Prevalence of Cytauxzoon felis Infection-Carriers in Eastern Kansas Domestic Cats
Abstract
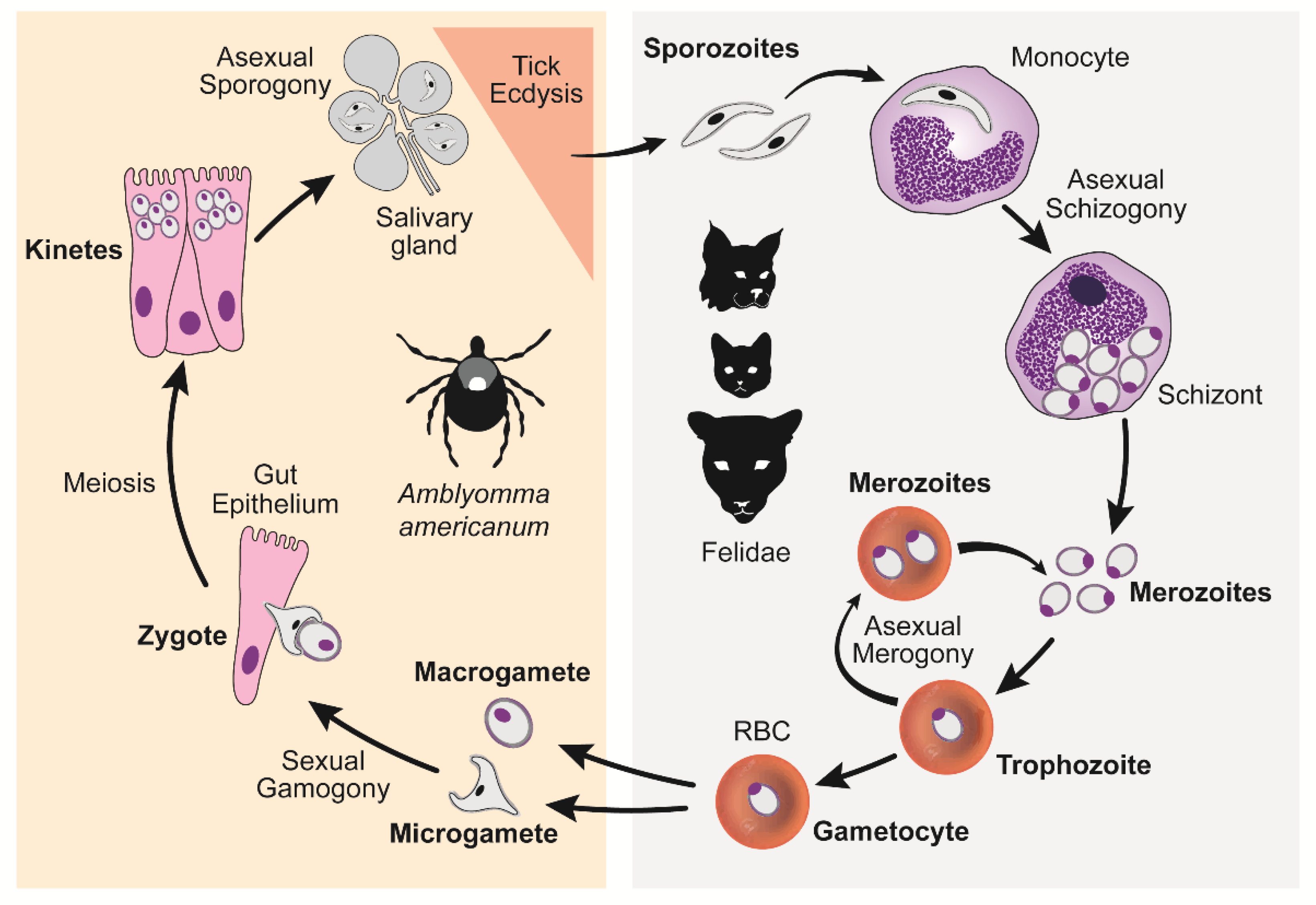
1. Introduction
2. Results
2.1. Cox3 Mitochondrial DNA (miDNA) qPCR Assay Limit of Detection (LOD) and Limit of Quantification (LOQ)
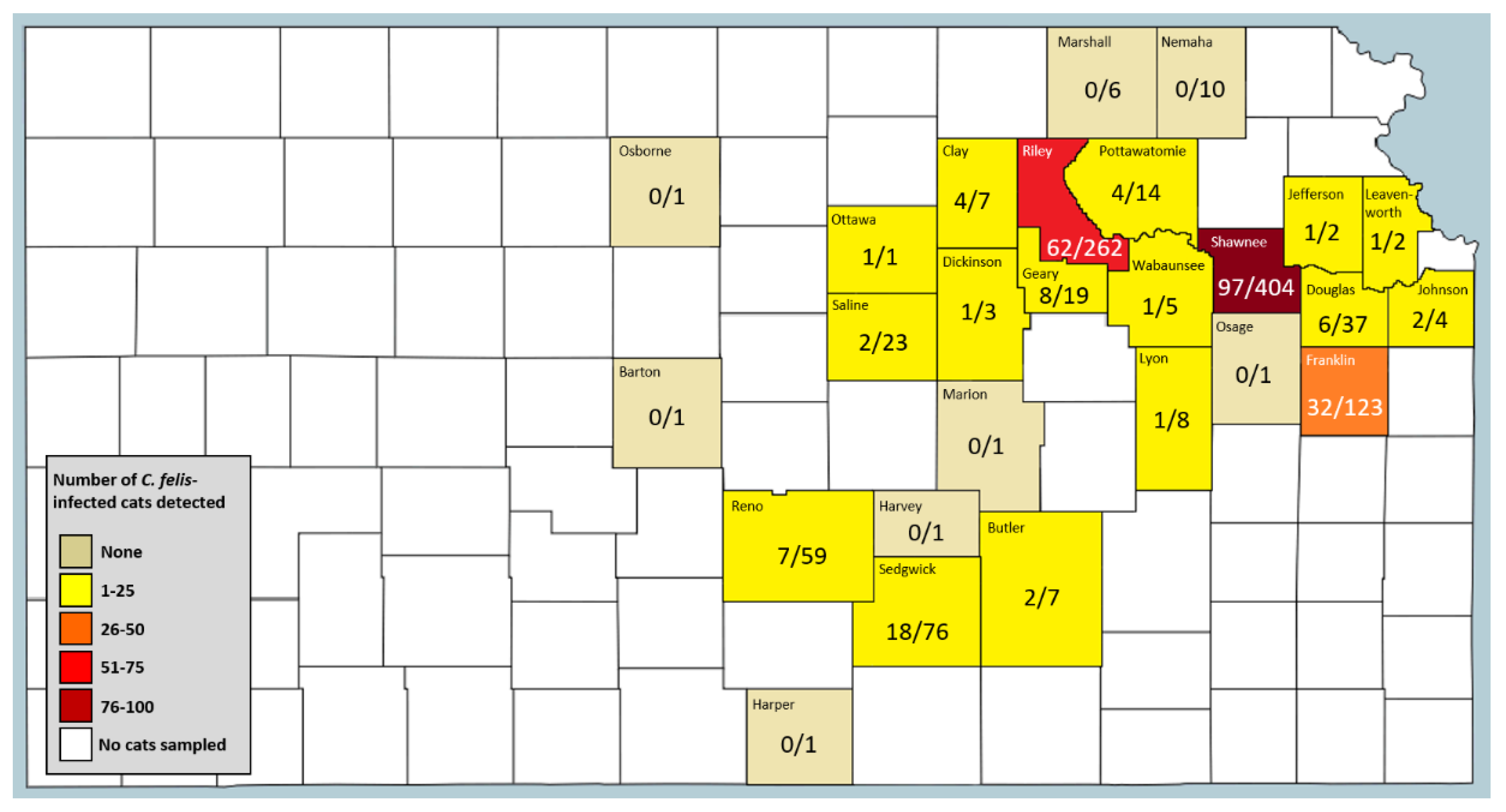
2.2. C. felis Infection Prevalence in Domestic Cats in Eastern Kansas
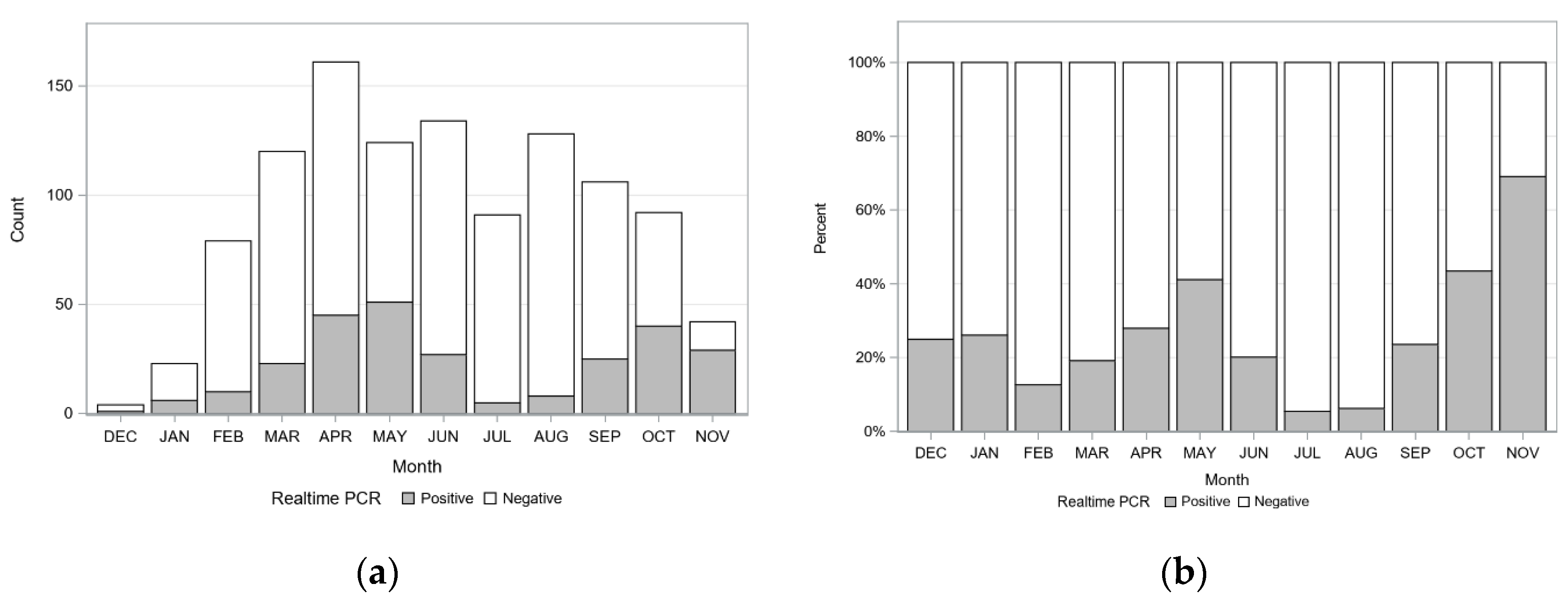
2.3. Seasonal Variation of Detecting C. felis-Carrier Cats
2.4. Evaluation of C. felis-Carriers among Cats with Different Lifestyles
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Study Design
5.2. Blood Sample Sources and Collection
5.3. DNA Extraction
5.4. Quantitative Real-Time PCR (qPCR)
5.5. C. felis Cox3 qPCR Assay Limit of Detection (LOD)
