Molecular Typing of Neisseria gonorrhoeae Clinical Isolates in Russia, 2018–2019: A Link Between penA Alleles and NG-MAST Types
Abstract
1. Introduction
2. Results
2.1. Susceptibility of Isolates Collected in Russia in 2018–2019 to β-Lactam Antibiotics
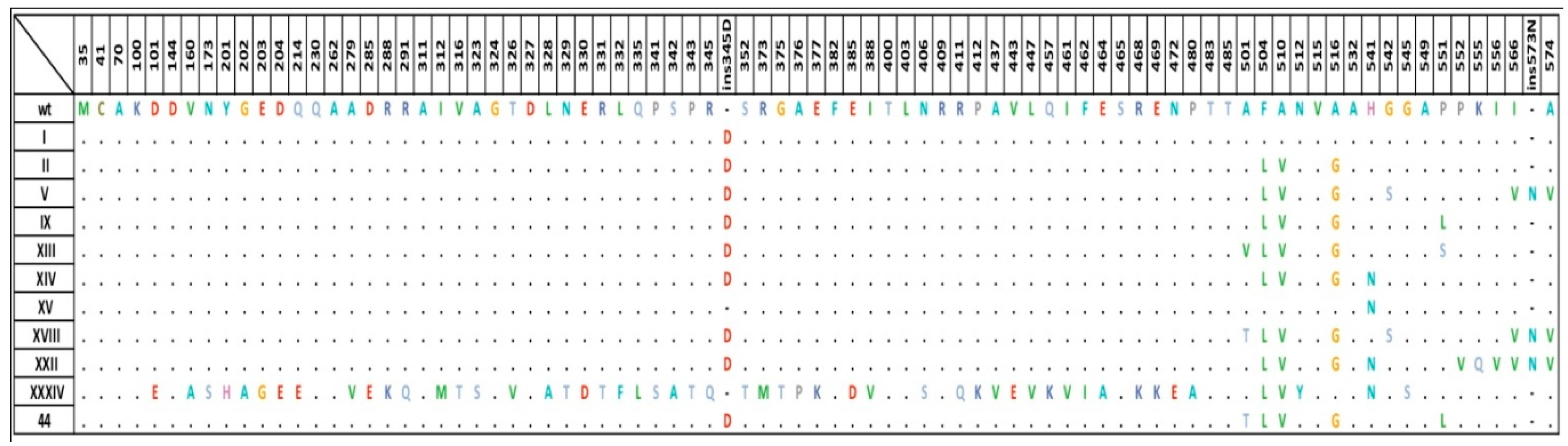
2.2. Diversity of penA Gene Alleles in the Modern Russian Population of N. gonorrhoeae
2.3. NG-MAST and Phylogenetic Analysis
2.4. Relationship between the penA Allele Type and NG-MAST Type
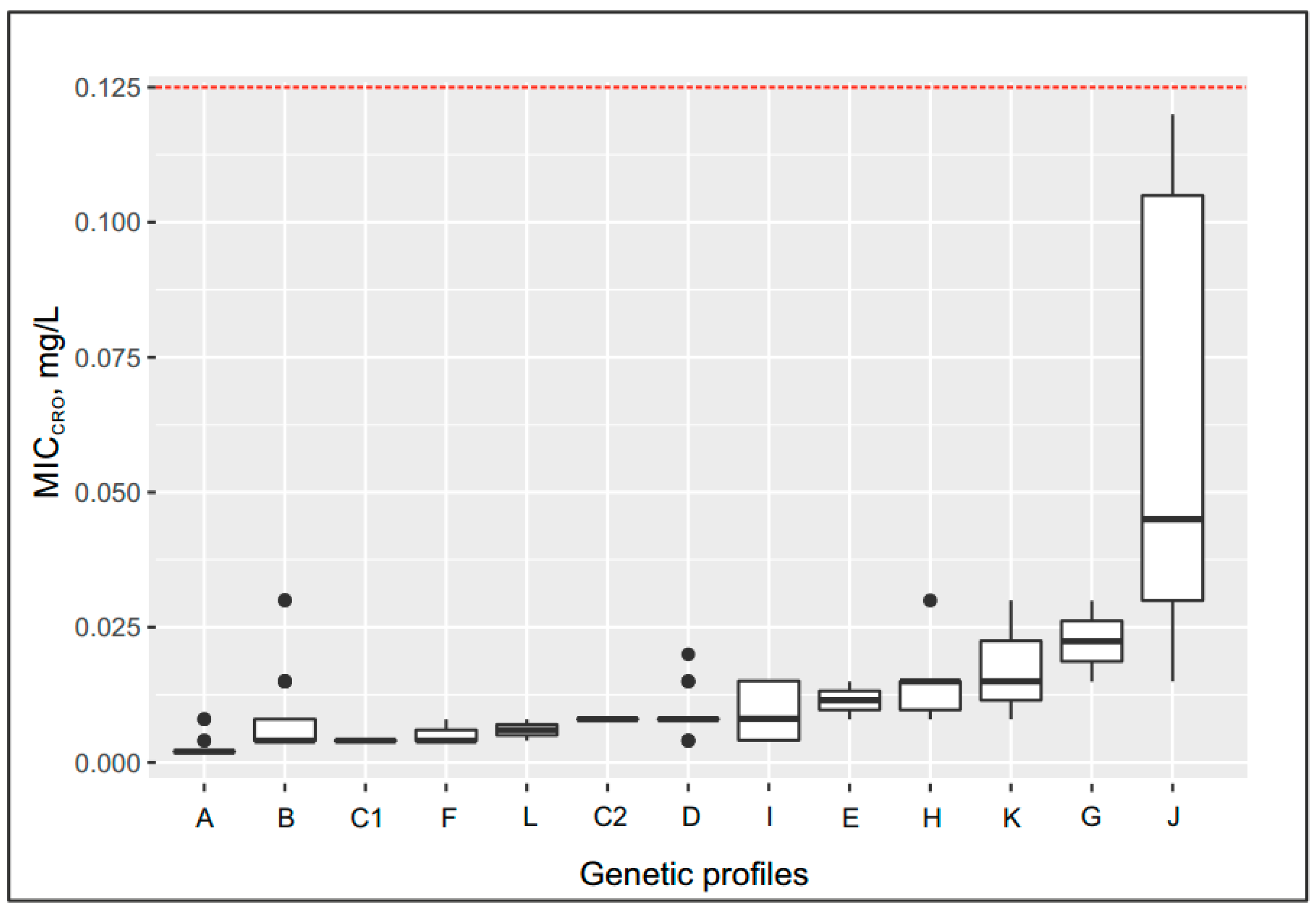
2.5. Effect of the Genetic Profile on the MICcro and MICpen
- −
- Up to 0.08 mg/L for the following genetic profiles: penA allele XXII, Leu421Pro replacement in PBP1 (ponA gene) (group C2); penA allele V, Leu421Pro replacement (ponA gene) or delA in the mtrR gene (groups D and I);
- −
- Up to 0.012–0.015 mg/L for the following genetic profiles: penA allele XIII, Leu421Pro replacement (ponA gene) (group E); penA allele V, Leu421Pro replacement plus mutations in the porB gene (group H); penA allele 44, Leu421Pro replacement plus delA in the mtrR gene, mutations in the porB gene (group K);
- −
- Up to 0.03 mg/L for the following genetic profile: penA allele IX, Leu421Pro replacement (ponA gene) plus mutations in the porB gene (group G);
- −
- Maximum increase in MICcro to 0.045 mg/L in group J, which contained isolates carrying the penA mosaic allele XXXIV, Leu421Pro replacement (ponA gene) plus delA in the mtrR gene, and mutations in the porB gene.
