Streptococcus suis in Brazil: Genotypic, Virulence, and Resistance Profiling of Strains Isolated from Pigs between 2001 and 2016
Abstract
1. Introduction
2. Results
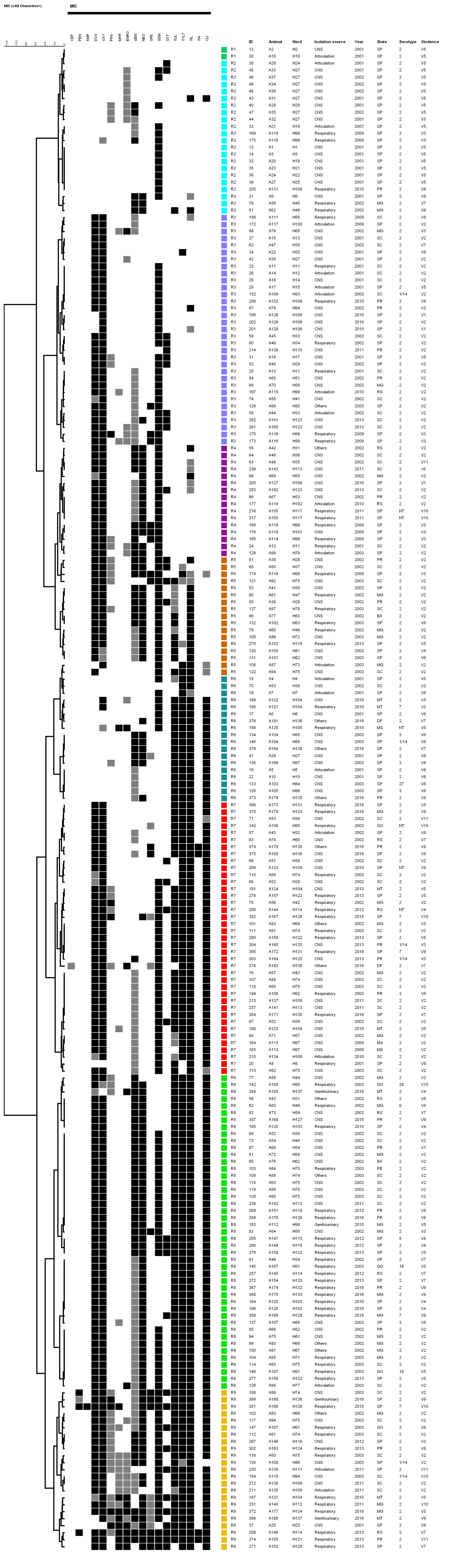
2.1. Molecular Serotyping
2.2. Virulence Profile Identification
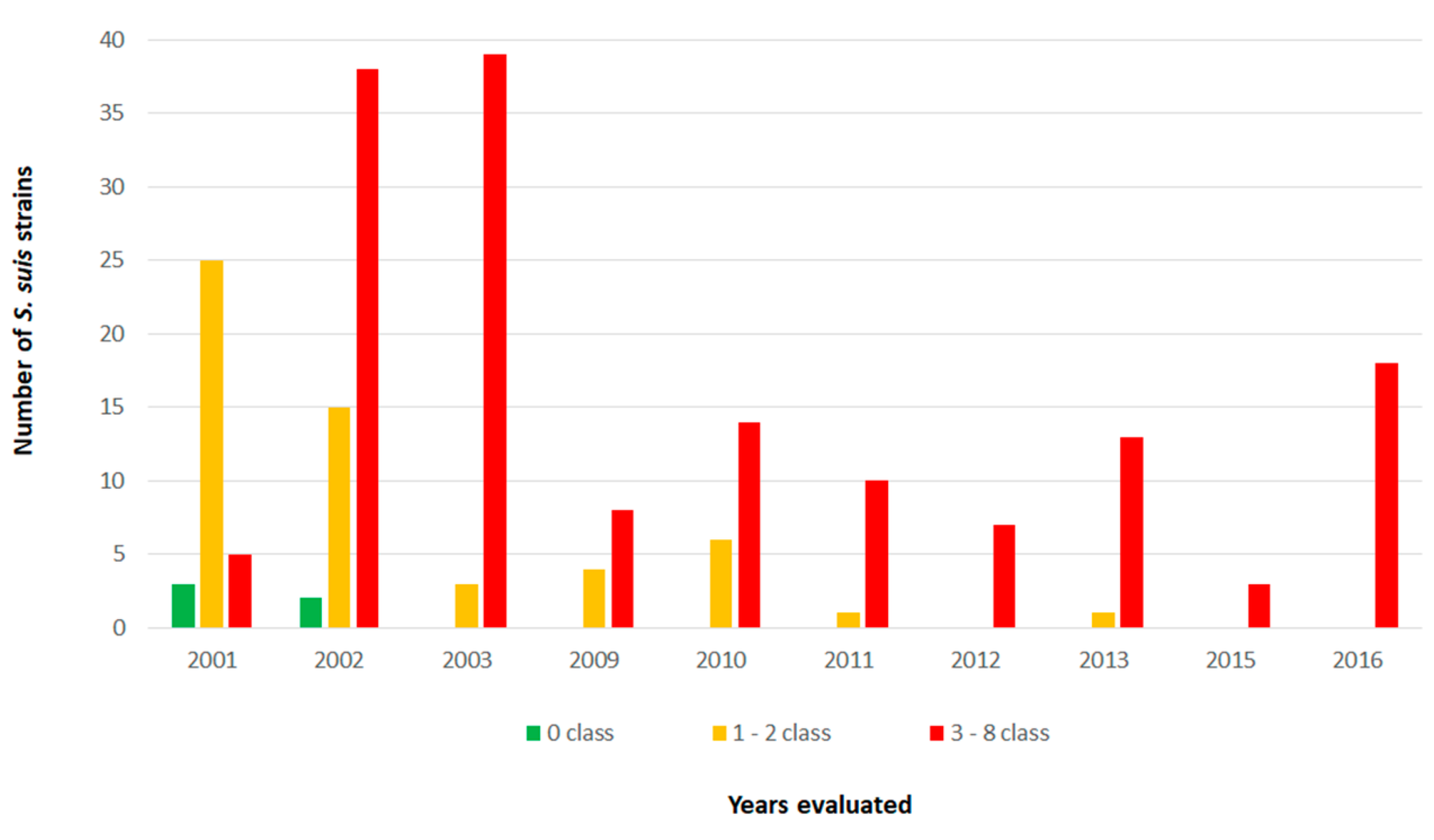
2.3. Antimicrobial Resistance Profiling
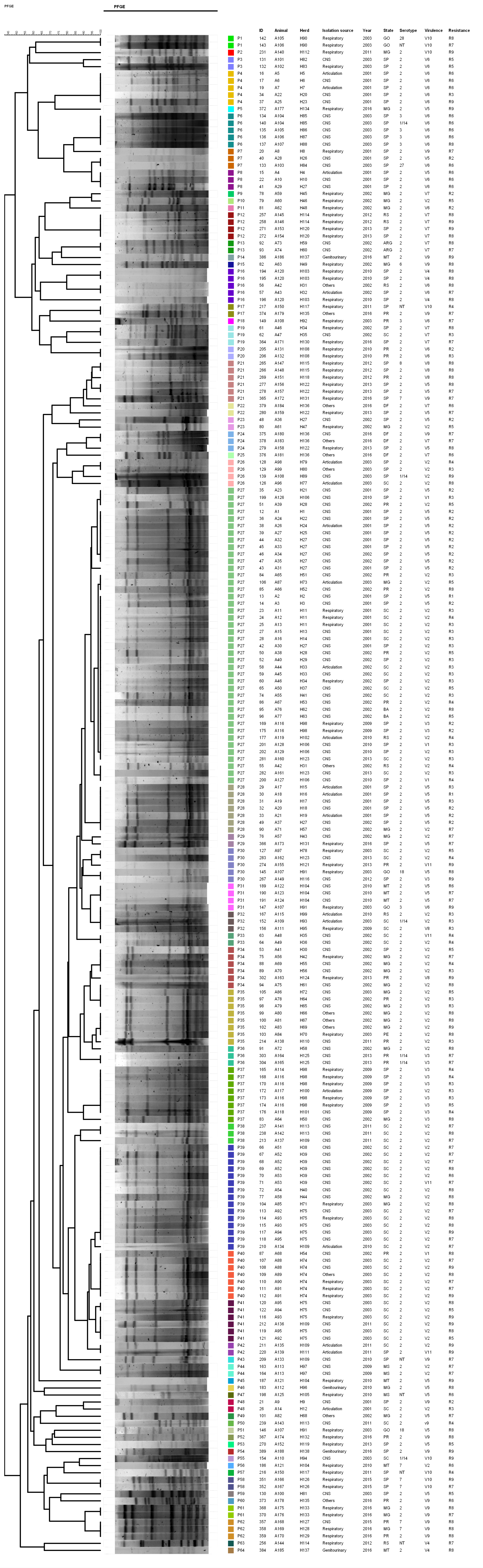
2.4. Pulsed-Field Electrophoresis Analysis
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains
4.2. Bacterial Reactivation
4.3. Identification by MALDI-TOF Mass Spectrometry
4.4. DNA Extraction
4.5. Molecular Serotyping
4.6. Virulence Gene Identification
4.7. Amplification and Electrophoresis
4.8. Pulsed-Field Gel Electrophoresis
4.9. Antimicrobial Resistance Profiling
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gottschalk, M.; Segura, M. The pathogenesis of the meningitis caused by Streptococcus suis: The unresolved questions. Vet. Microbiol. 2000, 86, 259–272. [Google Scholar] [CrossRef]
- Goyette-Desjardins, G.; Auger, J.P.; Xu, J.; Segura, M.; Gottschalk, M. Streptococcus suis, an important pig pathogen and emerging zoonotic agent-an update on the worldwide distribution based on serotyping and sequence typing. Emerg. Microbes Infect. 2014, 3, e45. [Google Scholar] [CrossRef]
- Huong, V.T.L.; Ha, N.; Huy, N.T.; Horby, P.; Nghia, H.D.T.; Thiem, V.D.; Zhu, X.; Hoa, N.T.; Hien, T.T.; Zamora, J.; et al. Epidemiology, clinical manifestations, and outcomes of Streptococcus suis infection in humans. Emerg. Infect. Dis. 2014, 20, 1105–1114. [Google Scholar]
- Soares, T.C.S.; Gottschalk, M.; Lacouture, S.; Megid, J.; Ribolla, P.E.M.; Pantoja, J.C.D.; Paes, A.C. Streptococcus suis in employees and the environment of swine slaughterhouses in São Paulo, Brazil: Occurrence, risk factors, serotype distribution, and antimicrobial susceptibility. Can. J. Vet. Res. 2015, 79, 279–284. [Google Scholar] [PubMed]
- Higgins, R.; Gottschalk, M.; Beaudoin, M.; Rawluk, S.A. Distribution of Streptococcus suis capsular types in Quebec and western Canada. Can. Vet. J. 1992, 33, 27–30. [Google Scholar] [PubMed]
