Assessment of Immunogenicity and Efficacy of a Zika Vaccine Using Modified Vaccinia Ankara Virus as Carriers
Abstract
1. Introduction
2. Results
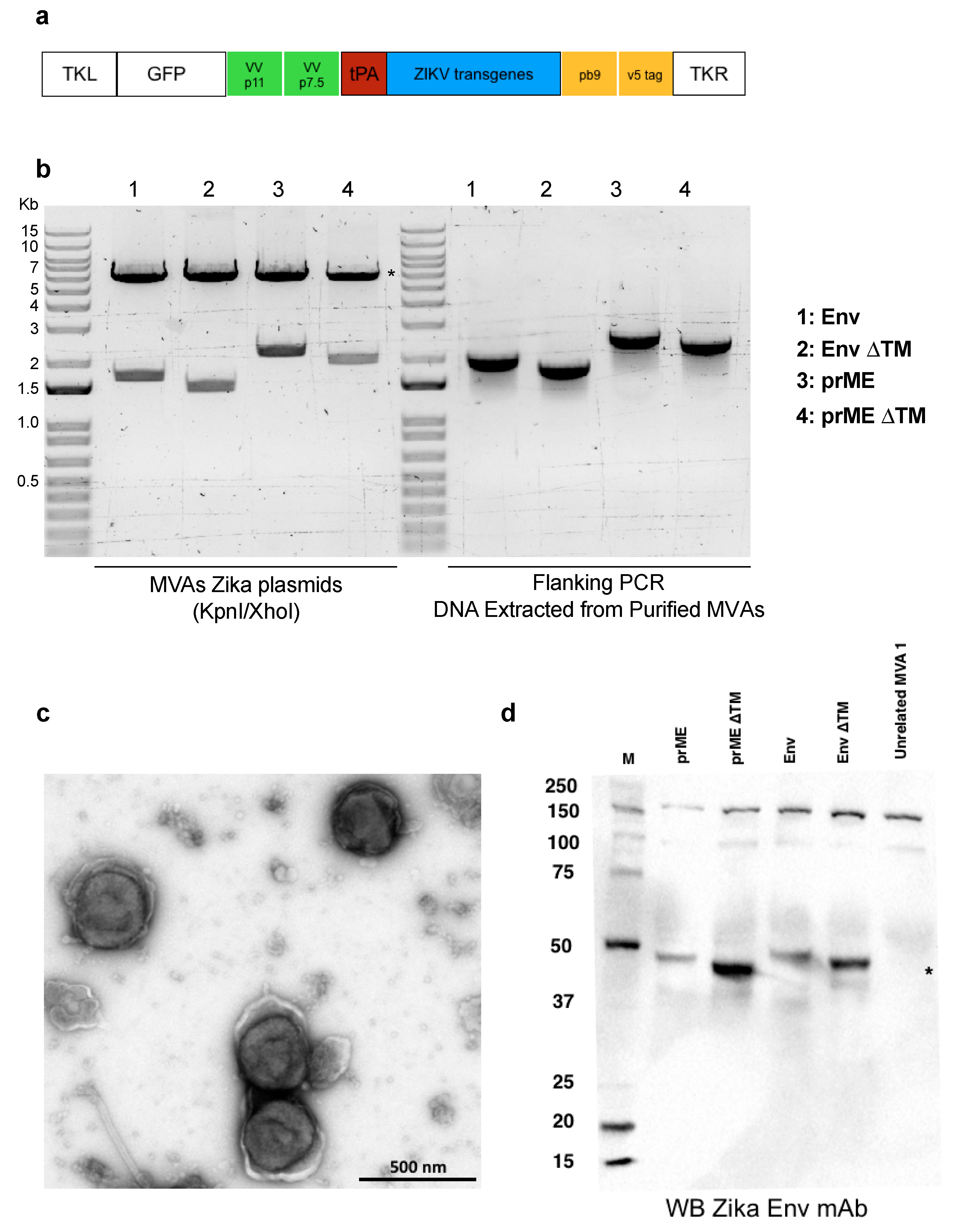
2.1. Modified Vaccinia Ankara (MVA) Expressing ZIKV Antigens
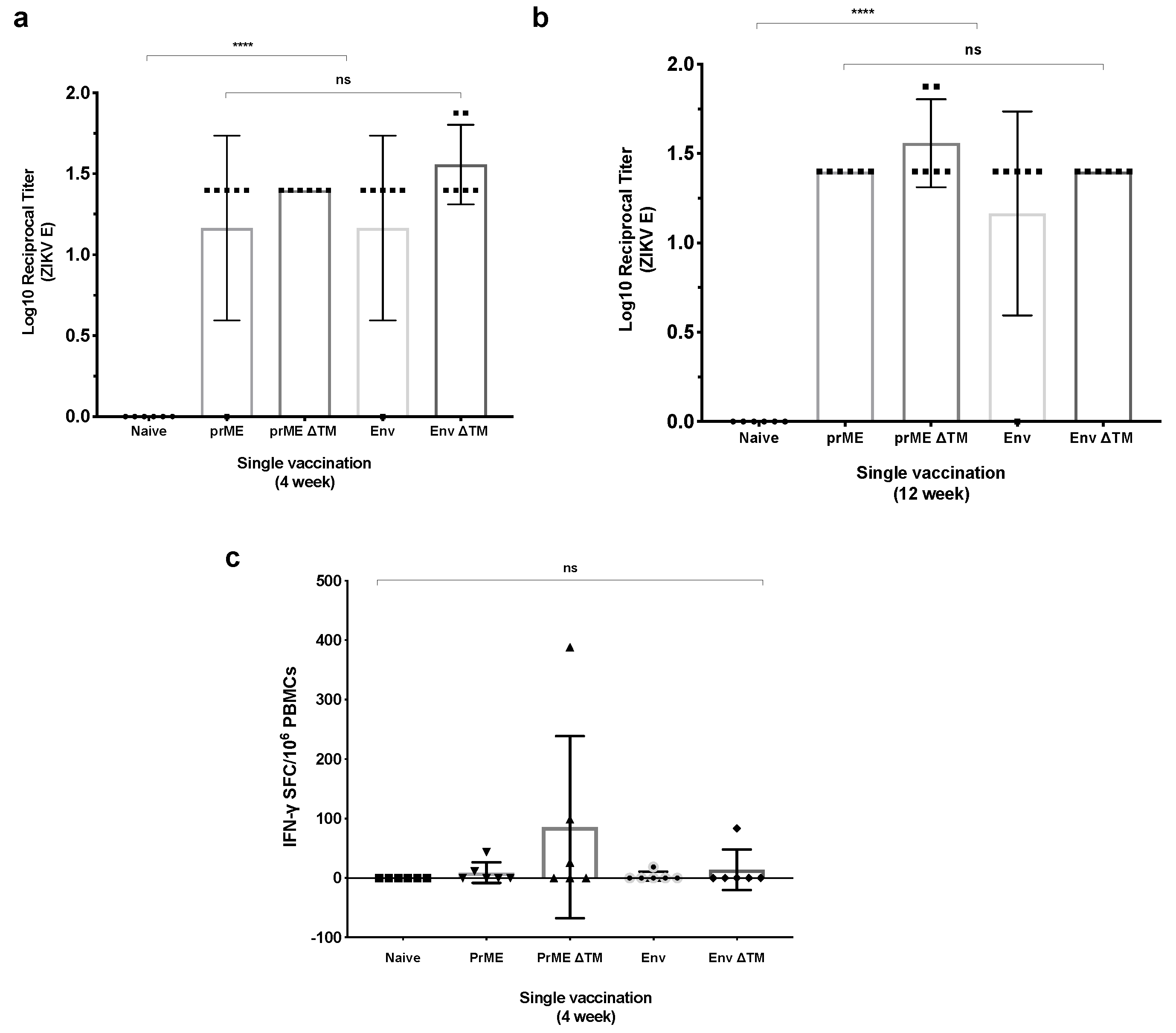
2.2. Assessment of Immunogenicity
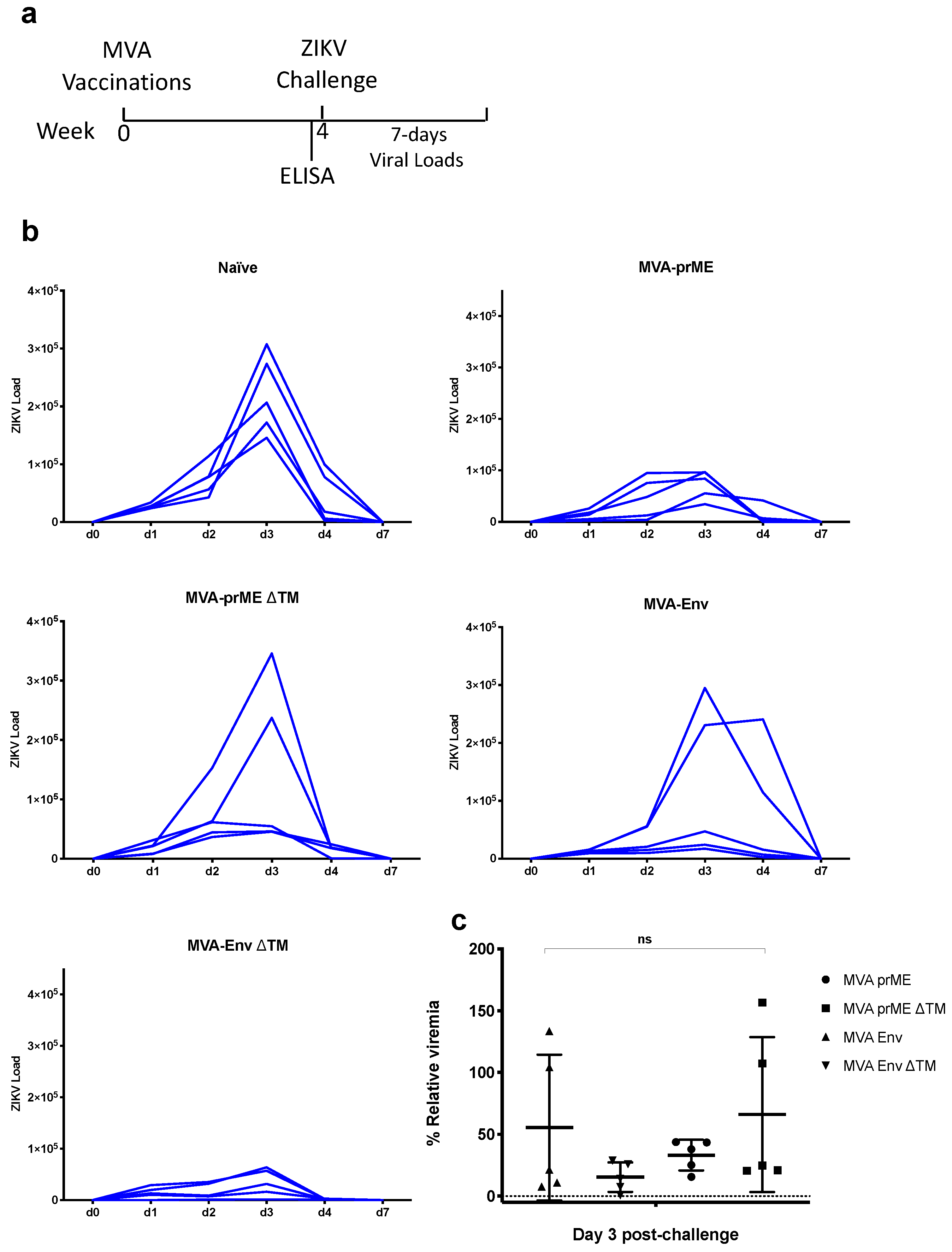
2.3. ZIKV Challenge in BALB/c Mice
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dick, G.W.A.; Kitchen, S.F.; Haddow, A.J. Zika Virus (I). Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 1952, 46, 509–520. [Google Scholar] [CrossRef]
- Duffy, M.R.; Chen, T.-H.; Hancock, W.T.; Powers, A.M.; Kool, J.L.; Lanciotti, R.S.; Pretrick, M.; Marfel, M.; Holzbauer, S.; Dubray, C.; et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N. Engl. J. Med. 