2.6. Contribution of Genetic Determinants to the Resistance of N. gonorrhoeae Isolates to β-Lactam Antibiotics: Regression Analysis
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Collection and Characterization of N. gonorrhoeae Isolates
4.3. Testing of N. gonorrhoeae Susceptibility to Penicillin and Ceftriaxone
4.4. Identification of Genetic Determinants of Antimicrobial Resistance Using an Oligonucleotide Microarray
4.5. Evaluation of PenA Gene Polymorphisms
4.6. NG-MAST and Definition of Genogroups
4.7. Construction of the Phylogenetic Tree
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Report on Global Sexually Transmitted Infection Surveillance, 2018; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Pandori, M.; Barry, P.M.; Wu, A.; Ren, A.; Whittington, W.L.H.; Liska, S.; Klausne, J.D. Mosaic penicillin- binding protein 2 in Neisseria gonorrhoeae isolates collected in 2008 in San Francisco, California. Antimicrob. Agents Chemother. 2009, 53, 4032–4034. [Google Scholar] [CrossRef] [PubMed]
- Martin, I.; Sawatzky, P.; Allen, V.; Hoang, L.; Lefebvre, B.; Mina, N.; Wong, T.; Gilmour, M. Emergence and characterization of Neisseria gonorrhoeae isolates with decreased susceptibilities to ceftriaxone and cefixime in Canada: 2001–2010. Sex. Transm. Dis. 2012, 39, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Camara, J.; Serra, J.; Ayats, J.; Bastida, T.; Carnicer-Pont, D.; Andreu, A.; Ardanuy, C. Molecular characterization of two high-level ceftriaxone-resistant Neisseria gonorrhoeae isolates detected in Catalonia, Spain. J. Antimicrob. Chemother. 2012, 67, 1858–1860. [Google Scholar] [CrossRef] [PubMed]
- Unemo, M.; Golparian, D.; Nicholas, R.; Ohnishi, M.; Gallay, A.; Sednaoui, P. High-level cefixime- and ceftriaxone-resistant N. gonorrhoeae in France: Novel penA mosaic allele in a successful international clone causes treatment failure. Antimicrob. Agents Chemother. 2012, 56, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Shimuta, K.; Watanabe, Y.; Nakayama, S.; Morita-Ishihara, T.; Kuroki, T.; Unemo, M.; Ohnishi, M. Emergence and evolution of internationally disseminated cephalosporin-resistant Neisseria gonorrhoeae clones from 1995 to 2005 in Japan. BMC Infect. Dis. 2015, 15, 378. [Google Scholar] [CrossRef] [PubMed]
- Suay-García, B.; Pérez-Gracia, M.T. Drug-resistant Neisseria gonorrhoeae: Latest developments. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1065–1071. [Google Scholar] [CrossRef]
