossRef] [PubMed]
- Booton, G.C.; Kelly, D.J.; Chu, Y.W.; Seal, D.V.; Houang, E.; Lam, D.S.C.; Byers, T.J.; Carpenter, A.E.; Jones, T.R.; Lamprecht, M.R.; et al. CellProfiler Image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006, 7, R100. [Google Scholar]
- Herron, G.S.; Banda, M.J.; Clark, E.J.; Gavrilovic, J.; Werb, Z. Secretion of metalloproteinases by stimulated capillary endothelial cells. J. Biol. Chem. 1986, 261, 2814–2818. [Google Scholar] [PubMed]
- Martín Navarro, C.M.; Lorenzo Morales, J.; Cabrera Serra, M.G.; Rancel, F.; Coronado Álvarez, N.M.; Piñero, J.E.; Valladares, B. The potential pathogenicity of chlorhexidine-sensitive Acanthamoeba strains isolated from contact lens cases from asymptomatic individuals in Tenerife, Canary Islands, Spain. J. Med. Microbiol. 2008, 57, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Martín Navarro, C.M.; Lorenzo Morales, J.; Machín, R.; López Arencibia, A.; Valladares, B.; Piñero, E. Acanthamoeba spp.: In vitro effects of clinical isolates on murine macrophages, osteosarcoma and HeLa cells. Exp. Parasitol. 2010, 126, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Parisse, P.; Rago, I.; Ulloa Severino, L.; Perissinotto, F.; Ambrosetti, E.; Paoletti, P.; Ricci, M.; Beltrami, A.P.; Cesselli, D.; Casalis, L. Atomic force microscopy analysis of extracellular vesicles. Eur. Biophys. J. 2017, 46, 813–820. [Google Scholar] [CrossRef]
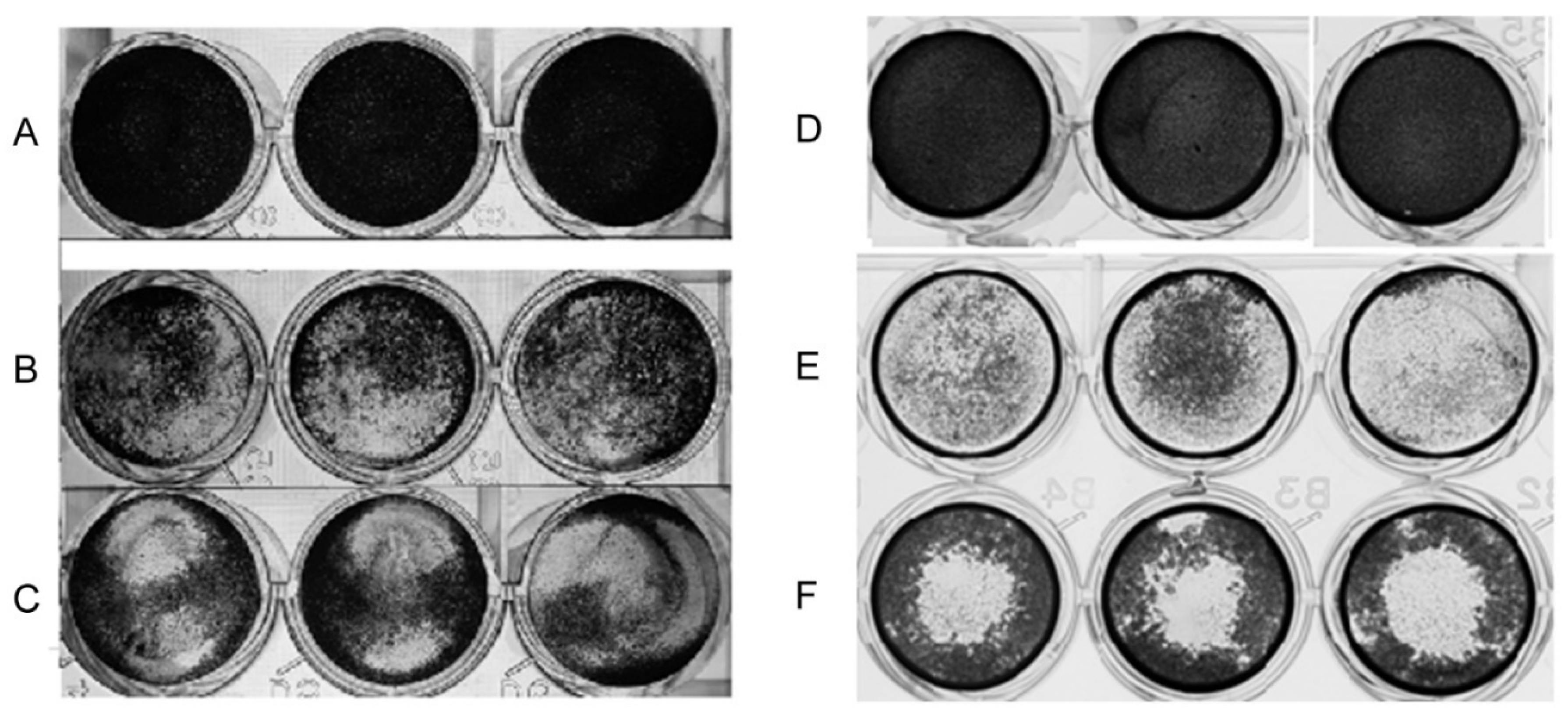
| Time p.i * (h) | Acanthamoeba CLC-16 (Genotype T3) | Acanthamoeba (Non-Pathogenic Genotype T4) | Acanthamoeba Genotype T5 | Cell Control |
|---|---|---|---|---|
| 0 | 100.0 | 100.0 | 100.0 | 100.0 |
| 4 | 98.7 (± 3.7) | 109.9 (± 2.6) | 101.8 (± 4.9) | 104.3 (± 1.5) |
| 8 | 67.4 (± 7.4) | 108.5 (± 2.3) | 88.9 (± 6.2) | 107.4 (± 1.5) |
