Immunohistochemical Expression Patterns of Inflammatory Cells Involved in Chronic Hyperplastic Candidosis
Abstract
1. Introduction
2. Results
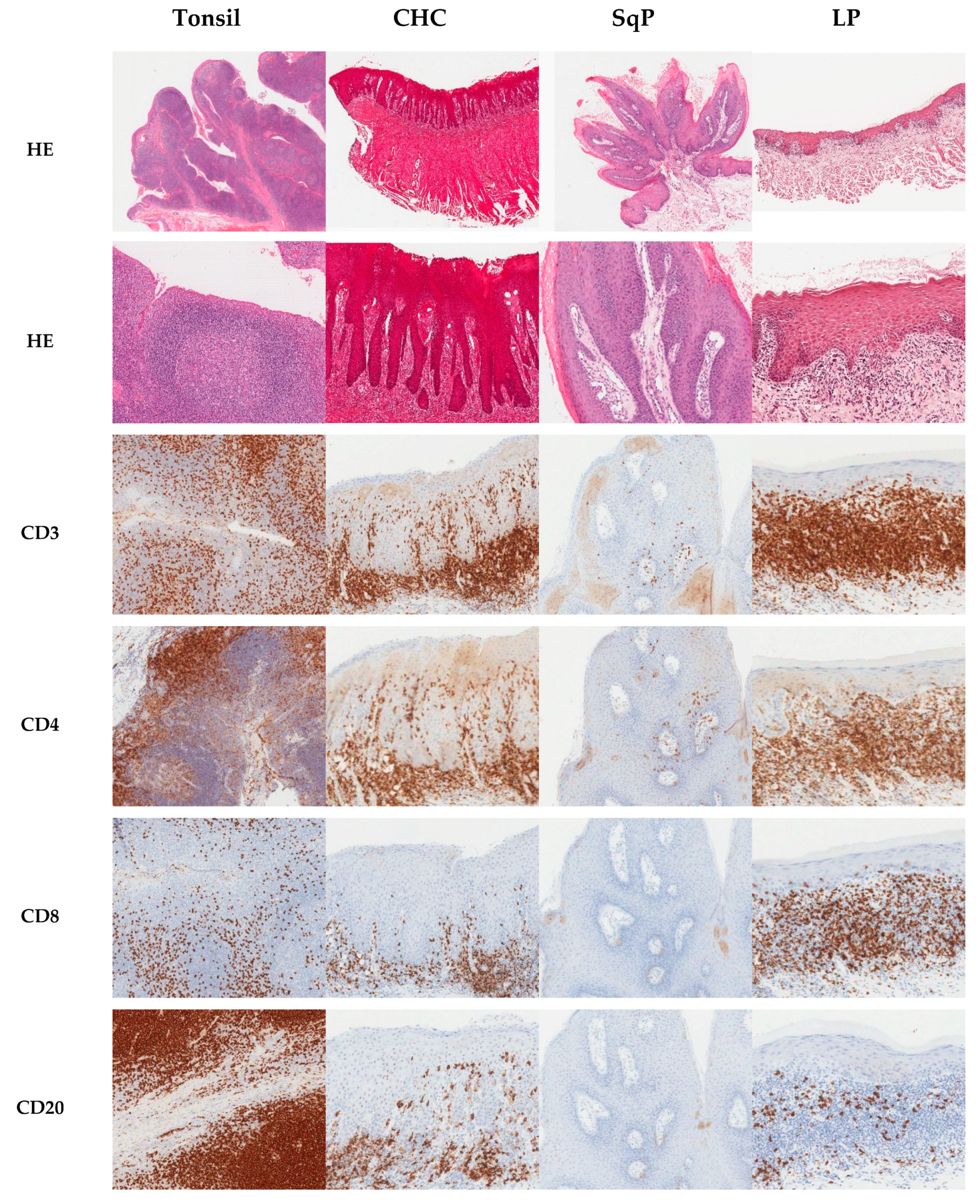
2.1. Immunohistochemical Analysis
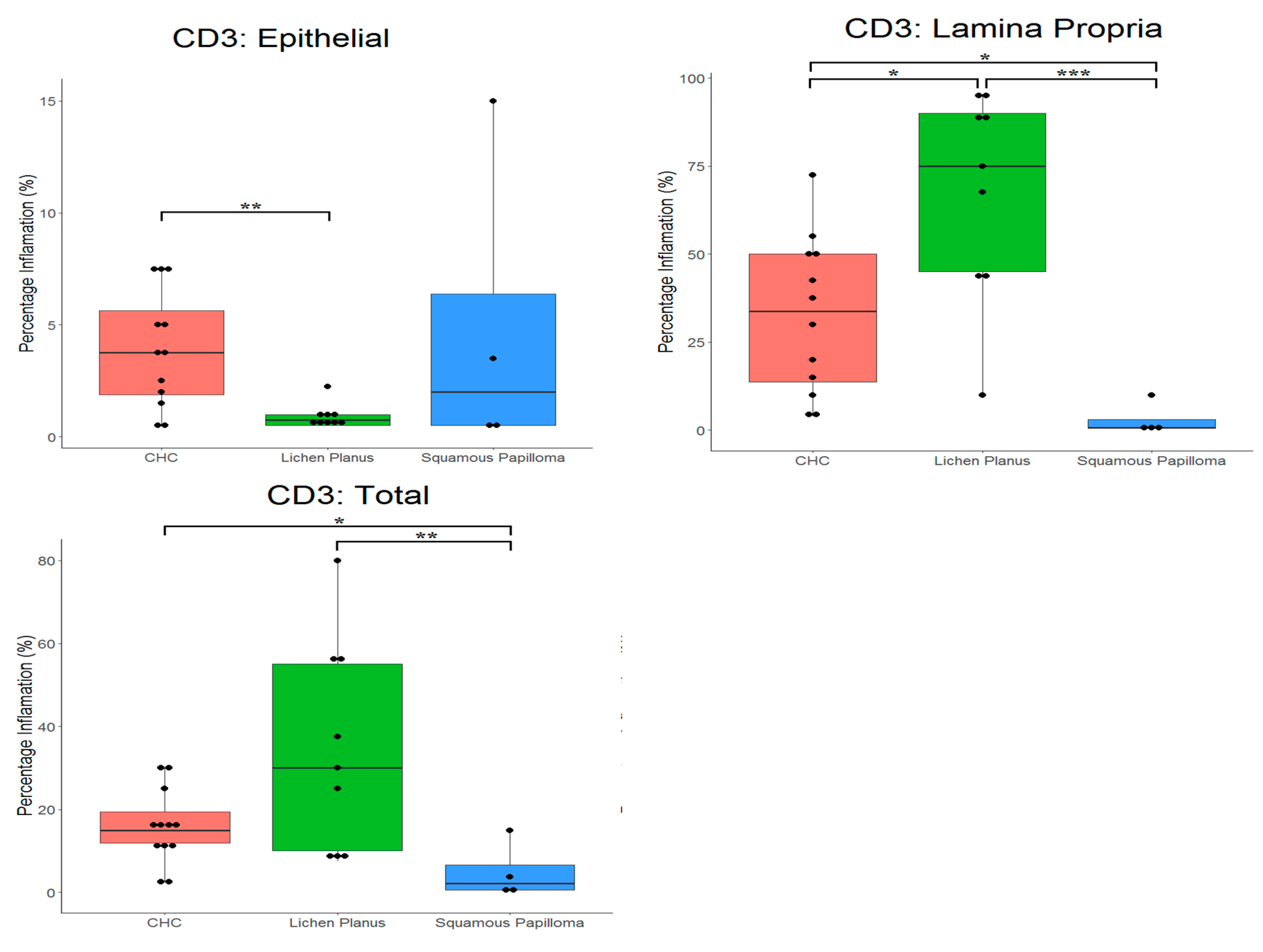
2.2. Proportion of CD3+ and CD20+ Cells in Tissues
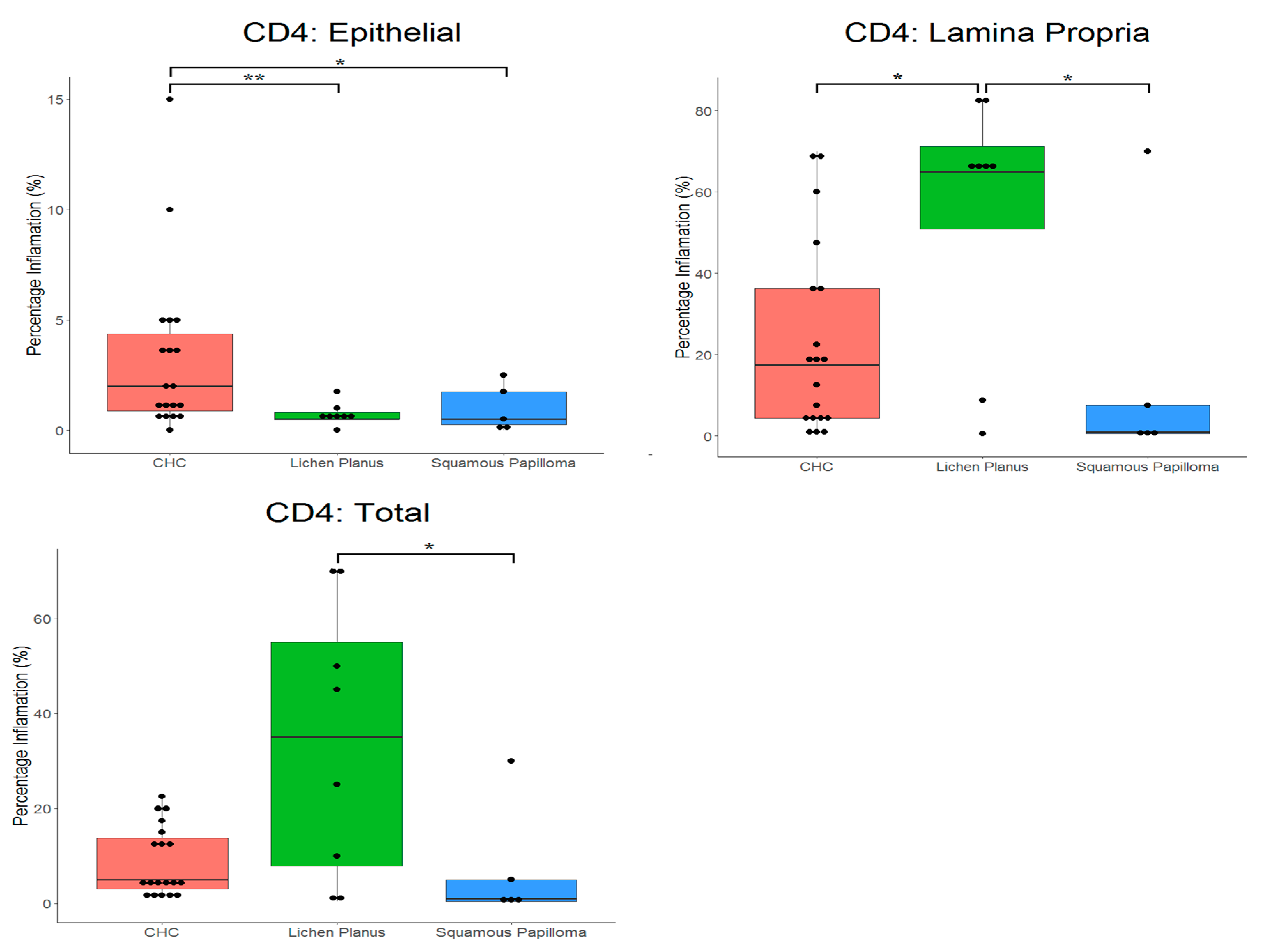
2.3. Proportion of CD4+ and CD8+ Cells in Tissues
3. Discussion
4. Materials and Methods
4.1. Tissue Samples
4.2. Immunohistochemistry
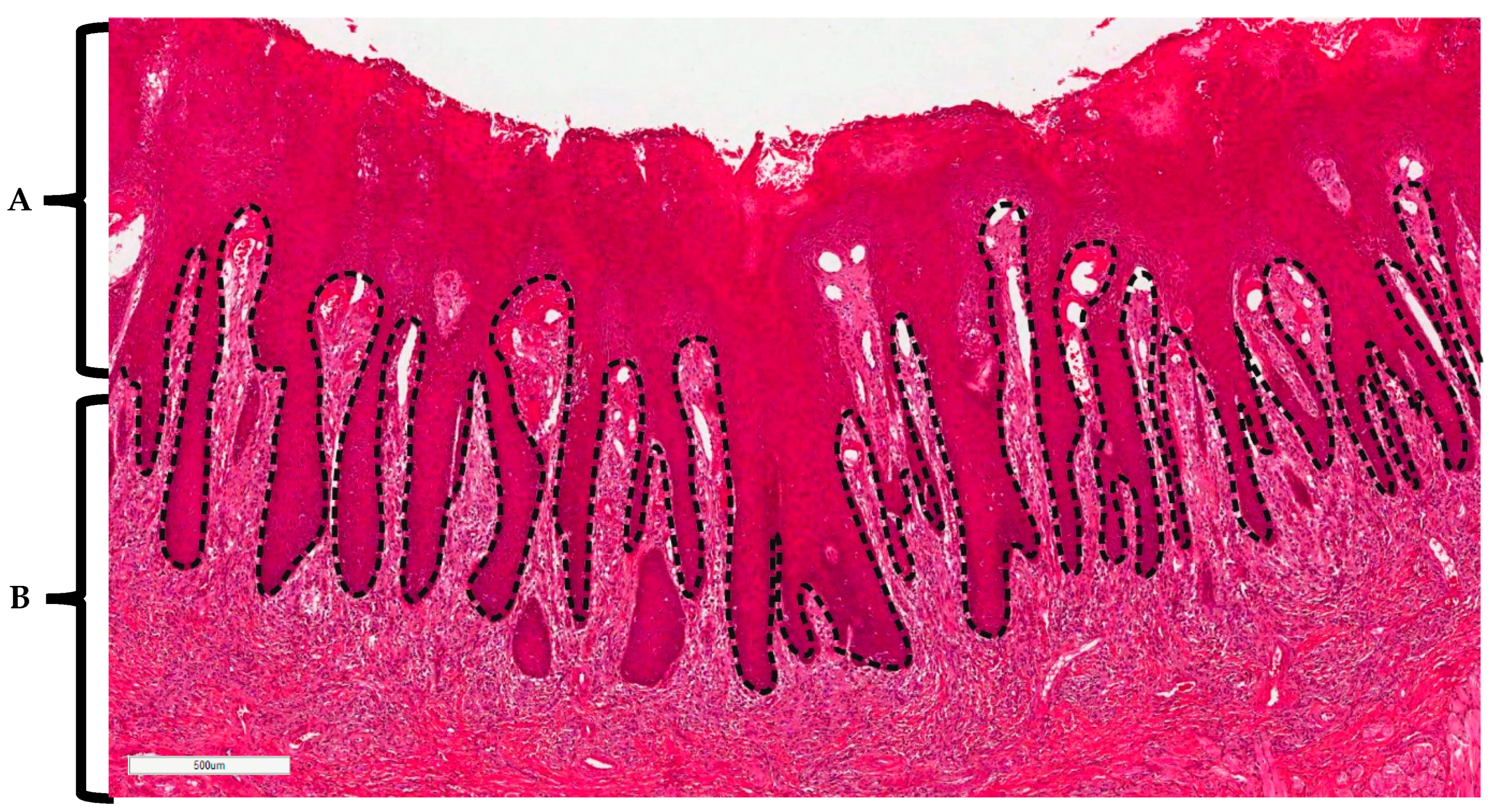
4.3. Histopathological Analysis of Stained Tissues
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tsui, C.; Kong, E.F.; Jabra-Rizk, M.A. Pathogenesis of Candida albicans biofilm. Pathog. Dis. 2016, 74, ftw018. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.; Lewis, M. Pathogenesis and treatment of oral candidosis. J. Oral Microbiol. 2011, 3. [Google Scholar] [CrossRef] [PubMed]
- Millsop, J.; Fazel, N. Oral candidiasis. Clin. Dermatol. 2016, 34, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Frimpong, P.; Amponsah, E.; Abebrese, J.; Kim, S. Oral manifestations and their correlation to baseline CD4 count of HIV/AIDS patients in Ghana. J. Korean Assoc. Oral Maxillofac. Surg. 2017, 43, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Bakri, M.M.; Hussaini, H.M.; Holmes, R.A.; Cannon, D.R.; Rich, M.A. Revisiting the association between candidal infection and carcinoma, particularly oral squamous cell carcinoma. J. Oral Microbiol. 