- Hill, J.E.; Gottschalk, M.; Brousseau, R.; Harel, J.; Hemmingsen, S.M.; Goh, S.H. Biochemical analysis, cpn60 and 16S rDNA sequence data indicate that Streptococcus suis serotypes 32 and 34, isolated from pigs, are Streptococcus orisratti. Vet. Microbiol. 2005, 107, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Tien, L.H.T.; Nishibori, T.; Nishitani, Y.; Nomoto, R.; Osawa, R. Reappraisal of the taxonomy of Streptococcus suis serotypes 20, 22, 26, and 33 based on DNA-DNA homology and sodA and recN phylogenies. Vet. Microbiol. 2013, 162, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Yongkiettrakul, S.; Maneerat, K.; Arechanajan, B.; Malila, Y.; Srimanote, P.; Gottschalk, M.; Visessanguan, W. Antimicrobial susceptibility of Streptococcus suis isolated from diseased pigs, asymptomatic pigs, and human patients in Thailand. BMC Vet. Res. 2019, 15, 5. [Google Scholar] [CrossRef] [PubMed]
- Gruening, P.; Fulde, M.; Valentin-Weigand, P.; Goethe, R. Structure, regulation, and putative function of the arginine deiminase system of Streptococcus suis. J. Bacteriol. 2006, 188, 361–369. [Google Scholar] [CrossRef]
- Hernandez-Garcia, J.; Wang, J.; Restif, O.; Holmes, M.A.; Mather, A.E.; Weinert, L.A.; Wileman, T.M.; Thomson, J.R.; Langford, P.R.; Wren, B.W.; et al. Patterns of antimicrobial resistance in Streptococcus suis isolates from pigs with or without streptococcal disease in England between 2009 and 2014. Vet. Microbiol. 2017, 207, 117–124. [Google Scholar] [CrossRef]
- Okura, M.; Lachance, C.; Osaki, M.; Sekizaki, T.; Maruyama, F.; Nozawa, T.; Nakagawa, I.; Hamada, S.; Rossignol, C.; Gottschalk, M.; et al. Development of a two-step multiplex PCR assay for typing of capsular polysaccharide synthesis gene clusters of Streptococcus suis. J. Clin. Microbiol. 2014, 52, 1714–1719. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.M.G.; Baums, C.G.; Rehm, T.; Wisselink, H.J.; Goethe, R.; Valentin-Weigand, P. Virulence-associated gene profiling of Streptococcus suis isolates by PCR. Vet. Microbiol. 2006, 209, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, S.; Silley, P.; Simjee, S.; Woodford, N.; van Duijkeren, E.; Johnson, A.P.; Gaastra, W. Editorial: Assessing the antimicrobial susceptibility of bacteria obtained from animals. J. Antimicrob. Chemother. 2010, 65, 601–604. [Google Scholar] [CrossRef] [PubMed]
