Molecular Characteristics of Rickettsia in Ticks Collected along the Southern Border of Mongolia
Abstract
1. Introduction
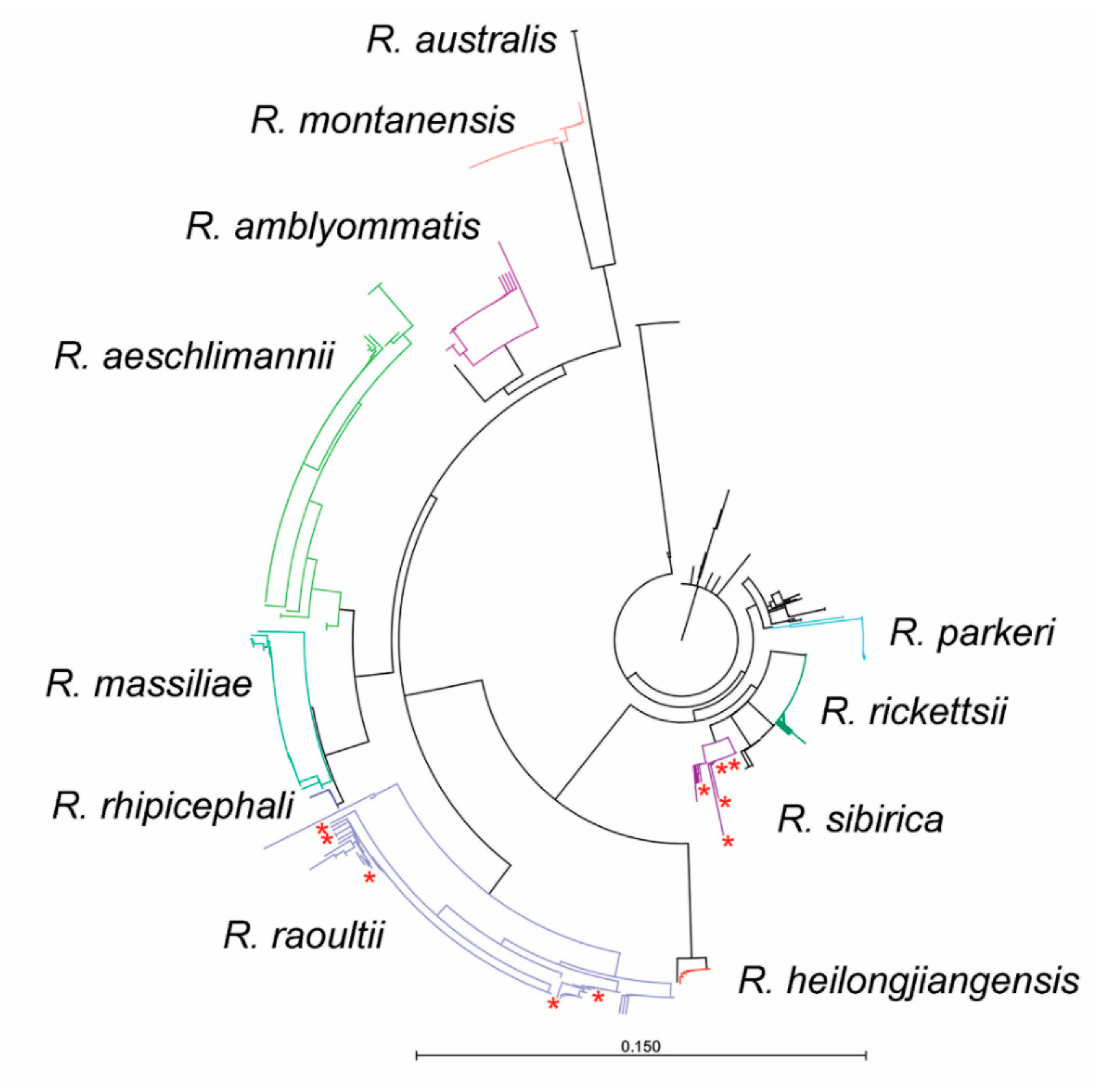
2. Results
3. Discussion
4. Materials and Methods
4.1. Sample Collection, Study Location, and Processing
4.2. Rickettsia spp. Testing
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abdad, M.Y.; Abou Abdallah, R.; Fournier, P.E.; Stenos, J.; Vasoo, S. A Concise Review of the Epidemiology and Diagnostics of Rickettsioses: Rickettsia and Orientia spp. J. Clin. Microbiol. 2018, 56. [Google Scholar] [CrossRef] [PubMed]
- Blanton, L.S. The Rickettsioses: A Practical Update. Infect. Dis. Clin. N. Am. 2019, 33, 213–229. [Google Scholar] [CrossRef] [PubMed]
- Parola, P.; Paddock, C.D.; Socolovschi, C.; Labruna, M.B.; Mediannikov, O.; Kernif, T.; Abdad, M.Y.; Stenos, J.; Bitam, I.; Fournier, P.-E.; et al. Update on tick-borne rickettsioses around the world: A geographic approach. Clin. Microbiol. Rev. 2013, 26, 657–702. [Google Scholar] [CrossRef] [PubMed]
- Galanakis, E.; Bitsori, M. When to think of Rickettsia. Pediatr. Infect. Dis. J. 2019, 38, S20–S23. [Google Scholar] [CrossRef]
- Pérez-Osorio, C.E.; Zavala-Velázquez, J.E.; Arias León, J.J.; Zavala-Castro, J.E. Rickettsia felis as emergent global threat for humans. Emerg. Infect. Dis. 2008, 4, 1019–1023. [Google Scholar] [CrossRef]
- Moore, T.C.; Pulscher, L.A.; Caddell, L.; von Fricken, M.E.; Anderson, B.D.; Gonchigoo, B.; Gray, G.C. Evidence for transovarial transmission of tick-borne rickettsiae circulating in Northern Mongolia. PLoS Negl. Trop. Dis. 2018, 12, e0006696. [Google Scholar] [CrossRef]
