Adhesins of Brucella: Their Roles in the Interaction with the Host
Abstract
1. Introduction
2. Brucella Infection and Clinical Manifestations
3. Brucella Entry into Host Cells
4. Bacterial Adhesins
5. Adhesins of Brucella
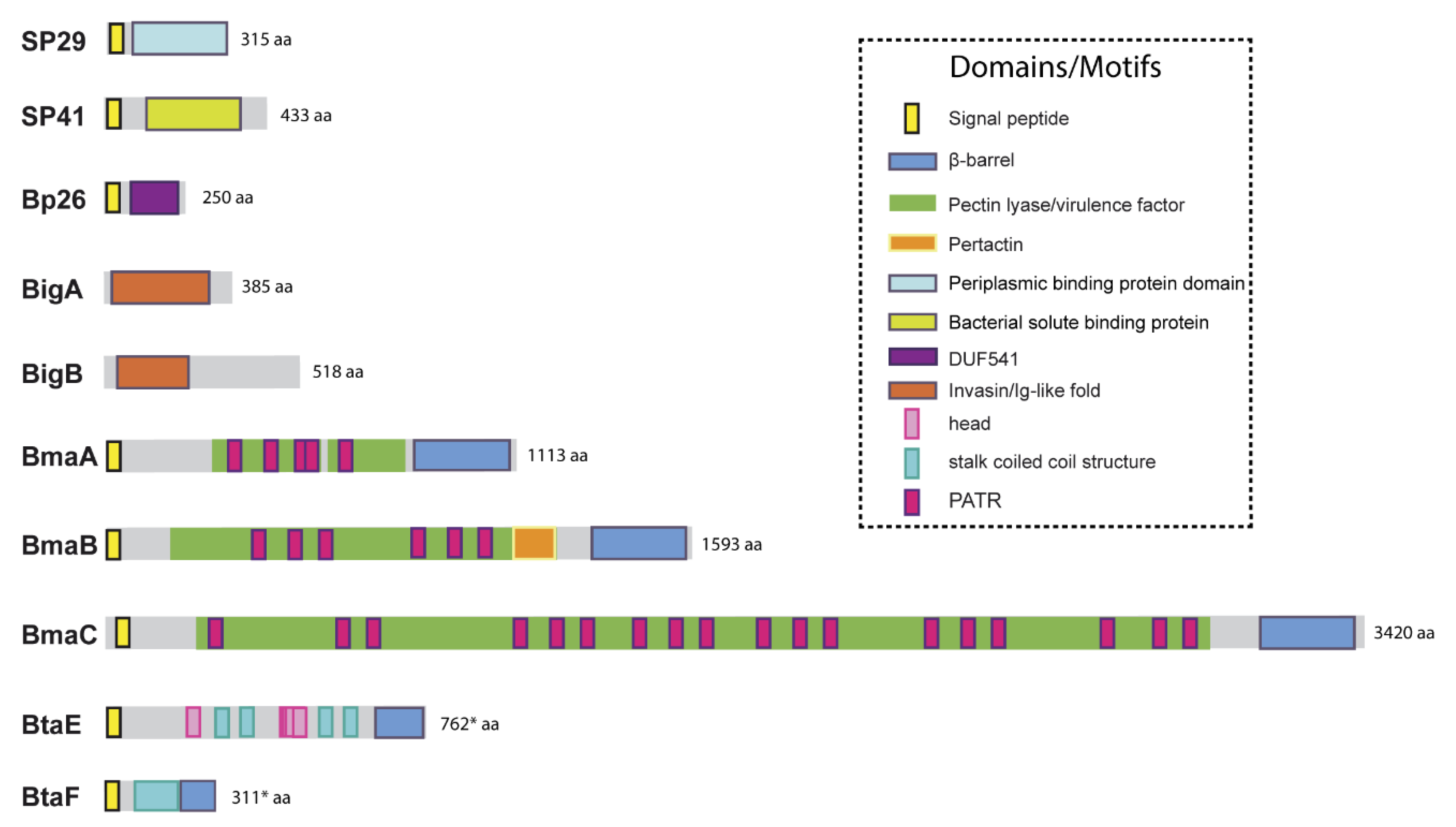
5.1. Unclassified Adhesins
5.2. Adhesins Containing Ig-Like Domains
5.3. Autotransporters
5.3.1. Monomeric Autotransporters
5.3.2. Trimeric Autotransporters
5.3.3. Autotransporters Insertion in the Outer Membrane
5.4. Brucella Adhesins as Vaccine Candidates
6. Summary and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pappas, G.; Papadimitriou, P.; Akritidis, N.; Christou, L.; Tsianos, E.V. The new global map of human brucellosis. Lancet Infect. Dis. 2006, 6, 91–99. [Google Scholar] [CrossRef]
- Traxler, R.M.; Lehman, M.W.; Bosserman, E.A.; Guerra, M.A.; Smith, T.L. A Literature Review of Laboratory-Acquired Brucellosis. J. Clin. Microbiol. 2013, 51, 3055–3062. [Google Scholar] [CrossRef] [PubMed]
- Strausbaugh, L.J.; Berkelman, R.L. Human Illness Associated with Use of Veterinary Vaccines. Clin. Infect. Dis. 2003, 37, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Ashford, D.A.; Di Pietra, J.; Lingappa, J.; Woods, C.; Noll, H.; Neville, B.; Weyant, R.; Bragg, S.L.; Spiegel, R.A.; Tappero, J.; et al. Adverse events in humans associated with accidental exposure to the livestock brucellosis vaccine RB51. Vaccine 2004, 22, 3435–3439. [Google Scholar] [CrossRef]
- Blasco, J.M.; Díaz, R. Brucella melitensis Rev-1 vaccine as a cause of human brucellosis. Lancet 1993, 342, 805. [Google Scholar] [CrossRef]
- Vincent, P.; Joubert, L.; Prave, M. 2 occupational cases of brucellar infection after inoculation of B 19 vaccine. Bull. Acad. Vet. Fr. 1970, 43, 89–97. [Google Scholar]
- Moreno, E. Retrospective and prospective perspectives on zoonotic brucellosis. Front. Microbiol. 2014, 5, 213. [Google Scholar] [CrossRef]
- Buzgan, T.; Karahocagil, M.K.; Irmak, H.; Baran, A.I.; Karsen, H.; Evirgen, O.; Akdeniz, H. Clinical manifestations and complications in 1028 cases of brucellosis: A retrospective evaluation and review of the literature. Int. J. Infect. Dis. 2010, 14, e469–e478. [Google Scholar] [CrossRef]
- Pourbagher, A.; Pourbagher, M.A.; Savas, L.; Turunc, T.; Demiroglu, Y.Z.; Erol, I.; Yalcintas, D. Epidemiologic, clinical, and imaging findings in brucellosis patients with osteoarticular involvement. Am. J. Roentgenol. 2006, 187, 873–880. [Google Scholar] [CrossRef]
- Godfroid, J.; Cloeckaert, A.; Liautard, J.P.; Kohler, S.; Fretin, D.; Walravens, K.; Garin-Bastuji, B.; Letesson, J.J. From the discovery of the Malta fever’s agent to the discovery of a marine mammal reservoir, brucellosis has continuously been a re-emerging zoonosis. Vet. Res. 2005, 36, 313–326. [Google Scholar] [CrossRef]
- Baldi, P.C.; Giambartolomei, G.H. Pathogenesis and pathobiology of zoonotic brucellosis in humans. OIE Rev. Sci. Tech. 2013, 32, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Arenas, G.N.; Staskevich, A.S.; Aballay, A.; Mayorga, L.S. Intracellular trafficking of Brucella abortus in J774 macrophages. Infect. Immun. 2000, 68, 4255–4263. [Google Scholar] [CrossRef] [PubMed]