- Fittipaldi, N.; Segura, M.; Grenier, D.; Gottschalk, M. Virulence factors involved in the pathogenesis of the infection caused by the swine pathogen and zoonotic agent Streptococcus suis. Future Microbiol. 2012, 7, 259–279. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhang, H.; Wu, Z.; Wang, S.; Cao, M.; Hu, D.; Wang, C. Streptococcus suis infection: An emerging/reemerging challenge of bacterial infectious diseases? Virulence 2014, 5, 477–497. [Google Scholar] [CrossRef] [PubMed]
- Martinez, G.; Pestana de Castro, A.F.; Ribeiro Pagnani, K.J.; Nakazato, G.; Dias da Silveira, W.; Gottschalk, M. Clonal distribution of an atypical MRP+, EF* and suilysin+ phenotype of virulent Streptococcus suis serotype 2 strains in Brazil. Can. J. Vet. Res. 2003, 67, 52–55. [Google Scholar] [PubMed]
- Costa, A.T.R.; Lobato, F.C.F.; Abreu, V.L.V.; Assis, R.A.; Reis, R.; Uzal, F.A. Serotyping and evaluation of the virulence in mice of Streptococcus suis strains isolated from diseased pigs. Rev. Inst. Med. Trop. Sao Paulo 2005, 47, 113–115. [Google Scholar] [CrossRef]
- Rocha, D.L.; Santos, L.F.; Santos, D.L.; Costa, W.M.T.; Santos, J.L. Sorotipos de Streptococcus suis identificados em suínos com meningite no estado do Paraná. Arq. Bras. Med. Vet. Zootec. 2012, 30, 457–462. [Google Scholar] [CrossRef][Green Version]
- Calderaro, F.F.; Moreno, L.Z.; Doto, D.S.; Matajira, C.E.C.; Gomes, V.T.M.; Ferreira, T.S.P.; Mesquita, R.E.; Moreno, A.M. Characterization of Streptococcus suis through serotyping, SE-AFLP and virulence profile. Pesqui. Vet. Bras. 2016, 36, 701–704. [Google Scholar] [CrossRef]
- Weinert, L.A.; Chaudhuri, R.R.; Wang, J.; Peters, S.E.; Corander, J.; Jombart, T.; Baig, A.; Howell, K.J.; Vehkala, M.; Välimäki, N.; et al. Genomic signatures of human and animal disease in the zoonotic pathogen Streptococcus suis. Nat. Commun. 2015, 6, 6740. [Google Scholar] [CrossRef]
