Adhesion-Regulating Molecule from Haemonchus contortus: Potential Antigen for Diagnosis of Early Infection in Goats
Abstract
1. Introduction
2. Results
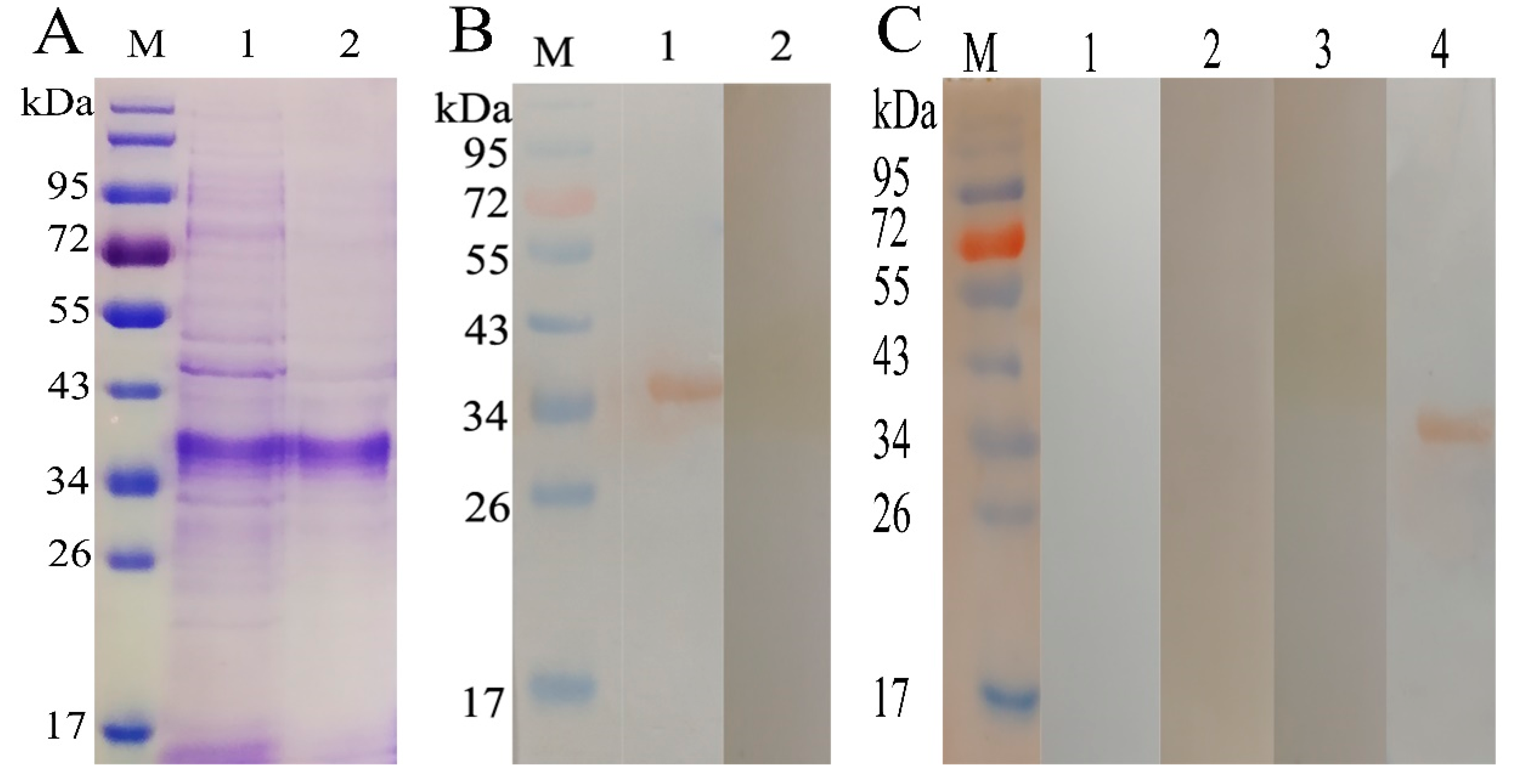
2.1. Expression and Purification of rHcADRM
2.2. Potential of rHcADRM in Diagnosis of H. contortus Infection
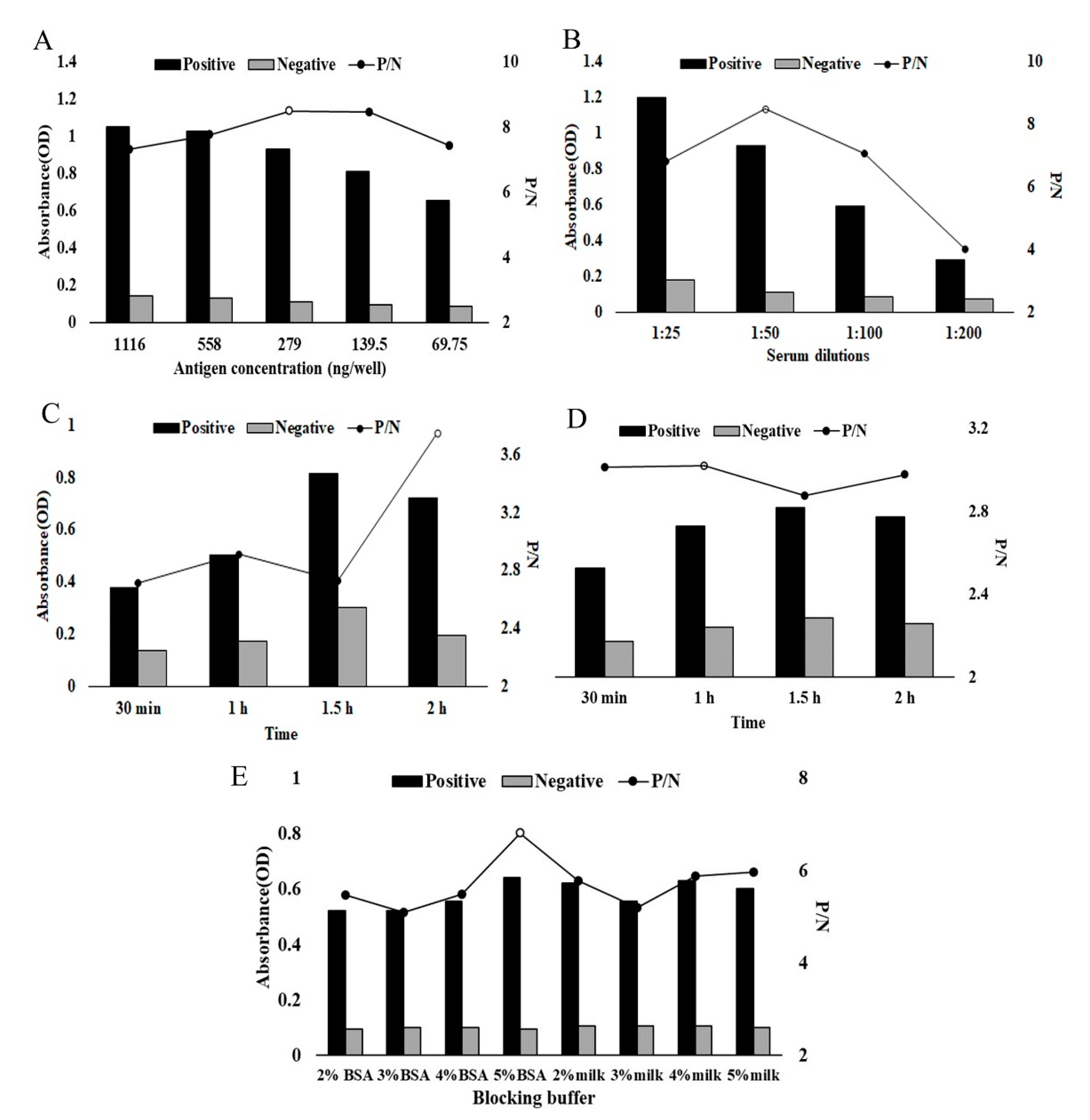
2.3. Indirect ELISA Constructed Based on rHcADRM
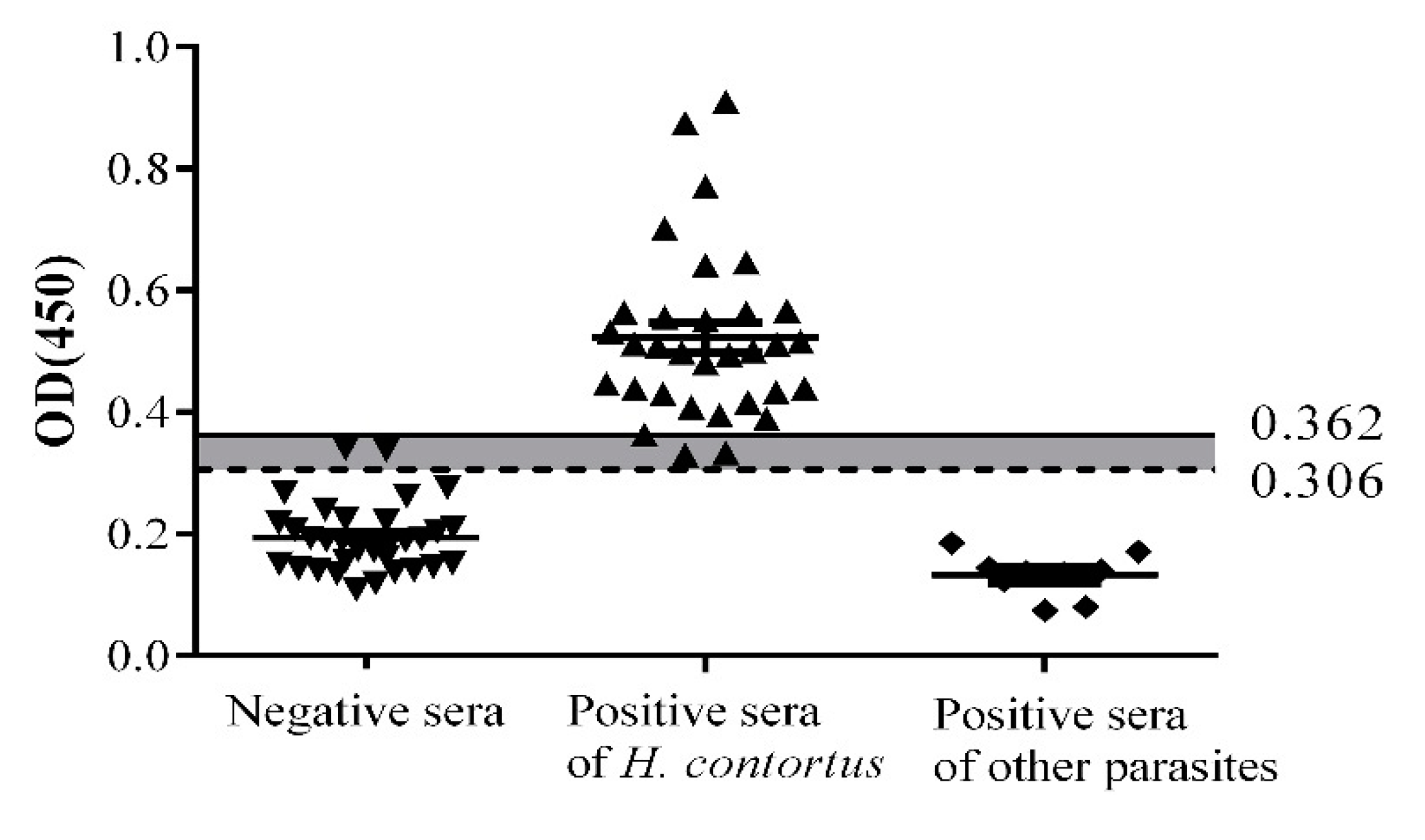
2.4. Sensitivity, Specificity, and Stability
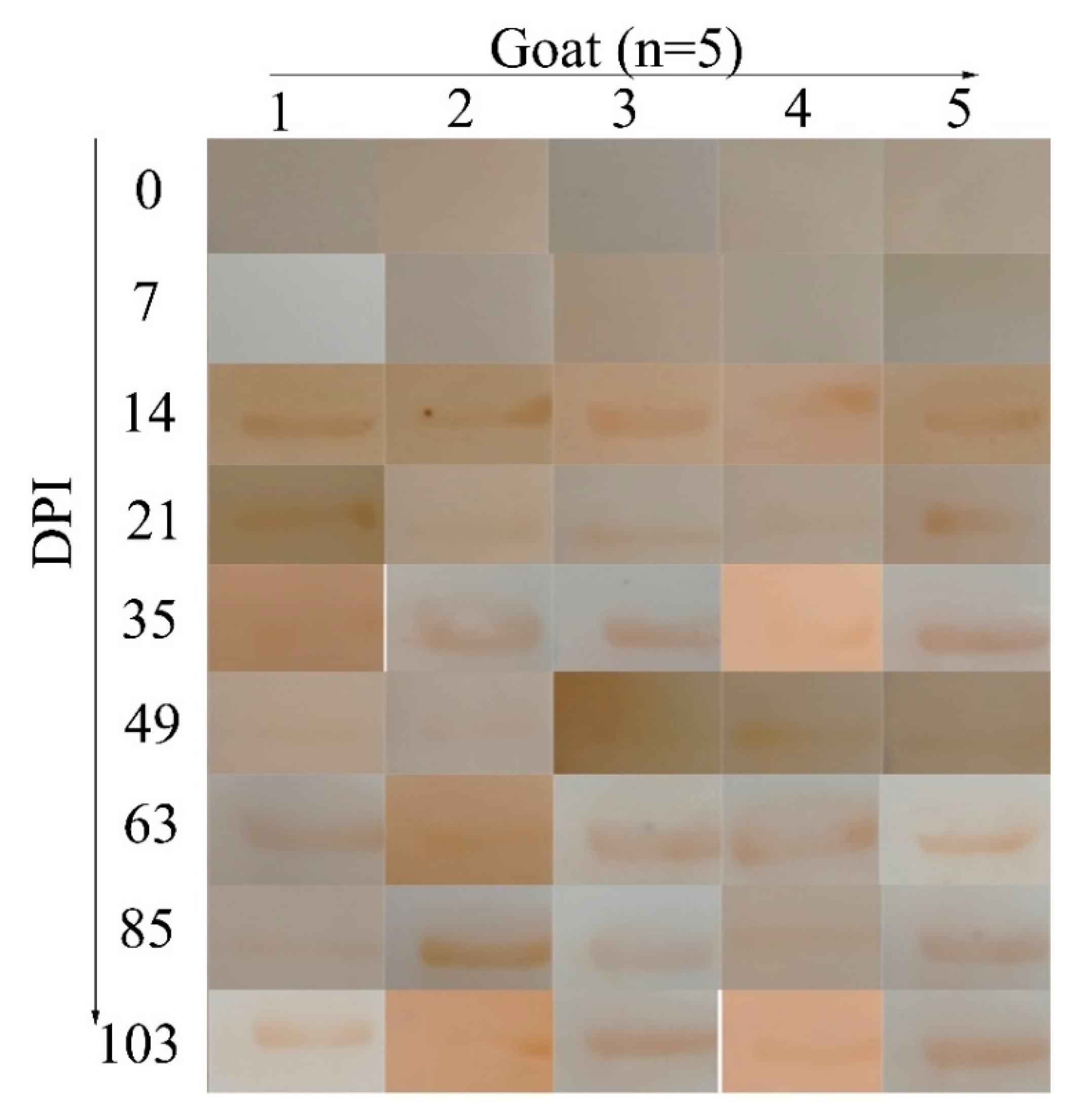
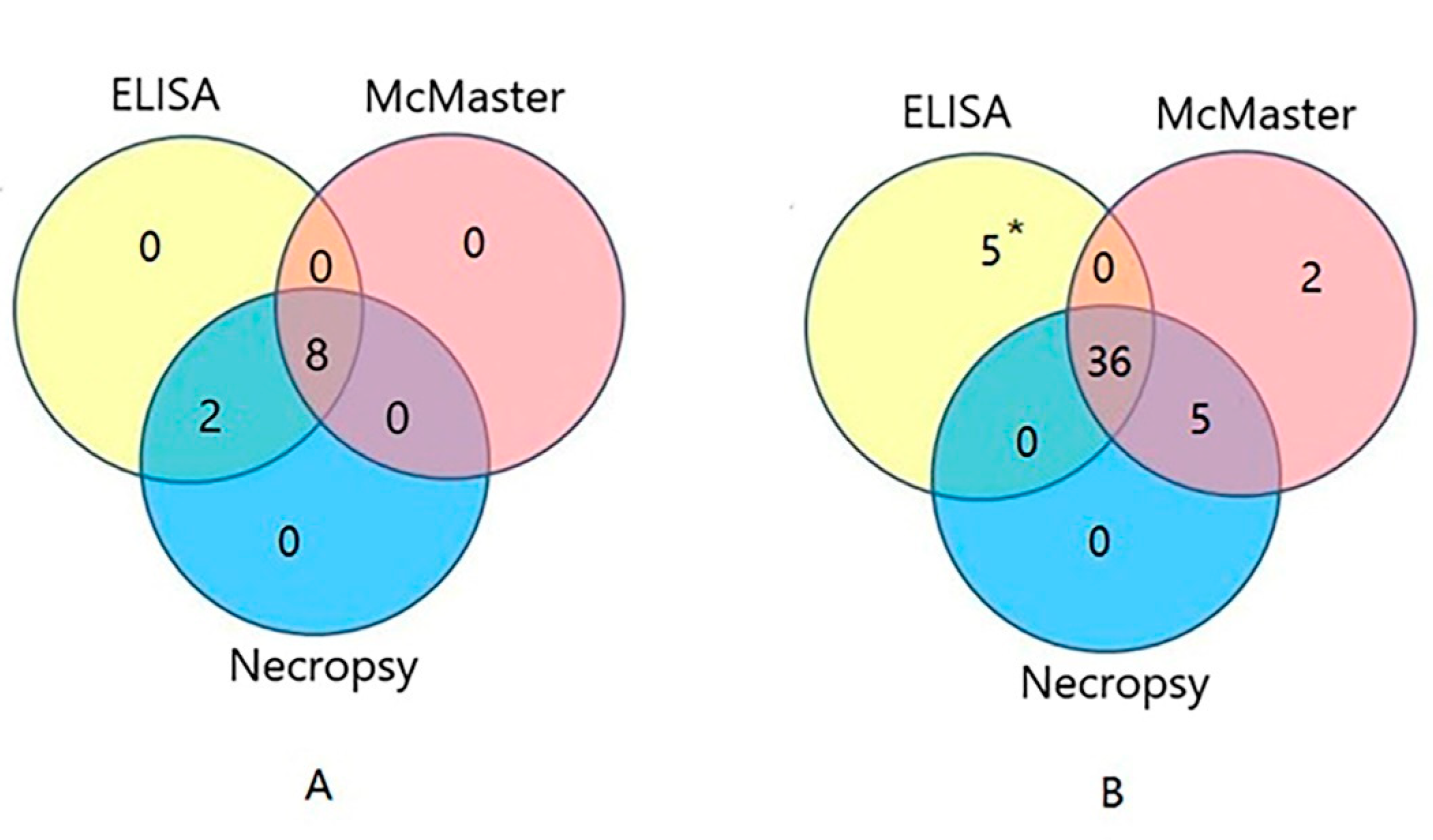
2.5. Diagnosis of H. contortus Infection in the Field
3. Discussion
4. Materials and Methods
4.1. Expression and Purification of rHcADRM
4.2. Parasite, Goat, and Serum Sample
4.3. Western Blot Analysis
4.4. Development of Indirect ELISA
4.5. Determination of Cut-Off Value
4.6. Determination of Sensitivity, Specificity, and Stability
4.7. Application of the Indirect ELISA
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Edwards, E.E.; Garner, B.C.; Williamson, L.H.; Storey, B.E.; Sakamoto, K. Pathology of Haemonchus contortus in New World camelids in the southeastern United States: A retrospective review. J. Vet. Diagn. Investig. 2016, 28, 105–109. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, L.; Liu, X.; Wang, S.; Ehsan, M.; Yan, R.; Song, X.; Xu, L.; Li, X. Characterization of a secreted cystatin of the parasitic nematode Haemonchus contortus and its immune-modulatory effect on goat monocytes. Parasit. Vectors 2017, 10, 425. [Google Scholar] [CrossRef]