5.6. C. felis Cox3 qPCR Assay Limit of Quantification (LOQ)
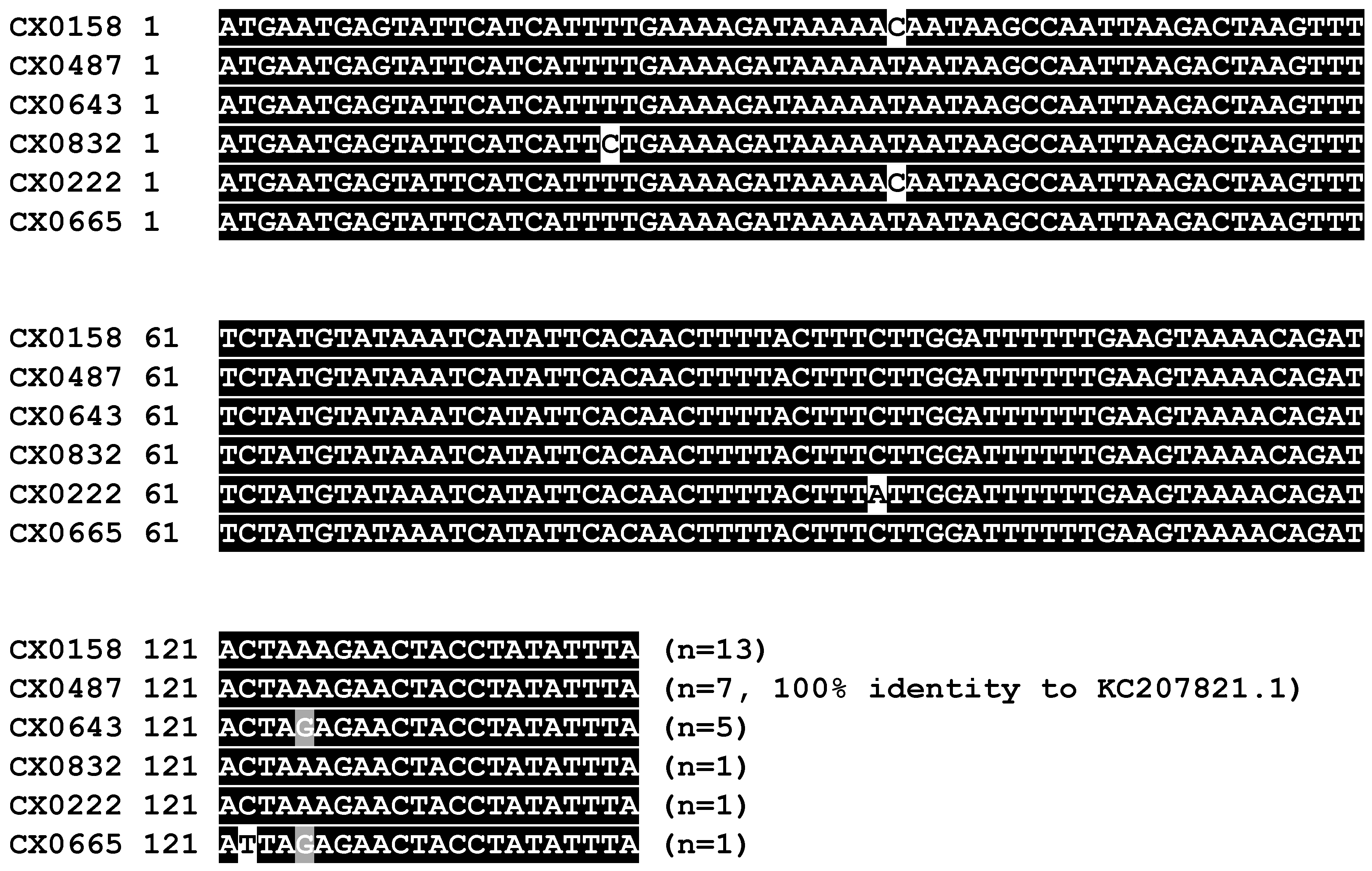
5.7. Amplicon Cloning and Sequence Analysis
5.8. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jalovecka, M.; Hajdusek, O.; Sojka, D.; Kopacek, P.; Malandrin, L. The Complexity of Piroplasms Life Cycles. Front. Cell. Infect. Microbiol. 2018, 8, 248. [Google Scholar] [CrossRef] [PubMed]
- Reichard, M.V.; Edwards, A.C.; Meinkoth, J.H.; Snider, T.; Meinkoth, K.R.; Heinz, R.E.; Little, S.E. Confirmation of Amblyomma americanum (Acari: Ixodidae) as a vector for Cytauxzoon felis (Piroplasmorida: Theileriidae) to domestic cats. J. Med. Èntomol. 