- Estrada, A.A.; Gottschalk, M.; Rossow, S.; Rendahl, A.; Gebhart, C.; Marthaler, D.G. Serotype and genotype (Multilocus Sequence Type) of Streptococcus suis isolates from the United States serve as predictors of pathotype. J. Clin. Microbiol. 2019, 57, 9. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, M.; Segura, M.; Xu, J. Streptococcus suis infections in humans: The Chinese experience and the situation in North America. Anim. Health Res. Rev. 2007, 8, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Han, K.; Oh, Y.; Kim, C.H.; Kang, I.; Lee, J.; Gottschalk, M.; Chae, C. Distribution of capsular serotypes and virulence markers of Streptococcus suis isolated from pigs with polyserositis in Korea. Can. J. Vet. Res. 2010, 74, 314–316. [Google Scholar] [PubMed]
- Gottschalk, M.; Lebrun, A.; Wisselink, H.; Dubreuil, J.D.; Smith, H.; Vecht, U. Production of virulence-related proteins by Canadian strains of Streptococcus suis capsular type 2. Can. J. Vet. Res. 1998, 62, 75–79. [Google Scholar]
- Zheng, H.; Qiu, X.; Roy, D.; Segura, M.; Du, P.; Xu, J.; Gottschalk, M. Genotyping and investigating capsular polysaccharide synthesis gene loci of non-serotypeable Streptococcus suis isolated from diseased pigs in Canada. Vet. Res. 2017, 48, 10. [Google Scholar] [CrossRef]
- Maneerat, K.; Yongkiettrakul, S.; Jiemsup, S.; Tongtawe, P.; Gottschalk, M.; Srimanote, P. Expression and characterization of serotype 2 Streptococcus suis Arginine Deiminase. J. Mol. Microbiol. Biotechnol. 2017, 184, 768–776. [Google Scholar] [CrossRef]
- Maneerat, K.; Yongkiettrakul, S.; Kramomtong, I.; Tongtawe, P.; Tapchaisri, P.; Luangsuk, P.; Chaicumpa, W.; Gottschalk, M.; Srimanote, P. Virulence genes and genetic diversity of Streptococcus suis serotype 2 isolates from Thailand. Transbound. Emerg. Dis. 2013, 60, 69–79. [Google Scholar] [CrossRef]
- Unterweger, C.; Ruczizka, U.; Spergser, J.; Baums, C.G.; Hennig-Pauka, I. Effect of early-life treatment of piglets with long-acting ceftiofur on colonization of Streptococcus suis serotype 7 and elicitation of specific humoral immunity in a farm dealing with streptococcal diseases. Pathogens 2018, 7, 34. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Z.; Song, L.; Fan, X.; Wen, F.; Xu, S.; Ning, Y. Antimicrobial resistance profile and genotypic characteristics of Streptococcus suis capsular type 2 isolated from clinical carrier sows and diseased pigs in China. BioMed Res. Int. 2015, 2015, 284303. [Google Scholar]