- Price, R.E.; Templeton, J.W.; Smith, R.; Adams, L.G. Ability of mononuclear phagocytes from cattle naturally resistant or susceptible to brucellosis to control in vitro intracellular survival of Brucella abortus. Infect. Immun. 1990, 58, 879–886. [Google Scholar] [CrossRef]
- Drazek, E.S.; Houng, H.S.; Crawford, R.M.; Hadfield, T.L.; Hoover, D.L.; Warren, R.L. Deletion of purE attenuates Brucella melitensis 16M for growth in human monocyte-derived macrophages. Infect. Immun. 1995, 63, 3297–3301. [Google Scholar] [CrossRef]
- Pizarro-Cerdá, J.; Méresse, S.; Parton, R.G.; Van Der Goot, G.; Sola-Landa, A.; Lopez-Goñi, I.; Moreno, E.; Gorvel, J.P. Brucella abortus transits through the autophagic pathway and replicates in the endoplasmic reticulum of nonprofessional phagocytes. Infect. Immun. 1998, 66, 5711–5724. [Google Scholar] [CrossRef]
- Detilleux, P.G.; Deyoe, B.L.; Cheville, N.F. Penetration and intracellular growth of Brucella abortus in nonphagocytic cells in vitro. Infect. Immun. 1990, 58, 2320–2328. [Google Scholar] [CrossRef] [PubMed]
- Delpino, M.V.; Fossati, C.A.; Baldi, P.C. Proinflammatory response of human osteoblastic cell lines and Osteoblast-monocyte interaction upon infection with Brucella spp. Infect. Immun. 2009, 77. [Google Scholar] [CrossRef]
- Scian, R.; Barrionuevo, P.; Giambartolomei, G.H.; De Simone, E.A.; Vanzulli, S.I.; Fossati, C.A.; Baldi, P.C.; Delpino, M.V. Potential role of fibroblast-like synoviocytes in joint damage induced by Brucella abortus infection through production and induction of matrix metalloproteinases. Infect. Immun. 2011, 79, 3619–3632. [Google Scholar] [CrossRef]
- Fernández, A.G.; Ferrero, M.C.; Hielpos, M.S.; Fossati, C.A.; Baldi, P.C. Proinflammatory response of human trophoblastic cells to Brucella abortus infection and upon interactions with infected phagocytes. Biol. Reprod. 2016, 94, 48. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, M.C.; Bregante, J.; Delpino, M.V.; Barrionuevo, P.; Fossati, C.A.; Giambartolomei, G.H.; Baldi, P.C. Proinflammatory response of human endothelial cells to Brucella infection. Microbes Infect. 2011, 13, 852–861. [Google Scholar] [CrossRef]
- Ferrero, M.C.; Fossati, C.A.; Baldi, P.C. Smooth Brucella strains invade and replicate in human lung epithelial cells without inducing cell death. Microbes Infect. 2009, 11, 476–483. [Google Scholar] [CrossRef]
- Billard, E.; Cazevieille, C.; Dornand, J.; Gross, A. High Susceptibility of Human Dendritic Cells to Invasion by the Intracellular Pathogens Brucella suis, B. abortus, and B. melitensis. Infect. Immun. 2005, 73, 8418–8424. [Google Scholar] [CrossRef]
- Delpino, M.V.; Barrionuevo, P.; Scian, R.; Fossati, C.A.; Baldi, P.C. Brucella-infected hepatocytes mediate potentially tissue-damaging immune responses. J. Hepatol. 2010, 53, 145–154. [Google Scholar] [CrossRef]
- Ferrero, M.C.; Hielpos, M.S.; Carvalho, N.B.; Barrionuevo, P.; Corsetti, P.P.; Giambartolomei, G.H.; Oliveira, S.C.; Baldi, P.C. Key role of toll-like receptor 2 in the inflammatory response and major histocompatibility complex class ii downregulation in Brucella abortus-infected alveolar macrophages. Infect. Immun. 2014, 82, 626–639. [Google Scholar] [CrossRef]
- Fernández, A.G.; Hielpos, M.S.; Ferrero, M.C.; Fossati, C.A.; Baldi, P.C. Proinflammatory response of canine trophoblasts to Brucella canis infection. PLoS ONE 2017, 12, e0186561. [Google Scholar] [CrossRef]