2010, 21, 2. [Google Scholar]
- Sitheeque, M.; Samaranayake, L. Chronic hyperplastic candidosis/candidiasis (candidal leukoplakia). Crit. Rev. Oral Biol. Med. 2003, 14, 253–267. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.; Potts, A.; Wilson, M.; Matthews, J.; Lewis, M. Characterisation of the inflammatory cell infiltrate in chronic hyperplastic candidosis of the oral mucosa. J. Oral Pathol. Med. 1997, 26, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Joosten, L.A.; van der Meer, J.W.; Kullberg, B.J.; van de Veerdonk, F.L. Immune defence against Candida fungal infections. Nat Rev. Immunol. 2015, 15, 630–642. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.P.; Moyes, D.L. Adaptive immune responses to Candida albicans infection. Virulence 2015, 6, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Trautwein-Weidner, K.; Gladiator, A.; Kirchner, F.; Becattini, S.; Rülicke, T.; Sallusto, F.; LeibundGut-Landmann, S. Antigen-specific Th17 cells are primed by distinct and complementary dendritic cell subsets in oropharyngeal candidiasis. PLoS Pathog. 2015, 11, e1005164. [Google Scholar] [CrossRef] [PubMed]
- Swidergall, M.; Solis, N.V.; Lionakis, M.S.; Filler, S.G. EphA2 is an epithelial cell pattern recognition receptor for fungal β-glucans. Nat. Microbiol. 2018, 3, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Moyes, D.L.; Runglall, M.; Murciano, C.; Shen, C.; Nayar, D.; Thavaraj, S.; Kohli, A.; Islam, A.; Mora-Montes, H.; Challacombe, S.J.; et al. A biphasic innate immune MAPK response discriminates between the yeast and hyphal forms of Candida albicans in epithelial cells. Cell Host Microbe 2010, 8, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Sekelova, Z.; Polansky, O.; Stepanova, H.; Fedr, R.; Faldynova, M.; Rychlik, I.; Vlasatikova, L. Different roles of CD4, CD8 and γδ T-lymphocytes in naive and vaccinated chickens during Salmonella enteritidis infection. Proteomics 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Conti, H.; Gaffen, S. Host responses to Candida albicans: Th17 cells and mucosal candidiasis. Microbes Infect. 2010, 12, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Levitz, S.M. Overview of host defenses in fungal infections. Clin. Infect. Dis. 1992, 14 (Suppl. 1), S37–S42. [Google Scholar] [CrossRef] [PubMed]
- Burnie, M.R.J. Antifungal antibodies: A new approach to the treatment of systemic candidiasis. Curr. Opin. Investig. Drugs 2001, 2, 472–476. [Google Scholar]