- Boldbaatar, B.; Jiang, R.R.; von Fricken, M.E.; Lkhagvatseren, S.; Nymadawa, P.; Baigalmaa, B.; Wang, Y.; Anderson, B.D.; Jiang, J.; Gray, G.C. Distribution and molecular characteristics of rickettsiae found in ticks across Central Mongolia. Parasites Vectors 2017, 10, 61. [Google Scholar] [CrossRef]
- von Fricken, M.E.; Lkhagvatseren, S.; Boldbaatar, B.; Nymadawa, P.; Weppelmann, T.A.; Baigalmaa, B.O.; Andersonbg, B.D.; Rellerbg, M.E.; Lantosh, P.M.; Graybg, G.C. Estimated seroprevalence of Anaplasma spp. and spotted fever group Rickettsia exposure among herders and livestock in Mongolia. Acta Trop. 2018, 177, 179–185. [Google Scholar] [CrossRef]
- Voorhees, M.A.; Padilla, S.L.; Jamsransuren, D.; Koehler, J.W.; Delp, K.L.; Adiyadorj, D.; Baasandagwa, U.; Jigjav, B.; Olschner, S.P.; Minogue, T.D.; et al. Crimean-Congo Hemorrhagic Fever Virus, Mongolia, 2013–2014. Emerg. Infect. Dis. 2018, 24, 2202–2209. [Google Scholar] [CrossRef]
- Lkhagvatseren, S.; Hogan, K.M.; Boldbaatar, B.; von Fricken, M.E.; Anderson, B.D.; Pulscher, L.A.; Caddell, L.; Nymadawa, P.; Gray, G.C. Discrepancies between self-reported tick bites and evidence of tick-borne disease exposure among nomadic Mongolian herders. Zoonoses Public Health 2019, 66, 480–486. [Google Scholar] [CrossRef]
- Byambaa, B. Nature-focal rickettsioses in Mongolia. Two decades of Russian-Mongolian scientific collaboration. Vestn Ross Akad Med Nauk 2008, 7, 44–45. [Google Scholar]
- Pulscher, L.A.; Moore, T.C.; Caddell, L.; Sukhbaatar, L.; von Fricken, M.E.; Anderson, B.D.; Gonchigoo, B.; Gray, G.C. A cross-sectional study of small mammals for tick-borne pathogen infection in northern Mongolia. Infect. Ecol. Epidemiol. 2018, 8, 1450591. [Google Scholar] [CrossRef] [PubMed]