- Sidhu-Muñoz, R.S.; Sancho, P.; Vizcaíno, N. Evaluation of human trophoblasts and ovine testis cell lines for the study of the intracellular pathogen Brucella ovis. FEMS Microbiol. Lett. 2018, 365. [Google Scholar] [CrossRef] [PubMed]
- Castaneda-Roldan, E.I.; Avelino-Flores, F.; Dall’Agnol, M.; Freer, E.; Cedillo, L.; Dornand, J.; Giron, J.A. Adherence of Brucella to human epithelial cells and macrophages is mediated by sialic acid residues. Cell. Microbiol. 2004, 6, 435–445. [Google Scholar] [CrossRef]
- Gomez, G.; Adams, L.G.; Rice-ficht, A.; Ficht, T.A. Host-Brucella interactions and the Brucella genome as tools for subunit antigen discovery and immunization against brucellosis. Front. Cell. Infect. Microbiol. 2013, 3, 1–15. [Google Scholar] [CrossRef]
- Watarai, M.; Makino, S.I.; Fujii, Y.; Okamoto, K.; Shirahata, T. Modulation of Brucella-induced macropinocytosis by lipid rafts mediates intracellular replication. Cell. Microbiol. 2002, 4, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Turse, J.E.; Ficht, T.A. Evidence of Brucella abortus OPS dictating uptake and restricting NF-k B activation in murine macrophages. Microbes Infect. 2008, 10, 582–590. [Google Scholar] [CrossRef]
- Naroeni, A.; Porte, F. Role of cholesterol and the ganglioside GM1 in entry and short-term survival of Brucella suis in murine macrophages. Infect. Immun. 2002, 70, 1640–1644. [Google Scholar] [CrossRef]
- Kim, S.; Watarai, M.; Suzuki, H.; Makino, S. Lipid raft microdomains mediate class A scavenger receptor-dependent infection of Brucella abortus. Microb. Pathog. 2004, 37, 11–19. [Google Scholar] [CrossRef]
- Martín-Martín, A.I.; Vizcaíno, N.; Fernández-Lago, L. Cholesterol, ganglioside GM1 and class A scavenger receptor contribute to infection by Brucella ovis and Brucella canis in murine macrophages. Microbes Infect. 2010, 12, 246–251. [Google Scholar] [CrossRef]
- Lapaque, N.; Moriyon, I.; Moreno, E.; Gorvel, J.P. Brucella lipopolysaccharide acts as a virulence factor. Curr. Opin. Microbiol. 2005, 8, 60–66. [Google Scholar] [CrossRef]
- Manterola, L.; Guzmán-Verri, C.; Chaves-Olarte, E.; Barquero-Calvo, E.; de Miguel, M.-J.; Moriyón, I.; Grilló, M.-J.; López-Goñi, I.; Moreno, E. BvrR/BvrS-controlled outer membrane proteins Omp3a and Omp3b are not essential for Brucella abortus virulence. Infect. Immun. 2007, 75, 4867–4874. [Google Scholar] [CrossRef]
- Martín-Martín, A.I.; Caro-Hernández, P.; Orduña, A.; Vizcaíno, N.; Fernández-Lago, L. Importance of the Omp25/Omp31 family in the internalization and intracellular replication of virulent B. ovis in murine macrophages and HeLa cells. Microbes Infect. 2008, 10, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Celli, J.; De Chastellier, C.; Franchini, D.M.; Pizarro-Cerda, J.; Moreno, E.; Gorvel, J.P. Brucella evades macrophage killing via VirB-dependent sustained interactions with the endoplasmic reticulum. J. Exp. Med. 2003, 198, 545–556. [Google Scholar] [CrossRef]
- Comerci, D.J.; Martínez-Lorenzo, M.J.; Sieira, R.; Gorvel, J.P.; Ugalde, R.A. Essential role of the virB machinery in the maturation of the Brucella abortus-containing vacuole. Cell. Microbiol. 2001, 3, 159–168. [Google Scholar] [CrossRef]