- Lavanya, N.; Rao, U.; Jayanthi, P.; Ranganathan, K. Oral lichen planus: An update on pathogenesis and treatment. J. Oral Maxillofac. Pathol. 2011, 15, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Barrett, A.; Kingsmill, V.; Speight, P. The frequency of fungal infection in biopsies of oral mucosal lesions. Oral Dis. 1998, 4, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Myers, T.; Leigh, J.; Arribas, A.; Hager, S.; Clark, R.; Lilly, E.; Fidel, P.L., Jr. Immunohistochemical evaluation of T cells in oral lesions from human immunodeficiency virus-positive persons with oropharyngeal candidiasis. Infect. Immun. 2003, 71, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Naglik, J.; Fidel, P.; Odds, F. Animal models of mucosal Candida infection. FEMS Microbiol. Lett. 2008, 283, 129–139. [Google Scholar] [CrossRef] [PubMed]


| CHC (n = 20) | LP (n = 10) | SqP (n = 4) | ||
|---|---|---|---|---|
| Epithelium | Mean | 3.9 | 0.9 | 4.9 |
| Median | 3.8 | 0.8 | 2.0 | |
| SD | 2.6 | 0.6 | 6.9 | |
| Lamina Propria | Mean | 32.6 | 67.2 | 2.9 |
| Median | 33.8 | 75.0 | 0.6 | |
| SD | 22.2 | 29.2 | 4.7 | |
| Total | Mean | 15.6 | 33.3 | 4.9 |
| Median | 15.0 | 30.0 | 2.1 | |
| SD | 9.1 | 24.1 | 6.9 | |