- Zhang, C.; Ning, Y.; Zhang, Z.; Song, L.; Qiu, H.; Gao, H. In vitro antimicrobial susceptibility of Streptococcus suis strains isolated from clinically healthy sows in China. Vet. Microbiol. 2008, 131, 386–389. [Google Scholar] [CrossRef]
- Thi Hoang Mai, N.; Thi Hoa, N.; Vu Thieu Nga, T.; Dieu Linh, L.; Thi Hong Chau, T.; Xuan Sinh, D.; Hoan Phu, N.; Van Chuong, L.; Song Diep, T.; Campbell, J.; et al. Streptococcus suis meningitis in adults in Vietnam. Clin. Infect. Dis. 2008, 46, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Tharavichitkul, P.; Wongsawan, K.; Takenami, N.; Pruksakorn, S.; Fongcom, A.; Gottschalk, M.; Khanthawa, B.; Supajatura, V.; Takai, S. Correlation between PFGE groups and mrp/epf/sly genotypes of human Streptococcus suis serotype 2 in Northern Thailand. J. Pathog. 2014, 2014, 350416. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Ma, J.; Shang, K.; Hu, X.; Liang, Y.; Li, D.; Wu, Z.; Dai, L.; Chen, L.; Wang, L. Evolution and diversity of the antimicrobial resistance associated mobilome in Streptococcus suis: A probable mobile genetic elements reservoir for other streptococci. Front. Cell. Infect. Microbiol. 2016, 6, 118. [Google Scholar] [CrossRef] [PubMed]
- Sánchez del Rey, V.; Fernández-Garayzábal, J.F.; Briones, V.; Iriso, A.; Domínguez, L.; Gottschalk, M.; Vela, A.I. Genetic analysis of streptococcus suis isolates from wild rabbits. Vet. Microbiol. 2013, 165, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Berthelot-Hérault, F.; Marois, C.; Gottschalk, M.; Kobisch, M. Genetic diversity of Streptococcus suis strains isolated from pigs and humans as revealed by pulsed-field gel electrophoresis. J. Clin. Microbiol. 2002, 40, 615–619. [Google Scholar] [CrossRef]
- Vela, A.I.; Goyache, J.; Tarradas, C.; Luque, I.; Mateos, A.; Moreno, M.A.; Borge, C.; Perea, J.A.; Domínguez, L.; Fernández-Garayzábal, J.F. Analysis of genetic diversity of Streptococcus suis clinical isolates from pigs in Spain by pulsed-field gel electrophoresis. J. Clin. Microbiol. 2003, 41, 2498–2502. [Google Scholar] [CrossRef]