- Starr, T.; Child, R.; Wehrly, T.D.; Hansen, B.; Hwang, S.; López-Otin, C.; Virgin, H.W.; Celli, J. Selective subversion of autophagy complexes facilitates completion of the Brucella intracellular cycle. Cell Host Microbe 2012, 11, 33–45. [Google Scholar] [CrossRef]
- Porte, F.; Naroeni, A.; Ouahrani-Bettache, S.; Liautard, J.P. Role of the Brucella suis lipopolysaccharide O antigen in phagosomal genesis and in inhibition of phagosome-lysosome fusion in murine macrophages. Infect. Immun. 2003, 71, 1481–1490. [Google Scholar] [CrossRef] [PubMed]
- Kline, K.A.; Fälker, S.; Dahlberg, S.; Normark, S.; Henriques-Normark, B. Bacterial Adhesins in Host-Microbe Interactions. Cell Host Microbe 2009, 5, 580–592. [Google Scholar] [CrossRef]
- Pizarro-Cerdá, J.; Cossart, P. Bacterial adhesion and entry into host cells. Cell 2006, 124, 715–727. [Google Scholar] [CrossRef]
- Gerlach, R.G.; Hensel, M. Protein secretion systems and adhesins: The molecular armory of Gram-negative pathogens. Int. J. Med. Microbiol. 2007, 297, 401–415. [Google Scholar] [CrossRef]
- Busch, A.; Waksman, G. Chaperone-usher pathways: Diversity and pilus assembly mechanism. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 1112–1122. [Google Scholar] [CrossRef]
- Giltner, C.L.; Nguyen, Y.; Burrows, L.L. Type IV Pilin Proteins: Versatile Molecular Modules. Microbiol. Mol. Biol. Rev. 2012, 76, 740–772. [Google Scholar] [CrossRef]
- Saldaña, Z.; Xicohtencatl-Cortes, J.; Avelino, F.; Phillips, A.D.; Kaper, J.B.; Puente, J.L.; Girón, J.A. Synergistic role of curli and cellulose in cell adherence and biofilm formation of attaching and effacing Escherichia coli and identification of Fis as a negative regulator of curli. Environ. Microbiol. 2009, 11, 992–1006. [Google Scholar] [CrossRef]
- Chapman, M.R.; Robinson, L.S.; Pinkner, J.S.; Roth, R.; Heuser, J.; Hammar, M.; Normark, S.; Hultgren, S.J. Role of Escherichia coli curli operons in directing amyloid fiber formation. Science 2002, 295, 851–855. [Google Scholar] [CrossRef]
- Delepelaire, P. Type I secretion in gram-negative bacteria. Biochim. Biophys. Acta Mol. Cell Res. 2004, 1694, 149–161. [Google Scholar] [CrossRef]
- Satchell, K.J.F. Structure and function of MARTX toxins and other large repetitive RTX proteins. Annu. Rev. Microbiol. 2011, 65, 71–90. [Google Scholar] [CrossRef]
- Meuskens, I.; Saragliadis, A.; Leo, J.C.; Linke, D. Type V secretion systems: An overview of passenger domain functions. Front. Microbiol. 2019, 10, 1163. [Google Scholar] [CrossRef]
- Babu, M.; Bundalovic-Torma, C.; Calmettes, C.; Phanse, S.; Zhang, Q.; Jiang, Y.; Minic, Z.; Kim, S.; Mehla, J.; Gagarinova, A.; et al. Global landscape of cell envelope protein complexes in Escherichia coli. Nat. Biotechnol. 2018, 36, 103–112. [Google Scholar] [CrossRef]
- Kiessling, A.R.; Malik, A.; Goldman, A. Recent advances in the understanding of trimeric autotransporter adhesins. Med. Microbiol. Immunol. 2020, 209, 233–242. [Google Scholar] [CrossRef]
- Bialer, M.G.; Ruiz-Ranwez, V.; Sycz, G.; Estein, S.M.; Russo, D.M.; Altabe, S.; Sieira, R.; Zorreguieta, A. MapB, the Brucella suis TamB homologue, is involved in cell envelope biogenesis, cell division and virulence. Sci. Rep. 2019, 9, 2158. [Google Scholar] [CrossRef]
- Albenne, C.; Ieva, R. Job contenders: Roles of the β-barrel assembly machinery and the translocation and assembly module in autotransporter secretion. Mol. Microbiol. 2017, 106, 505–517. [Google Scholar] [CrossRef]