| CHC (n = 20) | LP (n = 10) | SqP (n = 4) | ||
|---|---|---|---|---|
| Epithelium | Mean | 0.2 | 0.1 | 0.0 |
| Median | 0.0 | 0.0 | 0.0 | |
| SD | 0.6 | 0.3 | 0.0 | |
| Lamina Propria | Mean | 3.6 | 6.8 | 0.6 |
| Median | 0.9 | 3.8 | 0.0 | |
| SD | 6.8 | 7.7 | 1.0 | |
| Total | Mean | 1.8 | 2.9 | 0.6 |
| Median | 0.6 | 2.5 | 0.0 | |
| SD | 2.5 | 3.1 | 1.0 | |
| CHC (n = 20) | LP (n = 10) | SqP (n = 4) | ||
|---|---|---|---|---|
| Epithelium | Mean | 3.2 | 0.7 | 1.0 |
| Median | 2.0 | 0.5 | 0.5 | |
| SD | 3.8 | 0.5 | 1.1 | |
| Lamina Propria | Mean | 23.1 | 54.6 | 15.9 |
| Median | 17.5 | 65.0 | 1.0 | |
| SD | 23.3 | 31.8 | 30.4 | |
| Total | Mean | 9.0 | 34.0 | 7.4 |
| Median | 5.0 | 35.0 | 1.0 | |
| SD | 7.2 | 28.8 | 12.8 | |
| CHC (n = 20) | LP (n = 10) | SqP (n = 4) | ||
|---|---|---|---|---|
| Epithelium | Mean | 0.6 | 1.2 | 0.4 |
| Median | 0.5 | 1.0 | 0.5 | |
| SD | 0.6 | 0.9 | 0.3 | |
| Lamina Propria | Mean | 15.0 | 54.4 | 1.1 |
| Median | 12.5 | 60.0 | 1.1 | |
| SD | 18.1 | 27.3 | 1.1 | |
| Total | Mean | 4.4 | 29.1 | 1.0 |
| Median | 2.5 | 30.0 | 0.8 | |
| SD | 4.6 | 19.5 | 1.1 | |
| Number of Each Oral Pathology Type(s) * | |||
|---|---|---|---|
| Oral site of tissue | Chronic hyperplastic candidosis | Lichen planus | Squamous papiloma |
| Buccal mucosa | 4 | 6 | |