- Hijazin, M.; Hassan, A.A.; Alber, J.; Lämmler, C.; Timke, M.; Kostrzewa, M.; Prenger-Berninghoff, E.; Zschöck, M. Evaluation of matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) for species identification of bacteria of genera Arcanobacterium and Trueperella. Vet. Microbiol. 2012, 157, 243–245. [Google Scholar] [CrossRef]
- Boom, R.; Sol, C.J.A.; Salimans, M.M.M.; Jansen, C.L.; Wertheim-Van Dillen, P.M.E.; Van Der Noordaa, J. Rapid and simple method for purification of nucleic acids. J. Clin. Microbiol. 1990, 28, 495–503. [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals, 5th ed.; CLSI standard VET01; CLSI: Wayne, PA, USA, 2018. [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals, 4th ed.; CLSI supplement VET08; CLSI: Wayne, PA, USA, 2018. [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, 28th ed.; CLSI supplement M100; CLSI: Wayne, PA, USA, 2018. [Google Scholar]
- Van Belkum, A.; Tassios, P.T.; Dijkshoorn, L.; Haeggman, S.; Cookson, B.; Fry, N.K.; Fussing, V.; Green, J.; Feil, E.; Gerner-smidt, P.; et al. Guidelines for the validation and application of typing methods for use in bacterial epidemiology. Clin. Microbiol. Infect. 2007, 13, 1–46. [Google Scholar] [CrossRef]
| Serotype | Isolation Site | Total | ||||
|---|---|---|---|---|---|---|
| Joints | Genitourinary System | Other Sites | Respiratory | CNS | ||
| 2/½ | 19 (95.2) | 4 (100) | 13 (100) | 55 (76.3) | 94 (88.6) | 185 (86) |
| 3 | 0 | 0 | 0 | 2 (2.9) | 4 (3.7) | 6 (2.8) |
| 7 | 0 | 0 | 0 | 5 (6.9) | 1 (0.9) | 6 (2.8) |
| 1/14 | 1 (4.8) | 0 | 0 | 0 | 5 (4.6) | 6 (2.8) |
| NT | 0 | 0 | 0 | 5 (6.9) | 1 (0.9) | 6 (2.8) |
| 18 | 0 | 0 | 0 | 2 (2.9) | 0 | 2 (0.9) |
| 27 | 0 | 0 | 0 | 0 | 1 (0.9) | 1 (0.5) |
| 28 | 0 | 0 | 0 | 1 (1.5) | 0 | 1 (0.5) |
| 6 | 0 | 0 | 0 | 1 (1.5) | 0 | 1 (0.5) |
| 8 | 0 | 0 | 0 | 1 (1.5) | 0 | 1 (0.5) |
| Total | 20 (100) | 4 (100) | 13 (100) | 72 (100) | 106 (100) | 215 (100) |
| Profile | Virulence Genes | Frequency |
|---|---|---|
| V1 | sly+/arcA+/epf+/mrpV1 | 4 (1.9) |
| V2 | sly+/arcA+/epf+/mrpV2 | 86 (40.0) |
| V3 | sly+/arcA+/epf+/mrpV3 | 13 (6.0) |
| V4 | sly+/arcA+/epf-/mrpV1 | 5 (2.3) |
| V5 | sly+/arcA+/epf-/mrpV2 | 38 (17.7) |
| V6 | sly-/arcA+/epf-/mrpV1 | 22 (10.2) |
| V7 | sly-/arcA+/epf-/mrpV2 | 13 (6.0) |
| V8 | sly+/arcA+/epf+/mrp- | 5 (2.3) |
| V9 | sly+/arcA+/epf-/mrp- | 17 (7.9) |