2010, 47, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.J.; Ohmes, C.M.; Payton, E.M.; Hostetler, A.J.; Reichard, M.V. Minimum transmission time of Cytauxzoon felis by Amblyomma americanum to domestic cats in relation to duration of infestation, and investigation of ingestion of infected ticks as a potential route of transmission. J. Feline Med. Surg. 2017, 20, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.E.; Thomas, J.E.; Wohltjen, M.L.; Reichard, M.V. Transmission of Cytauxzoon felis to domestic cats by Amblyomma americanum nymphs. Parasites Vectors 2019, 12, 28. [Google Scholar] [CrossRef]
- Little, S.E.; Barrett, A.W.; Nagamori, Y.; Herrin, B.H.; Normile, D.; Heaney, K.; Armstrong, R. Ticks from cats in the United States: Patterns of infestation and infection with pathogens. Vet. Parasitol. 2018, 257, 15–20. [Google Scholar] [CrossRef]
- Chan, W.-H.; Kaufman, P.E. Common Name: American Dog Tick Scientific Name: Dermacentor variabilis (Say) (Arachnida: Ixodida: Ixodidae). Available online: http://entnemdept.ufl.edu/creatures/urban/medical/american_dog_tick.htm (accessed on 24 March 2020).
- Minigan, J.N.; Gedalof, Z.; Peregrine, A.S.; Newman, J.A. Current and potential future distribution of the American dog tick (Dermacentor variabilis, Say) in North America. Ticks Tick-Borne Dis. 2018, 9, 354–362. [Google Scholar] [CrossRef]
- Holderman, C.J.; Kaufman, P.E. Common Name: Lone Star Tick Scientific Name: Amblyomma americanum (Linnaeus) (Acari: Ixodidae). Available online: http://entnemdept.ufl.edu/creatures/urban/medical/lone_star_tick.htm (accessed on 15 March 2020).