- Emery, D.L.; Hunt, P.W.; Le Jambre, L.F. Haemonchus contortus: The then and now, and where to from here? Int. J. Parasitol. 2016, 46, 755–769. [Google Scholar] [CrossRef]
- Gadahi, J.A.; Ehsan, M.; Wang, S.; Zhang, Z.; Wang, Y.; Yan, R.; Song, X.; Xu, L.; Li, X. Recombinant protein of Haemonchus contortus 14-3-3 isoform 2 (rHcftt-2) decreased the production of IL-4 and suppressed the proliferation of goat PBMCs in vitro. Exp. Parasitol. 2016, 171, 57–66. [Google Scholar] [CrossRef]
- Fawzi, E.M.; Gonzalez-Sanchez, M.E.; Corral, M.J.; Cuquerella, M.; Alunda, J.M. Vaccination of lambs against Haemonchus contortus infection with a somatic protein (Hc23) from adult helminths. Int. J. Parasitol. 2014, 44, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Ma, G.; Ang, C.S.; Korhonen, P.K.; Xu, R.; Nie, S.; Koehler, A.V.; Simpson, R.J.; Greening, D.W.; Reid, G.E.; et al. Somatic proteome of Haemonchus contortus. Int. J. Parasitol. 2019, 49, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Schallig, H.D.; Hornok, S.; Cornelissen, J.B. Comparison of two enzyme immunoassays for the detection of Haemonchus contortus infections in sheep. Vet. Parasitol. 1995, 57, 329–338. [Google Scholar] [CrossRef]
- Yang, X.; Qi, M.W.; Zhang, Z.Z.; Gao, C.; Wang, C.Q.; Lei, W.Q.; Tan, L.; Zhao, J.L.; Fang, R.; Hu, M. Development and Evaluation of a Loop-Mediated Isothermal Amplification (Lamp) Assay for the Detection of Haemonchus contortus in Goat Fecal Samples. J. Parasitol. 2017, 103, 161–167. [Google Scholar] [CrossRef]