| V10 | sly-/arcA+/epf-/mrp- | 8 (3.7) |
| V11 | sly-/arcA-/epf-/mrp- | 4 (1.9) |
| Profile | Isolation Site | Total | ||||
|---|---|---|---|---|---|---|
| Joints | Genitourinary System | Other Sites | Respiratory System | CNS | ||
| V1 | 0 | 0 | 0 | 0 | 4 (100) | 4 (100) |
| V2 | 11 (12.8) | 0 | 6 (7.0) | 16 (18.6) | 53 (61.6) | 86 (100) |
| V3 | 0 (0) | 0 | 0 | 7 (53.8) | 6 (46.2) | 13 (100) |
| V4 | 0 | 1 (20.0) | 0 | 4 (80.0) | 0 | 5 (100) |
| V5 | 5 (13.2) | 1 (2.6) | 1 (2.6) | 11 (28.9) | 20 (52.6) | 38 (100) |
| V6 | 3 (13.6) | 0 | 1 (4.5) | 6 (27.3) | 12 (54.5) | 22 (100) |
| V7 | 0 | 0 | 3 (23.1) | 7 (53.8) | 3 (23.1) | 13 (100) |
| V8 | 0 | 0 | 0 | 5 (100) | 0 | 5 (100) |
| V9 | 0 | 2 (11.8) | 2 (11.8) | 8 (47.1) | 5 (29.4) | 17 (100) |
| V10 | 0 | 0 | 0 | 7 (87.5) | 1 (12.5) | 8 (100) |
| V11 | 1 (25.0) | 0 | 0 | 1 (25.0) | 2 (50.0) | 4 (100) |
| Antimicrobial | MIC Range (µg/mL) 1 | MIC50 (µg/mL) | MIC90 (µg/mL) | Resistance N (%) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | 512 | ||||
| Ampicillin | 0 | 0 | 214 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.25 | 0.25 | 1 (0.5) |
| Ceftiofur | 0 | 0 | 212 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.25 | 0.25 | 0 |
| Penicillin | 0 | 209 | 3 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.12 | 0.12 | 3 (1.4) |
| Doxycycline | 0 | 0 | 0 | 49 | 7 | 20 | 45 | 69 | 25 | 0 | 0 | 0 | 0 | 0 | 4.0 | >16.0 | 159 (74.0) |
| Oxytetracycline | 0 | 0 | 0 | 42 | 5 | 7 | 22 | 30 | 109 | 0 | 0 | 0 | 0 | 0 | >16.0 | >16.0 | 168 (78.1) |
| Marbofloxacin | 3 | 5 | 13 | 79 | 93 | 13 | 1 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 1.0 | 2.0 | 9 (4.2) |
| Enrofloxacin | 0 | 17 | 72 | 91 | 24 | 3 | 0 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0.5 | 1.0 | 12 (5.6) |
| Florfenicol | 0 | 0 | 3 | 8 | 64 | 99 | 34 | 5 | 2 | 0 | 0 | 0 | 0 | 0 | 2.0 | 4.0 | 7 (3.3) |
| Spectinomycin | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 73 | 88 | 28 | 9 | 3 | 14 | 14 | 16.0 | 64.0 | 17 (7.9) |
| Gentamicin | 0 | 0 | 0 | 0 | 31 | 39 | 80 | 52 | 12 | 1 | 0 | 0 | 0 | 0 | 4.0 | 8.0 | 65 (30.2) |
| Neomycin | 0 | 0 | 0 | 0 | 0 | 0 | 68 | 41 | 47 | 37 | 22 | 0 | 0 | 0 | 8.0 | >64.0 | 106 (49.3) |