- Monzón, J.D.; Atkinson, E.G.; Henn, B.M.; Benach, J.L. Population and Evolutionary Genomics of Amblyomma americanum, an Expanding Arthropod Disease Vector. Genome Biol. Evol. 2016, 8, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Reichard, M.V.; Baum, K.A.; Cadenhead, S.C.; Snider, T. Temporal occurrence and environmental risk factors associated with cytauxzoonosis in domestic cats. Vet. Parasitol. 2008, 152, 314–320. [Google Scholar] [CrossRef]
- Ferris, D. A progress report on the status of a new disease of American cats: Cytauxzoonosis. Comp. Immunol. Microbiol. Infect. Dis. 1979, 1, 269–276. [Google Scholar] [CrossRef]
- Meinkoth, J.; Kocan, A.A.; Whitworth, L.; Murphy, G.; Fox, J.C.; Woods, J.P. Cats surviving natural infection with Cytauxzoon felis: 18 cases (1997–1998). J. Vet. Intern. Med. 2000, 14, 521–525. [Google Scholar] [CrossRef]
- Birkenheuer, A.J.; Le, J.A.; Valenzisi, A.M.; Tucker, M.D.; Levy, M.G.; Breitschwerdt, E.B. Cytauxzoon felis infection in cats in the mid-Atlantic states: 34 cases (1998–2004). J. Am. Vet. Med. Assoc. 2006, 228, 568–571. [Google Scholar] [CrossRef] [PubMed]
- Snider, T.; Confer, A.W.; Payton, M.E. Pulmonary Histopathology of Cytauxzoon felis Infections in the Cat. Vet. Pathol. 2010, 47, 698–702. [Google Scholar] [CrossRef] [PubMed]
- Frontera-Acevedo, K. Feline Immune Response to Infection with Cytauxzoon felis and the Role of CD18 in the Pathogenesis of Cytauxzoonosis; University of Georgia: Athens, GA, USA, 2013. [Google Scholar]
- Frontera-Acevedo, K.; Sakamoto, K. Local pulmonary immune responses in domestic cats naturally infected with Cytauxzoon felis. Vet. Immunol. Immunopathol. 2015, 163, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Conner, B.J.; Hanel, R.M.; Brooks, M.B.; Cohn, L.A.; Birkenheuer, A.J. Coagulation abnormalities in 5 cats with naturally occurring cytauxzoonosis. J. Vet. Emerg. Crit. Care 2015, 25, 538–545. [Google Scholar] [CrossRef] [PubMed]
- A Cohn, L.; Birkenheuer, A.; Brunker, J.; Ratcliff, E.; Craig, A. Efficacy of Atovaquone and Azithromycin or Imidocarb Dipropionate in Cats with Acute Cytauxzoonosis. J. Vet. Intern. Med. 2010, 25, 55–60. [Google Scholar] [CrossRef]
- Wang, J.-L.; Li, T.-T.; Liu, G.-H.; Zhu, X.-Q.; Yao, C. Two Tales of Cytauxzoon felis Infections in Domestic Cats. Clin. Microbiol. Rev. 2017, 30, 861–885. [Google Scholar] [CrossRef]
- Zou, F.-C.; Li, Z.; Yang, J.-F.; Chang, J.-Y.; Liu, G.-H.; Lv, Y.; Zhu, X.-Q. Cytauxzoon felis Infection in Domestic Cats, Yunnan Province, China, 2016. Emerg. Infect. Dis. 2019, 25, 353–354. [Google Scholar] [CrossRef]
- Furtado, M.M.; Taniwaki, S.A.; Metzger, B.; Paduan, K.D.S.; O’Dwyer, H.L.; Jácomo, A.T.D.A.; Porfirio, G.; Silveira, L.; Sollmann, R.; Torres, N.; et al. Is the free-ranging jaguar (Panthera onca) a reservoir for Cytauxzoon felis in Brazil? Ticks Tick-Borne Dis. 2017, 8, 470–476. [Google Scholar] [CrossRef]
- Legroux, J.-P.; Halos, L.; René-Martellet, M.; Servonnet, M.; Pingret, J.-L.; Bourdoiseau, G.; Baneth, G.; Chabanne, L. First clinical case report of Cytauxzoon sp. infection in a domestic cat in France. BMC Veter- Res. 2017, 13, 81. [Google Scholar] [CrossRef]
- Veronesi, F.; Ravagnan, S.; Cerquetella, M.; Carli, E.; Olivieri, E.; Santoro, A.; Pesaro, S.; Berardi, S.; Rossi, G.; Ragni, B.; et al. First detection of Cytauxzoon spp. infection in European wildcats (Felis silvestris silvestris) of Italy. Ticks Tick-Borne Dis. 2016, 7, 853–858. [Google Scholar] [CrossRef]
- Glenn, B.L.; Kocan, A.A.; Blouin, E.F. Cytauxzoonosis in bobcats. J. Am. Vet. Med. Assoc. 1983, 183, 1155–1158. [Google Scholar] [PubMed]