- Attram, N.; Agbodzi, B.; Dela, H.; Behene, E.; Nyarko, E.O.; Kyei, N.N.A.; Larbi, J.A.; Lawson, B.W.L.; Addo, K.K.; Newman, M.J.; et al. Antimicrobial resistance (AMR) and molecular characterization of Neisseria gonorrhoeae in Ghana, 2012–2015. PLoS ONE 2019, 14, e0223598. [Google Scholar] [CrossRef]
- Xiu, L.; Yuan, Q.; Li, Y.; Zhang, C.; Tang, L.; Peng, J. Emergence of ceftriaxone-resistant Neisseria gonorrhoeae strains harbouring a novel mosaic penA gene in China. J. Antimicrob. Chemother. 2020, 75, 907–910. [Google Scholar] [CrossRef]
- Dong, Y.; Yang, Y.; Wang, Y.; Martin, I.; Demczuk, W.; Gu, W. Shanghai Neisseria gonorrhoeae isolates exhibit resistance to extended-spectrum cephalosporins and clonal distribution. Front. Microbiol. 2020. [Google Scholar] [CrossRef]
- Lindberg, R.; Fredlund, H.; Nicholas, R.; Unemo, M. Neisseria gonorrhoeae isolates with reduced susceptibility to cefixime and ceftriaxone: Association with genetic polymorphisms in penA, mtrR, porB1b, and ponA. Antimicrob. Agents Chemother. 2007, 51, 2117–2122. [Google Scholar] [CrossRef]
- Unemo, M.; Shafer, W.M. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: Past, evolution, and future. Clin. Microbiol. Rev. 2014, 27, 587–613. [Google Scholar] [CrossRef] [PubMed]
- Ameyama, S.; Onodera, S.; Takahata, M.; Minami, S.; Maki, N.; Endo, K.; Goto, H.; Suzuki, H.; Oishi, Y. Mosaic-like structure of penicillin binding protein 2 gene (penA) in clinical isolates of Neisseria gonorrhoeae with reduced susceptibility to cefixime. Antimicrob. Agents Chemother. 2002, 46, 3744–3749. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Deguchi, T.; Mizutani, K.S.; Yasuda, M.; Yokoi, S.; Ito, S.; Takahashi, Y.; Ishihara, S.; Kawamura, Y.; Ezaki, T. Emergence and spread of Neisseria gonorrhoeae clinical isolates harboring mosaic-like structure of penicillin-binding protein 2 in central Japan. Antimicrob. Agents Chemother. 2005, 49, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Osaka, K.; Takakura, T.; Narukawa, K.; Takahata, M.; Endo, K.; Kiyota, H.; Onodera, S. Analysis of amino acid sequences of penicillin-binding protein 2 in clinical isolates of Neisseria gonorrhoeae with reduced susceptibility to cefixime and ceftriaxone. J. Infect. Chemother. 2008, 14, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Demczuk, W.; Sidhu, S.; Unemo, M.; Whiley, D.M.; Allen, V.G.; Dillon, J.R.; Cole, M.; Seah, C.; Trembizki, E.; Trees, D.L.; et al. Neisseria gonorrhoeae sequence typing for antimicrobial resistance, a novel antimicrobial resistance multilocus typing scheme for tracking global dissemination of N. gonorrhoeae strains. J. Clin. Microbiol. 2017, 55, 1454–1468. [Google Scholar] [CrossRef] [PubMed]