| Tongue | 8 | 3 | 3 |
| Commisure | 7 | ||
| Lips | 1 | 1 | |
| Gingiva | 1 | ||
| Total | 20 | 10 | 4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Williams, A.; Williams, D.; Rogers, H.; Wei, X.; Lewis, M.; Wozniak, S.; Farnell, D.; Jones, A. Immunohistochemical Expression Patterns of Inflammatory Cells Involved in Chronic Hyperplastic Candidosis. Pathogens 2019, 8, 232. https://doi.org/10.3390/pathogens8040232
Williams A, Williams D, Rogers H, Wei X, Lewis M, Wozniak S, Farnell D, Jones A. Immunohistochemical Expression Patterns of Inflammatory Cells Involved in Chronic Hyperplastic Candidosis. Pathogens. 2019; 8(4):232. https://doi.org/10.3390/pathogens8040232
Chicago/Turabian StyleWilliams, Ailish, David Williams, Helen Rogers, Xiaoqing Wei, Michael Lewis, Sue Wozniak, Damian Farnell, and Adam Jones. 2019. "Immunohistochemical Expression Patterns of Inflammatory Cells Involved in Chronic Hyperplastic Candidosis" Pathogens 8, no. 4: 232. https://doi.org/10.3390/pathogens8040232
APA StyleWilliams, A., Williams, D., Rogers, H., Wei, X., Lewis, M., Wozniak, S., Farnell, D., & Jones, A. (2019). Immunohistochemical Expression Patterns of Inflammatory Cells Involved in Chronic Hyperplastic Candidosis. Pathogens, 8(4), 232. https://doi.org/10.3390/pathogens8040232