- Sandagdorj, N.; Punsantsogvoo, M.; Davaasuren, P.; Enkhtaivan, B.; Battsetsegn, B.; Banzragch, B. Molecular biological detection of emerging tick-borne zoonotic pathogens in ixodid tick species. Mong. J. Agric. Sci. 2015, 13. [Google Scholar] [CrossRef]
- Speck, S.; Derschum, H.; Damdindorj, T.; Dashdavaa, O.; Jiang, J.; Kaysser, P.; Jigjav, B.; Nyamdorj, E.; Baatar, U.; Munkhbat, E.; et al. Rickettsia raoultii, the predominant Rickettsia found in Mongolian Dermacentor nuttalli. Ticks Tick Borne Dis. 2012, 3, 227–231. [Google Scholar] [CrossRef]
- Angelakis, E.; Pulcini, C.; Waton, J.; Imbert, P.; Socolovschi, C.; Edouard, S.; Dellamonica, P.; Raoult, D. Scalp eschar and neck lymphadenopathy caused by Bartonella henselae after Tick Bite. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2010, 50, 549–551. [Google Scholar] [CrossRef]
- Jia, N.; Zheng, Y.C.; Jiang, J.F.; Ma, L.; Cao, W.C. Human infection with Candidatus Rickettsia tarasevichiae. N. Engl. J. Med. 2013, 369, 1178–1180. [Google Scholar] [CrossRef]
- Jia, N.; Zheng, Y.C.; Ma, L.; Huo, Q.B.; Ni, X.B.; Jiang, B.G.; Chu, Y.L.; Jiang, R.R.; Jiang, J.F.; Cao, W.C. Human infections with Rickettsia raoultii, China. Emerg. Infect. Dis. 2014, 20, 866–868. [Google Scholar] [CrossRef]
- Li, H.; Fu, X.Y.; Jiang, J.F.; Liu, R.X.; Li, R.; Zheng, Y.C.; Qi, W.J.; Liu, W.; Cao, W.C. Severe illness caused by Rickettsia sibirica subspecies sibirica BJ-90 infection, China. Emerg. Microbes Infect. 2017, 6, e107. [Google Scholar] [CrossRef]