- Van Ulsen, P.; ur Rahman, S.; Jong, W.S.P.; Daleke-Schermerhorn, M.H.; Luirink, J. Type V secretion: From biogenesis to biotechnology. Biochim. Biophys. Acta Mol. Cell Res. 2014, 1843, 1592–1611. [Google Scholar] [CrossRef]
- Emsley, P.; Charles, I.G.; Fairweather, N.F.; Isaacs, N.W. Structure of Bordetella pertussis virulence factor P.69 pertactin. Nature 1996, 381, 90–92. [Google Scholar] [CrossRef]
- Oberhettinger, P.; Leo, J.C.; Linke, D.; Autenrieth, I.B.; Schütz, M.S. The inverse autotransporter intimin exports its passenger domain via a hairpin intermediate. J. Biol. Chem. 2015, 290, 1837–1849. [Google Scholar] [CrossRef]
- Leo, J.C.; Oberhettinger, P.; Schütz, M.; Linke, D. The inverse autotransporter family: Intimin, invasin and related proteins. Int. J. Med. Microbiol. 2015, 305, 276–282. [Google Scholar] [CrossRef]
- Leibiger, K.; Schweers, J.M.; Schütz, M. Biogenesis and function of the autotransporter adhesins YadA, intimin and invasin. Int. J. Med. Microbiol. 2019, 309, 331–337. [Google Scholar] [CrossRef]
- del Rocha-Gracia, R.C.; Castañeda-Roldán, E.I.; Giono-Cerezo, S.; Girón, J.A. Brucella sp. bind to sialic acid residues on human and animal red blood cells. FEMS Microbiol. Lett. 2002, 213, 219–224. [Google Scholar] [CrossRef]
- Castañeda-Roldán, E.I.; Ouahrani-Bettache, S.; Saldaña, Z.; Avelino, F.; Rendón, M.A.; Dornand, J.; Girón, J.A. Characterization of SP41, a surface protein of Brucella associated with adherence and invasion of host epithelial cells. Cell. Microbiol. 2006, 8, 1877–1887. [Google Scholar] [CrossRef] [PubMed]
- Sidhu-Muñoz, R.S.; Sancho, P.; Vizcaíno, N. Brucella ovis PA mutants for outer membrane proteins Omp10, Omp19, SP41, and BepC are not altered in their virulence and outer membrane properties. Vet. Microbiol. 2016, 186, 59–66. [Google Scholar] [CrossRef]
- Czibener, C.; Ugalde, J.E. Identification of a unique gene cluster of Brucella spp. that mediates adhesion to host cells. Microbes Infect. 2012, 14, 79–85. [Google Scholar] [CrossRef]
- Czibener, C.; Merwaiss, F.; Guaimas, F.; Del Giudice, M.G.; Serantes, D.A.R.; Spera, J.M.; Ugalde, J.E. BigA is a novel adhesin of Brucella that mediates adhesion to epithelial cells. Cell. Microbiol. 2016, 18, 500–513. [Google Scholar] [CrossRef]
- Lopez, P.; Guaimas, F.; Czibener, C.; Ugalde, J.E. A genomic island in Brucella involved in the adhesion to host cells: Identification of a new adhesin and a translocation factor. Cell. Microbiol. 2020, e13245. [Google Scholar] [CrossRef]
- Eltahir, Y.; Al-Araimi, A.; Nair, R.; Autio, K.J.; Tu, H.; Leo, J.C.; Al-Marzooqi, W.; Johnson, E. Binding of Brucella protein, Bp26, to select extracellular matrix molecules. BMC Mol. Cell Biol. 2019, 20, 55. [Google Scholar] [CrossRef]
- Posadas, D.M.; Ruiz-Ranwez, V.; Bonomi, H.R.; Martín, F.A.; Zorreguieta, A. BmaC, a novel autotransporter of Brucella suis, is involved in bacterial adhesion to host cells. Cell. Microbiol. 2012, 14, 965–982. [Google Scholar] [CrossRef]
- Bialer, M.G.; Ferrero, M.C.; Delpino, M.V.; Ruiz-Ranwez, V.; Posadas, D.M.; Baldi, P.C.; Zorreguieta, A. Adhesive functions or pseudogenization of monomeric autotransporters in Brucella species. Unpublished.