- Whiley, D.M.; Goire, N.; Lambert, S.B.; Ray, S.; Limnios, E.A.; Nissen, M.D.; Sloots, T.P.; Tapsall, J.W. Reduced susceptibility to ceftriaxone in Neisseria gonorrhoeae is associated with mutations G542S, P551S and P551L in the gonococcal penicillin-binding protein 2. J. Antimicrob. Chemother. 2010, 65, 1615–1618. [Google Scholar] [CrossRef] [PubMed]
- Unemo, M.; Jensen, J.S. Antimicrobial-resistant sexually transmitted infections: Gonorrhoea and Mycoplasma genitalium. Nat. Rev. Urol. 2017, 14, 139–152. [Google Scholar] [CrossRef]
- Zapun, A.; Morlot, C.; Taha, M. Resistance to beta-lactams in Neisseria ssp. due to chromosomally encoded penicillin-binding proteins. Antibiotics 2016, 5, 35. [Google Scholar] [CrossRef]
- Kubanov, A.; Solomka, V.; Plakhova, X.; Chestkov, A.; Petrova, N.; Shaskolskiy, B.; Dementieva, E.; Leinsoo, A.; Gryadunov, D.; Deryabin, D. Summary and trends of the Russian Gonococcal Antimicrobial Surveillance Programme, 2005–2016. J. Clin. Microbiol. 2019, 5, e02024-18. [Google Scholar] [CrossRef]
- Shaskolskiy, B.; Dementieva, E.; Kandinov, I.; Filippova, M.; Petrova, N.; Chestkov, A.; Kubanov, A.; Deryabin, D.; Gryadunov, D. Resistance of Neisseria gonorrhoeae isolates to beta-lactams (benzylpenicillin and ceftriaxone) in Russia, 2015–2017. PLoS ONE 2019, 14, e0220339. [Google Scholar] [CrossRef]
- Shaskolskiy, B.; Dementieva, E.; Kandinov, I.; Chestkov, A.; Kubanov, A.; Deryabin, D.; Gryadunov, D. Genetic diversity of Neisseria gonorrhoeae multiantigen sequence types in Russia and Europe. Int. J. Infect. Dis. 2020, 93, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Unemo, M. Current and future antimicrobial treatment of gonorrhea—The rapidly evolving Neisseria gonorrhoeae continues to challenge. BMC Infect. Dis. 2015, 15, 364. [Google Scholar] [CrossRef]
- Dowson, C.G.; Jephcott, A.E.; Gough, K.R.; Spratt, B.G. Penicillin binding protein 2 genes of non-beta-lactamase-producing, penicillin resistant strains of Neisseria gonorrhoeae. Mol. Microbiol. 1989, 3, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Brannigan, J.A.; Tirodimos, I.A.; Zhang, Q.Y.; Dowson, C.G.; Spratt, B.G. Insertion of an extra amino acid is the main cause of the low affinity of penicillin-binding protein 2 in penicillin-resistant strains of Neisseria gonorrhoeae. Mol. Microbiol. 1990, 4, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Powell, A.J.; Tomberg, J.; Deacon, A.M.; Nicholas, R.A.; Davies, C. Crystal structures of penicillin-binding protein 2 from penicillin-susceptible and -resistant strains of Neisseria gonorrhoeae reveal an unexpectedly subtle mechanism for antibiotic resistance. J. Biol. Chem. 2009, 284, 1202–1212. [Google Scholar] [CrossRef]