- Blouin, E.F.; Kocan, A.A.; Glenn, B.L.; Kocan, K.M.; Hair, J.A. Transmission of Cytauxzoon felis by Kier, 1979 from Bobcats, Felis rufus (Schreber), to Domestic Cats by Dermacentor veriabilis (Say). J. Wildl. 1984, 20, 241–242. [Google Scholar] [CrossRef] [PubMed]
- Birkenheuer, A.J.; Marr, H.S.; Warren, C.; Acton, A.E.; Mucker, E.M.; Humphreys, J.G.; Tucker, M.D. Cytauxzoon felis infections are present in bobcats (Lynx rufus) in a region where cytauxzoonosis is not recognized in domestic cats. Vet. Parasitol. 2008, 153, 126–130. [Google Scholar] [CrossRef]
- Zieman, E.A.; Jiménez, F.A.; Nielsen, C.K. Concurrent Examination of Bobcats and Ticks Reveals High Prevalence of Cytauxzoon felis in Southern Illinois. J. Parasitol. 2017, 103, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Zieman, E.A.; Nielsen, C.K.; Jiménez, F.A. Chronic Cytauxzoon felis infections in wild-caught bobcats (Lynx rufus). Vet. Parasitol. 2018, 252, 67–69. [Google Scholar] [CrossRef]
- Shock, B.C.; Murphy, S.M.; Patton, L.L.; Shock, P.M.; Olfenbuttel, C.; Beringer, J.; Prange, S.; Grove, D.M.; Peek, M.; Butfiloski, J.W.; et al. Distribution and prevalence of Cytauxzoon felis in bobcats (Lynx rufus), the natural reservoir, and other wild felids in thirteen states. Vet. Parasitol. 2011, 175, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Brown, H.M.; Lockhart, J.M.; Latimer, K.S.; Peterson, D.S. Identification and genetic characterization of Cytauxzoon felis in asymptomatic domestic cats and bobcats. Vet. Parasitol. 2010, 172, 311–316. [Google Scholar] [CrossRef]
- Lewis, K.; Cohn, A.L.; Marr, H.; Birkenheuer, A. Diminazene Diaceturate for Treatment of Chronic Cytauxzoon felis Parasitemia in Naturally Infected Cats. J. Vet. Intern. Med. 2012, 26, 1490–1493. [Google Scholar] [CrossRef]
- Butt, M.T.; Bowman, D.; Barr, M.C.; Roelke, M.E. Iatrogenic Transmission of Cytauxzoon felis from a Florida Panther (Felix concolor coryi) to a Domestic Cat. J. Wildl. Dis. 1991, 27, 342–347. [Google Scholar] [CrossRef]
- Rotstein, D.S.; Taylor, S.K.; Harvey, J.W.; Bean, J. Hematologic Effects of Cytauxzoonosis in Florida Panthers and Texas Cougars in Florida. J. Wildl. Dis. 1999, 35, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Harvey, J.W.; Dunbar, M.R.; Norton, T.M.; Yabsley, M.J. Laboratory Findings in Acute Cytauxzoon felis Infection in Cougars (Puma concolor couguar) in Florida. J. Zoo Wildl. Med. 2007, 38, 285–291. [Google Scholar] [CrossRef]
- Lewis, K.M.; A Cohn, L.; Downey, M.E.; Whitney, M.S.; Birkenheuer, A.J. Evaluation of Cytauxzoon felis infection status in captive-born wild felids housed in an area endemic for the pathogen. J. Am. Vet. Med. Assoc. 2012, 241, 1088–1092. [Google Scholar] [CrossRef] [PubMed]
- E Wagner, J. A fatal cytauxzoonosis-like disease in cats. J. Am. Veter- Med Assoc. 1976, 168, 585–588. [Google Scholar]
- Hoover, J.P.; Walker, D.B.; Hedges, J.D. Cytauxzoonosis in cats: Eight cases (1985–1992). J. Am. Vet. Med. Assoc. 1994, 205. [Google Scholar]
- Reichard, M.V.; Meinkoth, J.H.; Edwards, A.C.; Snider, T.; Kocan, K.M.; Blouin, E.F.; Little, S.E. Transmission of Cytauxzoon felis to a domestic cat by Amblyomma americanum. Vet. Parasitol. 2009, 161, 110–115. [Google Scholar] [CrossRef]
- E Rizzi, T.; Reichard, M.V.; A Cohn, L.; Birkenheuer, A.J.; Taylor, J.D.; Meinkoth, J.H. Prevalence of Cytauxzoon felis infection in healthy cats from enzootic areas in Arkansas, Missouri, and Oklahoma. Parasites Vectors 2015, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Haber, M.D.; Tucker, M.D.; Marr, H.S.; Levy, J.K.; Burgess, J.; Lappin, M.R.; Birkenheuer, A.J. The detection of Cytauxzoon felis in apparently healthy free-roaming cats in the USA. Vet. Parasitol. 2007, 146, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Nagamori, Y.; E Slovak, J.; Reichard, M.V. Prevalence of Cytauxzoon felis infection in healthy free-roaming cats in north-central Oklahoma and central Iowa. J. Feline Med. Surg. Open Rep. 2016, 2, 1–4. [Google Scholar] [CrossRef]