- Li, H.; Zhang, P.H.; Huang, Y.; Du, J.; Cui, N.; Yang, Z.D.; Tang, F.; Fu, F.X.; Li, X.-M.; Cui, X.-M.; et al. Isolation and Identification of Rickettsia raoultii in Human Cases: A Surveillance Study in 3 Medical Centers in China. Clin. Infect. Dis. 2018, 66, 1109–1115. [Google Scholar] [CrossRef]
- Liu, L.; Chen, Q.; Yang, Y.; Wang, J.; Cao, X.; Zhang, S.; Li, H.; Hou, Y.; Wang, F.; Xu, B. Investigations on Rickettsia in Ticks at the Sino-Russian and Sino-Mongolian Borders, China. Vector Borne Zoonotic Dis. 2015, 15, 785–789. [Google Scholar] [CrossRef]
- Liu, Q.H.; Walker, D.H.; Zhou, G.F. Serologic survey for antibodies to Rickettsia sibirica in Inner Mongolia, People’s Republic of China. Ann. N. Y. Acad. Sci. 1990, 590, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Lado, P.; Qurollo, B.; Williams, C.; Junge, R.; Klompen, H. The microbiome of Haemaphysalis lemuris (Acari: Ixodidae), a possible vector of pathogens of endangered lemur species in Madagascar. Ticks Tick Borne Dis. 2018, 9, 1252–1260. [Google Scholar] [CrossRef] [PubMed]
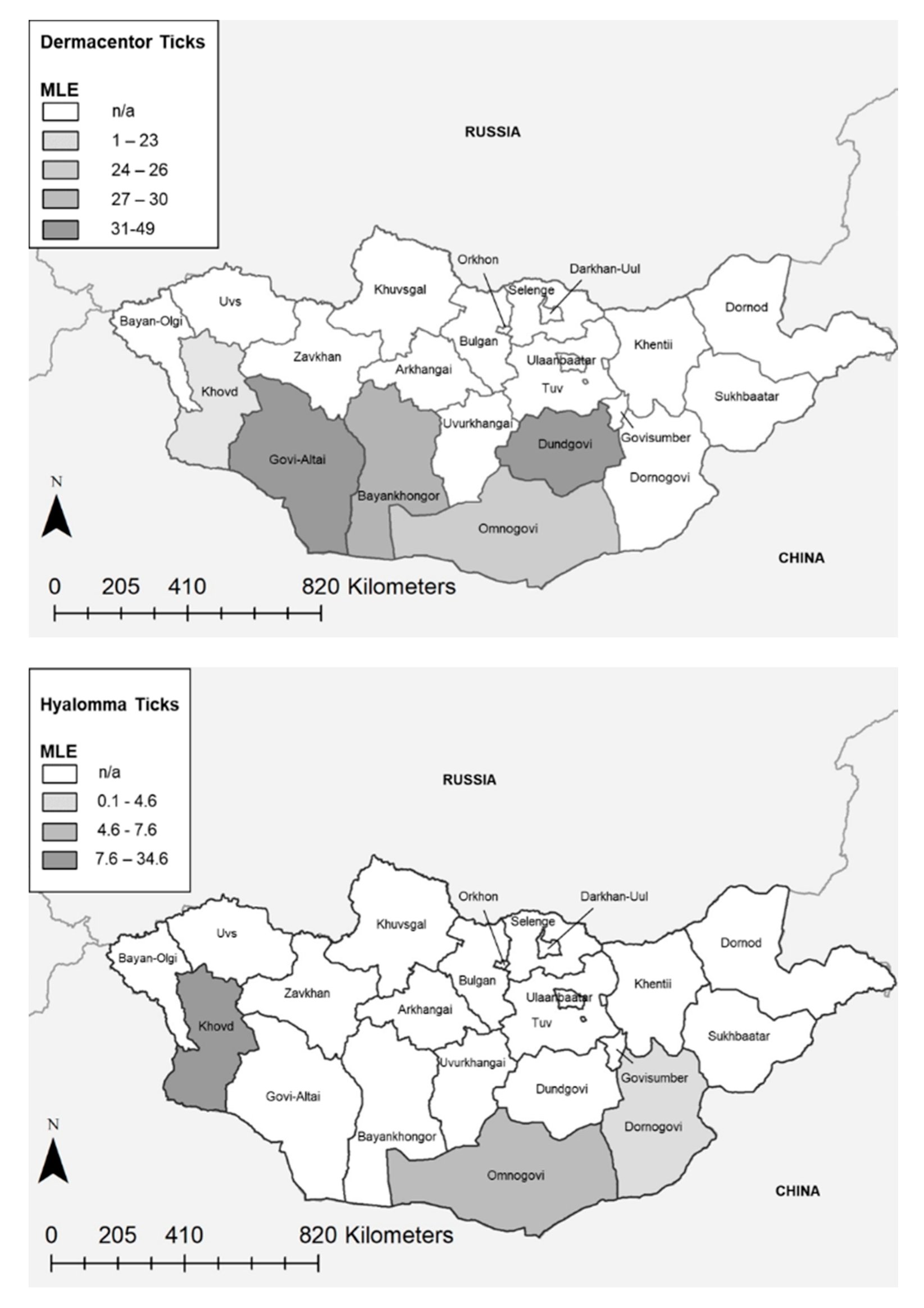
| Province | Genus | Positive Pools (%) | Total Ticks | MLE | MIR | ||||
|---|---|---|---|---|---|---|---|---|---|
| Point | Low | High | Point | Low | High | ||||
| Bayankhongor | Dermacentor | 55/67 (82%) | 334 | 30.1 | 22.9 | 37.3 | 16.5 | 12.5 | 20.4 |
| Dornogovi | Dermacentor | 10/12 (83%) | 58 | 39.6 | 17.4 | 61.3 | 17.2 | 7.5 | 27.0 |
| Govi-Altai | Dermacentor | 195/204 (96%) | 1058 | 48.9 | 41.2 | 55.8 | 18.4 | 16.1 | 20.8 |
| Khovd | Dermacentor | 34/46 (74%) | 238 | 23.2 | 16.4 | 30.4 | 14.3 | 9.8 | 18.7 |
| Omnogovi | Dermacentor | 107/138 (78%) | 708 | 26.9 | 21.6 | 30.8 | 15.1 | 12.5 | 17.8 |
| Dornogovi | Hyalomma | 6/27 (22%) | 144 | 4.6 | 2.1 | 9.2 | 4.2 | 1.0 | 7.4 |