- Ruiz-Ranwez, V.; Posadas, D.M.; Van der Henst, C.; Estein, S.M.; Arocena, G.M.; Abdian, P.L.; Martín, F.A.; Sieira, R.; De Bolle, X.; Zorreguieta, A. BtaE, an Adhesin That Belongs to the Trimeric Autotransporter Family, Is Required for Full Virulence and Defines a Specific Adhesive Pole of Brucella suis. Infect. Immun. 2013, 81, 996–1007. [Google Scholar] [CrossRef]
- Ruiz-Ranwez, V.; Posadas, D.M.; Estein, S.M.; Abdian, P.L.; Martin, F.A.; Zorreguieta, A. The BtaF trimeric autotransporter of Brucella suis is involved in attachment to various surfaces, resistance to serum and virulence. PLoS ONE 2013, 8, e79770. [Google Scholar] [CrossRef] [PubMed]
- Muñoz González, F.; Sycz, G.; Alonso Paiva, I.M.; Linke, D.; Zorreguieta, A.; Baldi, P.C.; Ferrero, M.C. The BtaF Adhesin Is Necessary for Full Virulence During Respiratory Infection by Brucella suis and Is a Novel Immunogen for Nasal Vaccination Against Brucella Infection. Front. Immunol. 2019, 10, 1775. [Google Scholar] [CrossRef]
- Vitry, M.A.; Mambres, D.H.; Deghelt, M.; Hack, K.; Machelart, A.; Lhomme, F.; Vanderwinden, J.M.; Vermeersch, M.; De Trez, C.; Pérez-Morga, D.; et al. Brucella melitensis invades murine erythrocytes during infection. Infect. Immun. 2014, 82, 3927–3938. [Google Scholar] [CrossRef] [PubMed]
- Seco-Mediavilla, P.; Verger, J.M.; Grayon, M.; Cloeckaert, A.; Marín, C.M.; Zygmunt, M.S.; Fernández-Lago, L.; Vizcaíno, N. Epitope mapping of the Brucella melitensis BP26 immunogenic protein: Usefulness for diagnosis of sheep brucellosis. Clin. Diagn. Lab. Immunol. 2003, 10, 647–651. [Google Scholar] [CrossRef]
- Bodelón, G.; Palomino, C.; Fernández, L.Á. Immunoglobulin domains in Escherichia coli and other enterobacteria: From pathogenesis to applications in antibody technologies. FEMS Microbiol. Rev. 2013, 37, 204–250. [Google Scholar] [CrossRef]
- Posadas, D.M. Transport and Adhesion in Brucella suis: Role of a TolC Family Protein in the Eflux of Toxic Compounds and of Three Potential Adhesins Host in Colonization. Ph.D. Thesis, University of Buenos Aires, Buenos Aires, Argentina, 2010. [Google Scholar]
- Jain, S.; Van Ulsen, P.; Benz, I.; Schmidt, M.A.; Fernandez, R.; Tommassen, J.; Goldberg, M.B. Polar localization of the autotransporter family of large bacterial virulence proteins. J. Bacteriol. 2006, 188, 4841–4850. [Google Scholar] [CrossRef][Green Version]
- Goldberg, M.B.; Barzu, O.; Parsot, C.; Sansonetti, P.J. Unipolar localization and ATPase activity of IcsA, a Shigella flexneri protein involved in intracellular movement. J. Bacteriol. 1993, 175, 2189–2196. [Google Scholar] [CrossRef]
- Bandara, A.B.; Sriranganathan, N.; Schurig, G.G.; Boyle, S.M. Putative outer membrane autotransporter protein influences survival of Brucella suis in BALB/c mice. Vet. Microbiol. 2005, 109, 95–104. [Google Scholar] [CrossRef]
- Sieira, R.; Bialer, M.G.; Roset, M.S.; Ruiz-Ranwez, V.; Langer, T.; Arocena, G.M.; Mancini, E.; Zorreguieta, A. Combinatorial control of adhesion of Brucella abortus 2308 to host cells by transcriptional rewiring of the trimeric autotransporter btaE gene. Mol. Microbiol. 2017, 103, 553–565. [Google Scholar] [CrossRef]
- Sieira, R.; Arocena, G.M.; Bukata, L.; Comerci, D.J.; Ugalde, R.A. Metabolic control of virulence genes in Brucella abortus: HutC coordinates virB expression and the histidine utilization pathway by direct binding to both promoters. J. Bacteriol. 2010, 192, 217–224. [Google Scholar] [CrossRef]
- Szczesny, P.; Lupas, A. Domain annotation of trimeric autotransporter adhesins—daTAA. Bioinformatics 2008, 24, 1251–1256. [Google Scholar] [CrossRef]
- Linke, D.; (University of Oslo, Oslo, Norway). Personal communication, 2018.