- Schreeg, M.E.; Marr, H.S.; Griffith, E.H.; Tarigo, J.L.; Bird, D.M.; Reichard, M.V.; Cohn, L.A.; Levy, M.G.; Birkenheuer, A.J.; Schrîg, M.E. PCR amplification of a multi-copy mitochondrial gene (cox3) improves detection of Cytauxzoon felis infection as compared to a ribosomal gene (18S). Vet. Parasitol. 2016, 225, 123–130. [Google Scholar] [CrossRef]
- Schreeg, M.E.; Marr, H.S.; Tarigo, J.; Cohn, L.A.; Levy, M.G.; Birkenheuer, A.J. Pharmacogenomics of Cytauxzoon felis Cytochrome b: Implications for Atovaquone and Azithromycin Therapy in Domestic Cats with Cytauxzoonosis. J. Clin. Microbiol. 2013, 51, 3066–3069. [Google Scholar] [CrossRef]
- Brown, H.M.; Latimer, K.S.; Erikson, L.E.; Cashwell, M.E.; Britt, J.O.; Peterson, D.S. Detection of persistent Cytauxzoon felis infection by polymerase chain reaction in three asymptomatic domestic cats. J. Vet. Diagn. Investig. 2008, 20, 485–488. [Google Scholar] [CrossRef] [PubMed]
- Bondy, P.J.; Cohn, L.A.; Tyler, J.W.; Marsh, A.E. Polymerase Chain Reaction Detection of Cytauxzoon felis From Field-Collected Ticks and Sequence Analysis of the Small Subunit and Internal Transcribed Spacer 1 Region of the Ribosomal RNA Gene. J. Parasitol. 2005, 91, 458–461. [Google Scholar] [CrossRef] [PubMed]
- Moore Brown, H. Cytauxzoon felis: Assessing Genetic Variability in an Emerging Feline Pathogen; University of Georgia: Athens, GA, USA, 2010. [Google Scholar]
- Pollard, D.A.; Reichard, M.V.; Cohn, L.A.; James, A.M.; Holman, P.J. Genetic variability of cloned Cytauxzoon felis ribosomal RNA ITS1 and ITS2 genomic regions from domestic cats with varied clinical outcomes from five states. Vet. Parasitol. 2017, 244, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Shock, B.C.; Birkenheuer, A.J.; Patton, L.L.; Olfenbuttel, C.; Beringer, J.; Grove, D.M.; Peek, M.; Butfiloski, J.W.; Hughes, D.W.; Lockhart, J.M.; et al. Variation in the ITS-1 and ITS-2 rRNA genomic regions of Cytauxzoon felis from bobcats and pumas in the eastern United States and comparison with sequences from domestic cats. Vet. Parasitol. 2012, 190, 29–35. [Google Scholar] [CrossRef]
- Motzel, S.; Wagner, J. Treatment of experimentally induced cytauxzoonosis in cats with parvaquone and buparvaquone. Vet. Parasitol. 1990, 35, 131–138. [Google Scholar] [CrossRef]
- Tarigo, J.L.; Scholl, E.H.; Bird, D.M.; Brown, C.C.; Cohn, L.A.; Dean, G.; Levy, M.G.; Rn, D.M.D.; Trieu, A.; Nordone, S.K.; et al. A Novel Candidate Vaccine for Cytauxzoonosis Inferred from Comparative Apicomplexan Genomics. PLoS ONE 2013, 8, e71233. [Google Scholar] [CrossRef]
- A Cohn, L.; Shaw, D.; Shoemake, C.; Birkenheuer, A.J. Second illness due to subsequent Cytauxzoon felis infection in a domestic cat. J. Feline Med. Surg. Open Rep. 2020, 6, 1–5. [Google Scholar] [CrossRef]
- Raghavan, R.; Almes, K.; Goodin, D.G.; Harrington, J.A.; Stackhouse, P.W. Spatially Heterogeneous Land Cover/Land Use and Climatic Risk Factors of Tick-Borne Feline Cytauxzoonosis. Vector Borne Zoonotic Dis. 2014, 14, 486–495. [Google Scholar] [CrossRef]
- Pets by the Numbers: Data and Statistics on Pet Ownership, Community Cat, and Shelter Population in the United States. Available online: https://www.animalsheltering.org/page/pets-by-the-numbers (accessed on 10 August 2020).