- Kandil, O.M.; Gamil, I.S.; Hendawy, S.H.M.; Medhat, F.; El-Habit, O.H. Efficacy of glutathione-S-transferase purified antigen of the gastro-intestinal nematode Haemonchus contortus in diagnosis of sheep haemonchosis. J. Parasit. Dis. 2017, 41, 968–975. [Google Scholar] [CrossRef]
- Yatsuda, A.P.; Krijgsveld, J.; Cornelissen, A.W.; Heck, A.J.; de Vries, E. Comprehensive analysis of the secreted proteins of the parasite Haemonchus contortus reveals extensive sequence variation and differential immune recognition. J. Biol. Chem. 2003, 278, 16941–16951. [Google Scholar] [CrossRef]
- Gadahi, J.A.; Yongqian, B.; Ehsan, M.; Zhang, Z.C.; Wang, S.; Yan, R.F.; Song, X.K.; Xu, L.X.; Li, X.R. Haemonchus contortus excretory and secretory proteins (HcESPs) suppress functions of goat PBMCs in vitro. Oncotarget 2016, 7, 35670–35679. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, M.A.; Naqvi, S.Z.; Memon, M.A.; Aimulajiang, K.; Haseeb, M.; Xu, L.; Song, X.; Li, X.; Yan, R. Combined Use of Indirect ELISA and Western Blotting with Recombinant Hepatocellular Carcinoma-Associated Antigen 59 Is a Potential Immunodiagnostic Tool for the Detection of Prepatent Haemonchus contortus Infection in Goat. Animals 2019, 13, 8. [Google Scholar] [CrossRef] [PubMed]
- Schallig, H.D.; van Leeuwen, M.A.; Hendrikx, W.M. Immune responses of Texel sheep to excretory/secretory products of adult Haemonchus contortus. Parasitology 1994, 108, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Husnjak, K.; Elsasser, S.; Zhang, N.; Chen, X.; Randles, L.; Shi, Y.; Hofmann, K.; Walters, K.J.; Finley, D.; Dikic, I. Proteasome subunit Rpn13 is a novel ubiquitin receptor. Nature 2008, 453, 481–488. [Google Scholar] [CrossRef]