- Eisenschenk, F.C.; Houle, J.J.; Hoffmann, E.M. Mechanism of serum resistance among Brucella abortus isolates. Vet. Microbiol. 1999, 68, 235–244. [Google Scholar] [CrossRef]
- Fernandez-Prada, C.M.; Nikolich, M.; Vemulapalli, R.; Sriranganathan, N.; Boyle, S.M.; Schurig, G.G.; Hadfield, T.L.; Hoover, D.L. Deletion of wboA enhances activation of the lectin pathway of complement in Brucella abortus and Brucella melitensis. Infect. Immun. 2001, 69, 4407–4416. [Google Scholar] [CrossRef]
- Mora-Cartín, R.; Chacón-Díaz, C.; Gutiérrez-Jiménez, C.; Gurdián-Murillo, S.; Lomonte, B.; Chaves-Olarte, E.; Barquero-Calvo, E.; Moreno, E. N -Formyl-Perosamine Surface Homopolysaccharides Hinder the Recognition of Brucella abortus by Mouse Neutrophils. Infect. Immun. 2016, 84, 1712–1721. [Google Scholar] [CrossRef]
- Dotreppe, D.; Mullier, C.; Letesson, J.J.; De Bolle, X. The alkylation response protein AidB is localized at the new poles and constriction sites in Brucella abortus. BMC Microbiol. 2011, 11, 257. [Google Scholar] [CrossRef]
- Hallez, R.; Mignolet, J.; Van Mullem, V.; Wery, M.; Vandenhaute, J.; Letesson, J.J.; Jacobs-Wagner, C.; De Bolle, X. The asymmetric distribution of the essential histidine kinase PdhS indicates a differentiation event in Brucella abortus. EMBO J. 2007, 26, 1444–1455. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.J.B.; De Pedro, M.A.; Kysela, D.T.; Van Der Henst, C.; Kim, J.; De Bolle, X.; Fuqua, C.; Brun, Y.V. Polar growth in the Alphaproteobacterial order Rhizobiales. Proc. Natl. Acad. Sci. USA 2012, 109, 1697–1701. [Google Scholar] [CrossRef]
- Hallez, R.; Bellefontaine, A.F.; Letesson, J.J.; De Bolle, X. Morphological and functional asymmetry in α-proteobacteria. Trends Microbiol. 2004, 12, 361–365. [Google Scholar] [CrossRef]
- Van Der Henst, C.; De Barsy, M.; Zorreguieta, A.; Letesson, J.J.; De Bolle, X. The Brucella pathogens are polarized bacteria. Microbes Infect. 2013, 15, 998–1004. [Google Scholar] [CrossRef]
- Tomlinson, A.D.; Fuqua, C. Mechanisms and regulation of polar surface attachment in Agrobacterium tumefaciens. Curr. Opin. Microbiol. 2009, 12, 708–714. [Google Scholar] [CrossRef]
- Merker, R.I.; Smit, J. Characterization of the Adhesive Holdfast of Marine and Freshwater Caulobacters. Appl. Environ. Microbiol. 1988, 54, 2078–2085. [Google Scholar] [CrossRef]
- Selkrig, J.; Mosbahi, K.; Webb, C.T.; Belousoff, M.J.; Perry, A.J.; Wells, T.J.; Morris, F.; Leyton, D.L.; Totsika, M.; Phan, M.D.; et al. Discovery of an archetypal protein transport system in bacterial outer membranes. Nat. Struct. Mol. Biol. 2012, 19, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Heinz, E.; Selkrig, J.; Belousoff, M.J.; Lithgow, T. Evolution of the translocation and assembly module (TAM). Genome Biol. Evol. 2015, 7, 1628–1643. [Google Scholar] [CrossRef]
- Davis, D.S.; Templeton, J.W.; Ficht, T.A.; Huber, J.D.; Angus, R.D.; Adams, L.G. Brucella abortus in Bison. II. Evaluation of strain 19 vaccination of pregnant cows. J. Wildl. Dis. 1991, 27, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Spink, W.W.; Hall, J.W.; Finstad, J.; Mallet, E. Immunization with viable Brucella organisms: Results of a safety test in humans. Bull. World Health Organ. 1962, 26, 409–419. [Google Scholar]
- Pappagianis, D.; Elberg, S.S.; Crouch, D. Immunization against brucella infections: Effects of graded doses of viable attenuated Brucella melitensis in humans. Am. J. Epidemiol. 1966, 84, 21–31. [Google Scholar] [CrossRef]
- Schurig, G.G.; Sriranganathan, N.; Corbel, M.J. Brucellosis vaccines: Past, present and future. Vet. Microbiol. 2002, 90, 479–496. [Google Scholar] [CrossRef]
- Neutra, M.R.; Kozlowski, P.A. Mucosal vaccines: The promise and the challenge. Nat. Rev. Immunol. 2006, 6, 148–158. [Google Scholar] [CrossRef]
- Pecha, B.; Low, D.; O’Hanley, P. Gal-Gal pili vaccines prevent pyelonephritis by piliated Escherichia coli in a murine model. Single-component Gal-Gal pili vaccines prevent pyelonephritis by homologous and heterologous piliated E. coli strains. J. Clin. Investig. 1989, 83, 2102–2108. [Google Scholar] [CrossRef]
- Langermann, S.; Palaszynski, S.; Barnhart, M.; Auguste, G.; Pinkner, J.S.; Burlein, J.; Barren, P.; Koenig, S.; Leath, S.; Jones, C.H.; et al. Prevention of mucosal Escherichia coli infection by FimH-adhesin-based systemic vaccination. Science 1997, 276, 607–611. [Google Scholar] [CrossRef]