| Khovd | Hyalomma | 2/3 (66%) | 10 | 34.6 | 7.3 | 69.7 | 20.0 | 0.0 | 44.8 |
| Omnogovi | Hyalomma | 96/289 (33%) | 1472 | 7.6 | 6.2 | 9.2 | 6.5 | 5.2 | 7.8 |
| Total | Dermacentor | 401/467 (86%) | 2396 | 33.2 | 30.1 | 36.2 | 16.7 | 15.2 | 18.2 |
| Total | Hyalomma | 104/319 (33%) | 1626 | 7.4 | 6.1 | 8.9 | 6.4 | 5.2 | 7.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
von Fricken, M.E.; Voorhees, M.A.; Koehler, J.W.; Asbun, C.; Lam, B.; Qurollo, B.; Hogan, K.M.; Baasandagva, U.; Jigjav, B.; Schoepp, R.J. Molecular Characteristics of Rickettsia in Ticks Collected along the Southern Border of Mongolia. Pathogens 2020, 9, 943. https://doi.org/10.3390/pathogens9110943
von Fricken ME, Voorhees MA, Koehler JW, Asbun C, Lam B, Qurollo B, Hogan KM, Baasandagva U, Jigjav B, Schoepp RJ. Molecular Characteristics of Rickettsia in Ticks Collected along the Southern Border of Mongolia. Pathogens. 2020; 9(11):943. https://doi.org/10.3390/pathogens9110943
Chicago/Turabian Stylevon Fricken, Michael E., Matthew A. Voorhees, Jeffrey W. Koehler, Carmen Asbun, Brandon Lam, Barbara Qurollo, Kathryn M. Hogan, Uyanga Baasandagva, Battsetseg Jigjav, and Randal J. Schoepp. 2020. "Molecular Characteristics of Rickettsia in Ticks Collected along the Southern Border of Mongolia" Pathogens 9, no. 11: 943. https://doi.org/10.3390/pathogens9110943
APA Stylevon Fricken, M. E., Voorhees, M. A., Koehler, J. W., Asbun, C., Lam, B., Qurollo, B., Hogan, K. M., Baasandagva, U., Jigjav, B., & Schoepp, R. J. (2020). Molecular Characteristics of Rickettsia in Ticks Collected along the Southern Border of Mongolia. Pathogens, 9(11), 943. https://doi.org/10.3390/pathogens9110943