- Kubanov, A.; Vorobyev, D.; Chestkov, A.; Leinsoo, A.; Shaskolskiy, B.; Dementieva, E.; Solomka, V.; Plakhova, X.; Gryadunov, D.; Deryabin, D. Molecular epidemiology of drug-resistant Neisseria gonorrhoeae in Russia (Current Status, 2015). BMC Infect. Dis. 2016, 16, 389. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Molecular Typing of Neisseria gonorrhoeae—A Study of 2013 Isolates; ECDC: Stockholm, Sweden, 2018; Available online: https://www.ecdc.europa.eu/sites/portal/files/documents/Molecular-typing-N-gonorrhoeae-web.pdf (accessed on 11 November 2020).
- Thakur, S.D.; Starnino, S.; Horsman, G.B.; Levett, P.N.; Dillon, J.R. Unique combined penA/mtrR/porB mutations and NG-MAST strain types associated with ceftriaxone and cefixime MIC increases in a ‘susceptible’ Neisseria gonorrhoeae population. Antimicrob. Chemother. 2014, 69, 1510–1516. [Google Scholar] [CrossRef]
- Demczuk, W.; Martin, I.; Peterson, S.; Bharat, A.; Van Domselaar, G.; Graham, M.; Lefebvre, B.; Allen, V.; Hoang, L.; Tyrrell, G.; et al. Genomic epidemiology and molecular resistance mechanisms of azithromycin-resistant Neisseria gonorrhoeae in Canada from 1997 to 2014. J. Clin. Microbiol. 2016, 54, 1304–1313. [Google Scholar] [CrossRef]
- Eyre, D.W.; De Silva, D.; Cole, K.; Peters, J.; Cole, M.J.; Grad, Y.H.; Demczuk, W.; Martin, I.; Mulvey, M.R.; Crook, D.W.; et al. WGS to predict antibiotic MICs for Neisseria gonorrhoeae. J. Antimicrob. Chemother. 2017, 72, 1937–1947. [Google Scholar] [CrossRef]
- Demczuk, W.; Martin, I.; Sawatzky, P.; Allen, V.; Lefebvre, B.; Hoang, L.; Naidu, P.; Minion, J.; Van Caeseele, P.; Haldane, D.; et al. Equations to predict antimicrobial MICs in Neisseria gonorrhoeae using molecular antimicrobial resistance determinants. Antimicrob. Agents Chemother. 2020, 64, e02005-19. [Google Scholar] [CrossRef]
- Shaskolskiy, B.L.; Kandinov, I.D.; Chestkov, A.V.; Solomka, V.S.; Kubanov, A.A.; Deryabin, D.G.; Gryadunov, D.A.; Dementieva, E.I. Comparative phylogenetic analysis of Neisseria gonorrhoeae clinical isolates in Russia, European Union, and Japan. Bull. Russ. State Med. Univ. 2020, 1, 76–94. [Google Scholar] [CrossRef]