- Samo, M.; Choudhary, N.R.; Riebe, K.J.; Shterev, I.; Staats, H.F.; Sempowski, G.D.; Leduc, I. Immunization with the Haemophilus ducreyi trimeric autotransporter adhesin DsrA with alum, CpG or imiquimod generates a persistent humoral immune response that recognizes the bacterial surface. Vaccine 2016, 34, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Comanducci, M.; Bambini, S.; Brunelli, B.; Adu-Bobie, J.; Aricò, B.; Capecchi, B.; Giuliani, M.M.; Masignani, V.; Santini, L.; Savino, S.; et al. NadA, a novel vaccine candidate of Neisseria meningitidis. J. Exp. Med. 2002, 195, 1445–1454. [Google Scholar] [CrossRef]
- Hung, M.C.; Heckels, J.E.; Christodoulides, M. The adhesin complex protein (ACP) of Neisseria meningitidis is a new adhesin with vaccine potential. MBio 2013, 4, e00041-13. [Google Scholar] [CrossRef] [PubMed]
- Al-Mariri, A.; Abbady, A.Q. Evaluation of the immunogenicity and the protective efficacy in mice of a DNA vaccine encoding SP41 from Brucella melitensis. J. Infect. Dev. Ctries. 2013, 7, 329–337. [Google Scholar] [CrossRef]

| Adhesin | Organism | KEGG Entry | NCBI Protein ID | Protein Class | Host Ligands Detected | Cellular Adhesion Role | In Vivo Infection Role | Reference |
|---|---|---|---|---|---|---|---|---|
| SP29 | B. abortus 9-941 | BruAb2_0373 | WP_002965789.1 | D-ribose ABC transporter substrate-binding protein | Sialic acid-containing proteins | Erythrocytes | ND | [60] |
| SP41 | B. abortus 9-941 | BruAb2_0571 | WP_002965982.1 | ATP-binding cassette transporter | Sialic acid-containing proteins | Epithelial (HeLa) | No role detected in B. ovis infections | [61,62] |
| BigA | B. abortus 2308 | BAB1_2009 | EEP62646.1 | Ig-like domain- containing protein | Cell adhesion molecules | Epithelial (HeLa, Caco.2, MDCK) | Potential role in oral infections * | [63,64] |
| BigB | B. abortus 2308 | BAB1_2012 | WP_002967016.1 | Ig-like domain- containing protein | Cell adhesion molecules | Epithelial (HeLa) | Potential role in oral infections * | [63,65] |
| Bp26 | B. melitensis 16M | BMEI0536 | WP_002964581.1 | Uncharacterized | Type I collagen, vitronectin, fibronectin | ND | ND | [66] |
| BmaC | B. suis 1330 | BRA1148 | WP_006191504.1 | Monomeric autotransporter | Fibronectin, type I collagen | Epithelial (HeLa, A549). Synoviocytes. Osteoblasts | ND | [67,68] |
| BmaA | B. suis 1330 | BR0173 | AAN33380.1 | Monomeric autotransporter | Fibronectin, type I collagen | Epithelial (HT 29, Caco.2). Synoviocytes. Osteoblasts. Trophoblasts | ND | [68] |
| BmaB | B. suis 1330 | BR2013 | AAN30903.1 | Monomeric autotransporter | Fibronectin | Synoviocytes. Osteoblasts. Trophoblasts | ND | [68] |
| BtaE | B. suis 1330 | BR0072 | WP_006191142.1 | Trimeric autotransporter | Fibronectin, hyaluronic acid | Epithelial (HeLa, A549) | Mutants display decreased colonization after oral infection | [69] |
| BtaF | B. suis 1330 | BR1846 | A0A0H3G4K1.1 | Trimeric autotransporter | Fibronectin, hyaluronic acid, fetuin, type I collagen | Epithelial (HeLa, A549) | Mutants display decreased colonization after oral or respiratory infection | [70,71] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bialer, M.G.; Sycz, G.; Muñoz González, F.; Ferrero, M.C.; Baldi, P.C.; Zorreguieta, A. Adhesins of Brucella: Their Roles in the Interaction with the Host. Pathogens 2020, 9, 942. https://doi.org/10.3390/pathogens9110942
Bialer MG, Sycz G, Muñoz González F, Ferrero MC, Baldi PC, Zorreguieta A. Adhesins of Brucella: Their Roles in the Interaction with the Host. Pathogens. 2020; 9(11):942. https://doi.org/10.3390/pathogens9110942
Chicago/Turabian StyleBialer, Magalí G., Gabriela Sycz, Florencia Muñoz González, Mariana C. Ferrero, Pablo C. Baldi, and Angeles Zorreguieta. 2020. "Adhesins of Brucella: Their Roles in the Interaction with the Host" Pathogens 9, no. 11: 942. https://doi.org/10.3390/pathogens9110942
APA StyleBialer, M. G., Sycz, G., Muñoz González, F., Ferrero, M. C., Baldi, P. C., & Zorreguieta, A. (2020). Adhesins of Brucella: Their Roles in the Interaction with the Host. Pathogens, 9(11), 942. https://doi.org/10.3390/pathogens9110942