| Clindamycin | 0 | 0 | 88 | 2 | 1 | 4 | 2 | 7 | 111 | 0 | 0 | 0 | 0 | 0 | >16.0 | >16.0 | 120 (55.8) |
| Tylosin | 0 | 0 | 0 | 73 | 11 | 4 | 0 | 4 | 7 | 6 | 110 | 0 | 0 | 0 | >64.0 | >64.0 | 126 (58.6) |
| Tilmicosin | 0 | 0 | 0 | 0 | 0 | 0 | 41 | 21 | 10 | 13 | 11 | 119 | 0 | 0 | >128.0 | >128.0 | 143 (66.5) |
| Tulathromycin | 0 | 0 | 0 | 0 | 57 | 9 | 8 | 6 | 5 | 9 | 7 | 113 | 0 | 0 | >128.0 | >128.0 | 120 (55.8) |
| Tiamulin | 0 | 0 | 0 | 122 | 33 | 31 | 11 | 8 | 6 | 3 | 1 | 0 | 0 | 0 | 0.5 | 4.0 | 4 (1.9) |
| Sulfamethoxazole | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 59 | 0 | 156 | 512 | 512 | 156 (60.9) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matajira, C.E.C.; Moreno, L.Z.; Poor, A.P.; Gomes, V.T.M.; Dalmutt, A.C.; Parra, B.M.; Oliveira, C.H.d.; Barbosa, M.R.F.; Sato, M.I.Z.; Calderaro, F.F.; et al. Streptococcus suis in Brazil: Genotypic, Virulence, and Resistance Profiling of Strains Isolated from Pigs between 2001 and 2016. Pathogens 2020, 9, 31. https://doi.org/10.3390/pathogens9010031
Matajira CEC, Moreno LZ, Poor AP, Gomes VTM, Dalmutt AC, Parra BM, Oliveira CHd, Barbosa MRF, Sato MIZ, Calderaro FF, et al. Streptococcus suis in Brazil: Genotypic, Virulence, and Resistance Profiling of Strains Isolated from Pigs between 2001 and 2016. Pathogens. 2020; 9(1):31. https://doi.org/10.3390/pathogens9010031
Chicago/Turabian StyleMatajira, Carlos E. C., Luisa Z. Moreno, Andre P. Poor, Vasco T. M. Gomes, Andressa C. Dalmutt, Beatriz M. Parra, Carolina H. de Oliveira, Mikaela R. F. Barbosa, Maria Inês Z. Sato, Franco F. Calderaro, and et al. 2020. "Streptococcus suis in Brazil: Genotypic, Virulence, and Resistance Profiling of Strains Isolated from Pigs between 2001 and 2016" Pathogens 9, no. 1: 31. https://doi.org/10.3390/pathogens9010031
APA StyleMatajira, C. E. C., Moreno, L. Z., Poor, A. P., Gomes, V. T. M., Dalmutt, A. C., Parra, B. M., Oliveira, C. H. d., Barbosa, M. R. F., Sato, M. I. Z., Calderaro, F. F., & Moreno, A. M. (2020). Streptococcus suis in Brazil: Genotypic, Virulence, and Resistance Profiling of Strains Isolated from Pigs between 2001 and 2016. Pathogens, 9(1), 31. https://doi.org/10.3390/pathogens9010031