- Leinsoo, A.T.; Shaskol’skii, B.L.; Dement’eva, E.I.; Gryadunov, D.A.; Kubanov, A.A.; Chestkov, A.V.; Obraztsova, O.A.; Shpilevaya, M.V.; Deryabin, D.G. Oligonucleotide microchip for the identification of infectious agents of reproductive system with simultaneous analysis of determinants of resistance to antimicrobial substances. Bull. Exp. Biol. Med. 2017, 164, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Gryadunov, D.A.; Shaskolskiy, B.L.; Nasedkina, T.V.; Rubina, A.Y.; Zasedatelev, A.S. The EIMB hydrogel microarray technology: Thirty years later. Acta Nat. 2018, 10, 4–18. [Google Scholar] [CrossRef]
- Olesky, M.; Zhao, S.; Rosenberg, R.L.; Nicholas, R.A. Porin-mediated antibiotic resistance in Neisseria gonorrhoeae: Ion, solute, and antibiotic permeation through PIB proteins with penB mutations. J. Bacteriol. 2006, 188, 2300–2308. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Duncan, M.; Tomberg, J.; Davies, C.; Unemo, M.; Nicholas, R.A. Genetics of chromosomally mediated intermediate resistance to ceftriaxone and cefixime in Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 2009, 53, 3744–3751. [Google Scholar] [CrossRef]
- Tamura, K.; Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 36, 1547–1549. [Google Scholar] [CrossRef]
- Unemo, M.; Dillon, J.A. Review and international recommendation of methods for typing Neisseria gonorrhoeae isolates and their implications for improved knowledge of gonococcal epidemiology, treatment, and biology. Clin. Microbiol. Rev. 2011, 24, 447–458. [Google Scholar] [CrossRef]
- Stamatakis, A. Using RAxML to infer phylogenies. Curr. Protoc. Bioinform. 2015, 51, 6.14.1–6.14.14. [Google Scholar] [CrossRef]
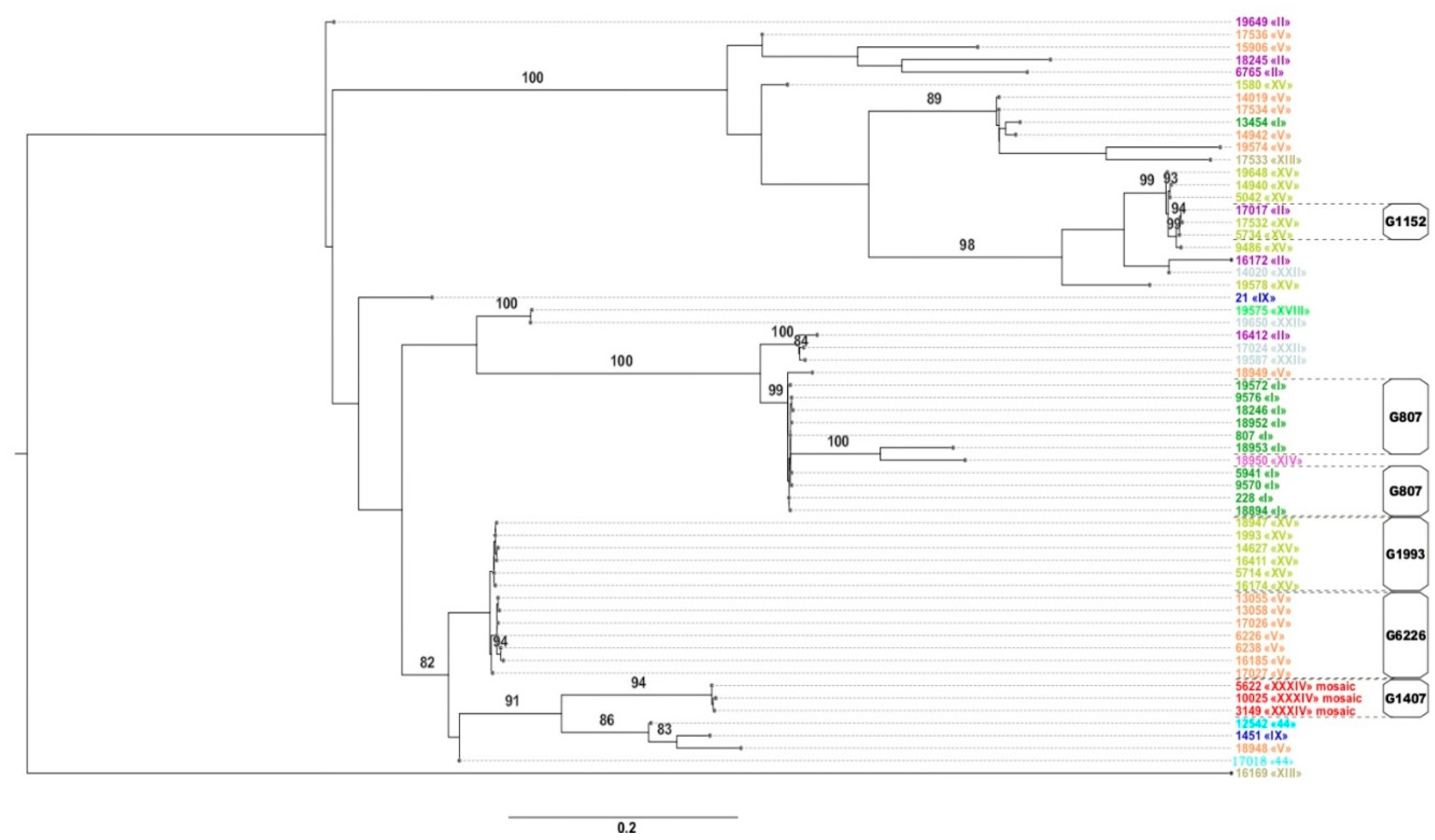

| Genogroup | NG-MAST Types | Number of Isolates in the Genogroup | Notes |
|---|---|---|---|
| G807 * | 228, 807, 5941, 9570, 9576, 18246, 18894, 18952, 19572 | 57 | Corresponds to the European genogroup G51 [28] |
| G1993 * | 1993, 5714, 14627, 16174, 16411, 17027, 18947 | 29 | European genogroup G1993 [28] |
| G6226 | 6226, 6238, 13055, 13058, 16185, 17026, 17027 | 19 | |
| G9486 * | 9486 | 13 | |
| G5042 | 5042,14940, 19648 | 7 | |
| G1407 (European) | 3149, 5622, 10025 | 6 | |
| G18948 | 18948 | 6 | |
| G14942 * | 14942 | 5 | |
| G1152 * | 5734, 17017, 17532 | 4 | Corresponds to the European genogroup G387 [28] |
| Group | Number of Isolates | NG-MAST | penA | MIC (mg/L), Group Median | Genetic Resistance Determinants | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| MICcro | MICpen | ponA | mtrR | porB | blaTEM | |||||
| −35 | −10 | |||||||||
| A | 55 | 1580, 1993, 5042, 5714, 5734, 9486, 14627, 14940, 16174, 16411, 17532, 18947, 19578, 19648 | XV | 0.002 | 0.03 | wt | wt | wt | wt | - |
| B | 59 | 228, 807, 5941, 9570, 9576, 18246, 18894, 18952, 18953, 19572 | I | 0.004 | 0.12 | wt | wt | wt | wt | - |