- Wang, C.; Li, F.; Zhang, Z.; Yang, X.; Ahmad, A.A.; Li, X.; Du, A.; Hu, M. Recent Research Progress in China on Haemonchus contortus. Front. Microbiol. 2017, 8, 1509. [Google Scholar] [CrossRef]
- Naeemipour, M.; Hashemitabar, G.R.; Dastjerdi, K.; Mojaver, M.J.; Mohammadi, H.R. Comparison of Fecal Egg Counts and ELISA for the Diagnosis of Dicrocoelium Dendriticum Infection. Pol. J. Vet. Sci. 2016, 19, 573–580. [Google Scholar] [CrossRef]
- Gasso, D.; Feliu, C.; Ferrer, D.; Mentaberre, G.; Casas-Diaz, E.; Velarde, R.; Fernandez-Aguilar, X.; Colom-Cadena, A.; Navarro-Gonzalez, N.; Lopez-Olvera, J.R.; et al. Uses and limitations of faecal egg count for assessing worm burden in wild boars. Vet. Parasitol. 2015, 209, 133–137. [Google Scholar] [CrossRef]
- Song, H.B.; Kim, J.; Jin, Y.; Lee, J.S.; Jeoung, H.G.; Lee, Y.H.; Saeed, A.A.W.; Hong, S.T. Comparison of ELISA and Urine Microscopy for Diagnosis of Schistosoma haematobium Infection. J. Korean Med. Sci. 2018, 33, e238. [Google Scholar] [CrossRef]
- Kandil, O.M.; Hendawy, S.; El Namaky, A.; Gabrashanska, M.; Nanev, V. Evaluation of different Haemonchus contortus antigens for diagnosis of sheep haemonchosis by ELISA and their cross reactivity with other helminthes. J. Parasit. Dis. 2017, 41, 678–683. [Google Scholar] [CrossRef]
- Xu, J.; Peeling, R.W.; Chen, J.X.; Wu, X.H.; Wu, Z.D.; Wang, S.P.; Feng, T.; Chen, S.H.; Li, H.; Guo, J.G.; et al. Evaluation of immunoassays for the diagnosis of Schistosoma japonicum infection using archived sera. PLoS Negl. Trop. Dis. 2011, 5, e949. [Google Scholar] [CrossRef]