- Pets by the Numbers: U.S. Pet Ownership, Community Cat and Shelter Population Estimates. Available online: https://www.humanesociety.org/resources/pets-numbers (accessed on 10 August 2020).
- Gedeon, J. Special Report: States with the Most and Least Cat Owners. Available online: https://247wallst.com/special-report/2017/07/19/states-with-the-most-and-least-cat-owners/ (accessed on 10 August 2020).
- Pet Statistics. Available online: https://www.aspca.org/animal-homelessness/shelter-intake-and-surrender/pet-statistics (accessed on 10 August 2020).
- History of National Feral Cat Day. Available online: https://nationaltoday.com/national-feral-cat-day/ (accessed on 10 August 2020).

| Sample Conc | Ct 1 Value | Melt Temp 2 (°C) |
|---|---|---|
| 1:105 | 40.36 | 75.50 |
| 1:105 | 37.62 | 75.00 |
| 1:105 | 41.11 | 75.50 |
| 1:105 | 40.69 | 75.50 |
| 1:105 | 37.10 | 75.00 |
| 1:105 | 35.77 | 75.00 |
| Sample Conc | Ct 1 Value | SQ 2 | Melt Temp 3 (°C) |
|---|---|---|---|
| 102 | 38.81 | 6 | 75.50 |
| 102 | 37.00 | 18 | 75.50 |
| 102 | 38.21 | 9 | 75.50 |
| 102 | 37.58 | 13 | 75.50 |
| 102 | 37.53 | 13 | 75.50 |
| 102 | 37.24 | 16 | 75.50 |
| Odds Ratio to (Adj. p-Value of Testing for Odds Ratio^ = 1) | |||||
|---|---|---|---|---|---|
| Season | Prevalence | SE 1 | Spring | Summer | Fall |
| Winter | 18.2% | 4.0% | 0.42 (0.016) | 1.46 (0.624) | 0.27 (<0.001) |
| Spring | 34.4% | 3.0% | 3.44 (<0.001) | 0.64 (0.048) | |
| Summer | 13.3% | 2.0% | 0.19 (<0.001) | ||
| Fall | 45.1% | 3.8% | |||
| Overall | 25.8% | 2.1% | |||
| Odds Ratio to (Adj. p-Value of Testing for Odds Ratio^ = 1) | ||||
|---|---|---|---|---|
| Lifestyle | Prevalence | SE 1 | Owned | Rescue |
| Feral | 27.2% | 3.2% | 1.12 (0.840) | 1.70 (0.015) |
| Owned | 25.0% | 2.5% | 1.51 (0.038) | |
| Rescue | 18.0% | 1.8% | ||
| Overall | 25.8% | 2.1% | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wikander, Y.M.; Anantatat, T.; Kang, Q.; Reif, K.E. Prevalence of Cytauxzoon felis Infection-Carriers in Eastern Kansas Domestic Cats. Pathogens 2020, 9, 854. https://doi.org/10.3390/pathogens9100854
Wikander YM, Anantatat T, Kang Q, Reif KE. Prevalence of Cytauxzoon felis Infection-Carriers in Eastern Kansas Domestic Cats. Pathogens. 2020; 9(10):854. https://doi.org/10.3390/pathogens9100854
Chicago/Turabian StyleWikander, Yvonne M., Tippawan Anantatat, Qing Kang, and Kathryn E. Reif. 2020. "Prevalence of Cytauxzoon felis Infection-Carriers in Eastern Kansas Domestic Cats" Pathogens 9, no. 10: 854. https://doi.org/10.3390/pathogens9100854
APA StyleWikander, Y. M., Anantatat, T., Kang, Q., & Reif, K. E. (2020). Prevalence of Cytauxzoon felis Infection-Carriers in Eastern Kansas Domestic Cats. Pathogens, 9(10), 854. https://doi.org/10.3390/pathogens9100854