| C1 | 4 | 14020, 17024, 19587 | XXII | 0.004 | 0.375 | wt | wt | wt | wt | - |
| C2 | 2 | 19650 | XXII | 0.008 | 0.25 | Leu421Pro | wt | wt | wt | - |
| D | 21 | 6226, 6238, 14019, 14942, 17026, 17027, 17536 | V | 0.008 | 0.5 | Leu421Pro | wt | wt | wt | - |
| E | 2 | 16169 | XIII | 0.012 | 2 | Leu421Pro | wt | wt | wt | - |
| F | 3 | 16172, 17017 | II | 0.004 | 0.25 | Leu421Pro | wt | wt | wt | - |
| G | 2 | 1451 | IX | 0.023 | 1 | Leu421Pro | wt | wt | Gly120Asp | - |
| H | 6 | 18948 | V | 0.015 | 1.5 | Leu421Pro | wt | wt | Gly120Lys, Ala121Gly | - |
| I | 7 | 13055, 13058, 16185, 17534, 18949 | V | 0.008 | 1 | Leu421Pro | delA | wt | wt | - |
| J | 6 | 3149, 5622, 10025 | XXXIV (mosaic) | 0.045 | 1.5 | Leu421Pro | delA | wt | Gly120Lys/Asp, Ala121Asn/Gly | - |
| K | 3 | 12542 | 44 | 0.015 | 1 | Leu421Pro | delA | wt | Gly120Asp | - |
| L | 2 | 18245, 18950 | II, XIV | 0.006 | 32 | wt | wt | wt | wt | blaTEM-1, African type |
| Un- grouped | 10 | 21, 6765, 13454, 15906, 16412, 17018, 17533, 19574, 19575, 19649 | I, II, V, IX, XIII, XVIII, 44 | 0.004–0.03 | 0.12–4 | wt or Leu421Pro | wt or delA | wt | wt or Gly120Lys/Asp, Ala121Asn/Asp | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kandinov, I.; Dementieva, E.; Kravtsov, D.; Chestkov, A.; Kubanov, A.; Solomka, V.; Deryabin, D.; Gryadunov, D.; Shaskolskiy, B. Molecular Typing of Neisseria gonorrhoeae Clinical Isolates in Russia, 2018–2019: A Link Between penA Alleles and NG-MAST Types. Pathogens 2020, 9, 941. https://doi.org/10.3390/pathogens9110941
Kandinov I, Dementieva E, Kravtsov D, Chestkov A, Kubanov A, Solomka V, Deryabin D, Gryadunov D, Shaskolskiy B. Molecular Typing of Neisseria gonorrhoeae Clinical Isolates in Russia, 2018–2019: A Link Between penA Alleles and NG-MAST Types. Pathogens. 2020; 9(11):941. https://doi.org/10.3390/pathogens9110941
Chicago/Turabian StyleKandinov, Ilya, Ekaterina Dementieva, Dmitry Kravtsov, Alexander Chestkov, Alexey Kubanov, Victoria Solomka, Dmitry Deryabin, Dmitry Gryadunov, and Boris Shaskolskiy. 2020. "Molecular Typing of Neisseria gonorrhoeae Clinical Isolates in Russia, 2018–2019: A Link Between penA Alleles and NG-MAST Types" Pathogens 9, no. 11: 941. https://doi.org/10.3390/pathogens9110941
APA StyleKandinov, I., Dementieva, E., Kravtsov, D., Chestkov, A., Kubanov, A., Solomka, V., Deryabin, D., Gryadunov, D., & Shaskolskiy, B. (2020). Molecular Typing of Neisseria gonorrhoeae Clinical Isolates in Russia, 2018–2019: A Link Between penA Alleles and NG-MAST Types. Pathogens, 9(11), 941. https://doi.org/10.3390/pathogens9110941