- Lv, C.; Hong, Y.; Fu, Z.; Lu, K.; Cao, X.; Wang, T.; Zhu, C.; Li, H.; Xu, R.; Jia, B.; et al. Evaluation of recombinant multi-epitope proteins for diagnosis of goat schistosomiasis by enzyme-linked immunosorbent assay. Parasit Vectors 2016, 9, 135. [Google Scholar] [CrossRef] [PubMed]
- Nuamtanong, S.; Reamtong, O.; Phuphisut, O.; Chotsiri, P.; Malaithong, P.; Dekumyoy, P.; Adisakwattana, P. Transcriptome and excretory-secretory proteome of infective-stage larvae of the nematode Gnathostoma spinigerum reveal potential immunodiagnostic targets for development. Parasite (Paris, France) 2019, 26, 34. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Q.; Liu, R.D.; Sun, G.G.; Song, Y.Y.; Jiang, P.; Zhang, X.; Cui, J. Proteomic Analysis of Trichinella spiralis Adult Worm Excretory-Secretory Proteins Recognized by Sera of Patients with Early Trichinellosis. Front. Microbiol. 2017, 8, 986. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.W.; Joo, H.N.; Lee, M.R.; Cho, S.H.; Cheun, H.I.; Kim, J.Y.; Lee, Y.H.; Lee, K.J.; Sohn, W.M.; Kim, D.M.; et al. Identification of a serodiagnostic antigen, legumain, by immunoproteomic analysis of excretory-secretory products of Clonorchis sinensis adult worms. Proteomics 2009, 9, 3066–3078. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.M.; Martin, S.; Hernandez, Y.I.; Gonzalez, J.F.; Ferrer, O.; Ruiz, A. Immunoprotective effect of cysteine proteinase fractions from two Haemonchus contortus strains adapted to sheep and goats. Vet. Parasitol. 2012, 188, 53–59. [Google Scholar] [CrossRef]