| 12 | 45.2 (± 6.3) | 109.2 (± 3.5) | 68.0 (± 1.6) | 105.9 (± 1.8) |
| 16 | 38.7 (± 4.7) | 107.1 (± 5.5) | 42.3 (± 5.5) | 105.5 (± 7.0) |
| 20 | 41.3 (± 3.7) | 103.2 (± 5.0) | 22.4 (± 2.3) | 106.4 (± 5.0) |
| 24 | 43.0 (± 3.2) | 102.1 (± 7.5) | 14.3 (± 2.7) | 103.9 (± 2.8) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Retana Moreira, L.; Vargas Ramírez, D.; Linares, F.; Prescilla Ledezma, A.; Vaglio Garro, A.; Osuna, A.; Lorenzo Morales, J.; Abrahams Sandí, E. Isolation of Acanthamoeba T5 from Water: Characterization of Its Pathogenic Potential, Including the Production of Extracellular Vesicles. Pathogens 2020, 9, 144. https://doi.org/10.3390/pathogens9020144
Retana Moreira L, Vargas Ramírez D, Linares F, Prescilla Ledezma A, Vaglio Garro A, Osuna A, Lorenzo Morales J, Abrahams Sandí E. Isolation of Acanthamoeba T5 from Water: Characterization of Its Pathogenic Potential, Including the Production of Extracellular Vesicles. Pathogens. 2020; 9(2):144. https://doi.org/10.3390/pathogens9020144
Chicago/Turabian StyleRetana Moreira, Lissette, Daniel Vargas Ramírez, Fátima Linares, Alexa Prescilla Ledezma, Annette Vaglio Garro, Antonio Osuna, Jacob Lorenzo Morales, and Elizabeth Abrahams Sandí. 2020. "Isolation of Acanthamoeba T5 from Water: Characterization of Its Pathogenic Potential, Including the Production of Extracellular Vesicles" Pathogens 9, no. 2: 144. https://doi.org/10.3390/pathogens9020144
APA StyleRetana Moreira, L., Vargas Ramírez, D., Linares, F., Prescilla Ledezma, A., Vaglio Garro, A., Osuna, A., Lorenzo Morales, J., & Abrahams Sandí, E. (2020). Isolation of Acanthamoeba T5 from Water: Characterization of Its Pathogenic Potential, Including the Production of Extracellular Vesicles. Pathogens, 9(2), 144. https://doi.org/10.3390/pathogens9020144