- Lin, A.V. Indirect ELISA. Methods Mol. Biol. 2015, 1318, 51–59. [Google Scholar]
- Gomez-Morales, M.A.; Ludovisi, A.; Amati, M.; Blaga, R.; Zivojinovic, M.; Ribicich, M.; Pozio, E. A distinctive Western blot pattern to recognize Trichinella infections in humans and pigs. Int. J. Parasitol. 2012, 42, 1017–1023. [Google Scholar] [CrossRef]
- Muller, N.; Frei, E.; Nunez, S.; Gottstein, B. Improved serodiagnosis of alveolar echinococcosis of humans using an in vitro-produced Echinococcus multilocularis antigen. Parasitology 2007, 134, 879–888. [Google Scholar] [CrossRef]
- Waritani, T.; Chang, J.; McKinney, B.; Terato, K. An ELISA protocol to improve the accuracy and reliability of serological antibody assays. MethodsX 2017, 4, 153–165. [Google Scholar] [CrossRef]
- Xiao, Y.; Isaacs, S.N. Enzyme-linked immunosorbent assay (ELISA) and blocking with bovine serum albumin (BSA)--not all BSAs are alike. J. Immunol. Methods 2012, 384, 148–151. [Google Scholar] [CrossRef]
- Azri, F.A.; Sukor, R.; Selamat, J.; Abu Bakar, F.; Yusof, N.A.; Hajian, R. Electrochemical Immunosensor for Detection of Aflatoxin B(1) Based on Indirect Competitive ELISA. Toxins 2018, 10, 196. [Google Scholar] [CrossRef]
- De-Simone, S.G.; Souza, A.L.A.; Aguiar, A.S.; Melgarejo, A.R.; Provance, D.W., Jr. Development of an elisa for the diagnosis of reactive IgE antibodies anti-therapeutic horse sera. Toxicon 2017, 138, 37–42. [Google Scholar] [CrossRef]
- Lu, M.; Tian, X.; Yang, X.; Yuan, C.; Ehsan, M.; Liu, X.; Yan, R.; Xu, L.; Song, X.; Li, X. The N- and C-terminal carbohydrate recognition domains of Haemonchus contortus galectin bind to distinct receptors of goat PBMC and contribute differently to its immunomodulatory functions in host-parasite interactions. Parasit Vectors 2017, 10, 409. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Wattanaphansak, S.; Asawakarn, T.; Gebhart, C.J.; Deen, J. Development and validation of an enzyme-linked immunosorbent assay for the diagnosis of porcine proliferative enteropathy. J. Vet. Diagn. Investig. 2008, 20, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Akao, T.; Kakehi, Y.; Wu, X.X.; Kinoshita, H.; Takahashi, T.; Ogawa, O.; Kato, T.; Yoshida, O. Semi-quantitative analysis of telomerase activity of exfoliated cells in urine of patients with urothelial cancers: Causative factors affecting sensitivity and specificity. Urol. Oncol. 1997, 3, 118–124. [Google Scholar] [CrossRef]
- Ghosh, D.; Bernstein, J. Development Of A Progesterone-Specific IgE Assay For Diagnosing Patients With Suspected Progestogen Hypersensitivity. Ann. Allergy Asthma Immunol. 2019, 6, 616–622. [Google Scholar] [CrossRef]
- Deo, V.K.; Inagaki, Y.; Murhandarwati, E.H.; Asmara, W.; Miyazaki, T.; Kato, T.; Park, E.Y. Sero-diagnostic potential of Plasmodium falciparum recombinant merozoite surface protein (MSP)-3 expressed in silkworm. Parasitol. Int. 2019, 72, 101938. [Google Scholar] [CrossRef]
- Lopes, L.G.; Silva, M.H.; Figueiredo, A.; Canuto, K.M.; Brito, E.S.; Ribeiro, P.R.V.; Souza, A.S.Q.; Barioni-Junior, W.; Esteves, S.N.; Chagas, A.C.S. The intake of dry cashew apple fiber reduced fecal egg counts in Haemonchus contortus-infected sheep. Exp. Parasitol. 2018, 195, 38–43. [Google Scholar] [CrossRef]
- Ljungstrom, S.; Melville, L.; Skuce, P.J.; Hoglund, J. Comparison of Four Diagnostic Methods for Detection and Relative Quantification of Haemonchus contortus Eggs in Feces Samples. Front. Vet. Sci. 2018, 4, 239. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aimulajiang, K.; Naqvi, M.A.-u.-H.; Chu, W.; Lu, M.; Tian, X.; Bu, Y.; Memon, M.A.; Li, X.; Xu, L.; Song, X.; et al. Adhesion-Regulating Molecule from Haemonchus contortus: Potential Antigen for Diagnosis of Early Infection in Goats. Pathogens 2020, 9, 34. https://doi.org/10.3390/pathogens9010034
Aimulajiang K, Naqvi MA-u-H, Chu W, Lu M, Tian X, Bu Y, Memon MA, Li X, Xu L, Song X, et al. Adhesion-Regulating Molecule from Haemonchus contortus: Potential Antigen for Diagnosis of Early Infection in Goats. Pathogens. 2020; 9(1):34. https://doi.org/10.3390/pathogens9010034
Chicago/Turabian StyleAimulajiang, Kalibixiati, Muhammad Ali-ul-Husnain Naqvi, Wen Chu, Mingmin Lu, Xiaowei Tian, Yongqian Bu, Muhammad Ali Memon, Xiangrui Li, Lixin Xu, Xiaokai Song, and et al. 2020. "Adhesion-Regulating Molecule from Haemonchus contortus: Potential Antigen for Diagnosis of Early Infection in Goats" Pathogens 9, no. 1: 34. https://doi.org/10.3390/pathogens9010034
APA StyleAimulajiang, K., Naqvi, M. A.-u.-H., Chu, W., Lu, M., Tian, X., Bu, Y., Memon, M. A., Li, X., Xu, L., Song, X., & Yan, R. (2020). Adhesion-Regulating Molecule from Haemonchus contortus: Potential Antigen for Diagnosis of Early Infection in Goats. Pathogens, 9(1), 34. https://doi.org/10.3390/pathogens9010034