2009, 360, 2536–2543. [Google Scholar] [CrossRef] [PubMed]
- Cao-Lormeau, V.-M.; Roche, C.; Teissier, A.; Robin, E.; Berry, A.-L.; Mallet, H.-P.; Sall, A.A.; Musso, D. Zika virus, French polynesia, South pacific, 2013. Emerg. Infect. Dis. 2014, 20, 1085–1086. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.A.; Jamieson, D.J.; Honein, M.A.; Petersen, L.R. Zika virus and birth defects---Reviewing the evidence for causality. N. Engl. J. Med. 2016, 374, 1981–1987. [Google Scholar] [CrossRef] [PubMed]
- WHO. Zika Situation Report. Available online: https://www.who.int/emergencies/zika-virus/situation-report/10-march-2017/en/ (accessed on 25 July 2019).
- Faye, O.; Faye, O.; Diallo, D.; Diallo, M.; Weidmann, M.; Sall, A.A. Quantitative real-time PCR detection of Zika virus and evaluation with field-caught Mosquitoes. Virol. J. 2013, 10, 311. [Google Scholar] [CrossRef]
- Petersen, L.R.; Jamieson, D.J.; Powers, A.M.; Honein, M.A. Zika Virus. N. Engl. J. Med. 2016, 374, 1552–1563. [Google Scholar] [CrossRef]
- Cauchemez, S.; Besnard, M.; Bompard, P.; Dub, T.; Guillemette-Artur, P.; Eyrolle-Guignot, D.; Salje, H.; Van Kerkhove, M.D.; Abadie, V.; Garel, C.; et al. Association between Zika virus and microcephaly in French Polynesia, 2013–2015: A retrospective study. Lancet 2016, 387, 2125–2132. [Google Scholar] [CrossRef]
- Mlakar, J.; Korva, M.; Tul, N.; Popović, M.; Poljšak-Prijatelj, M.; Mraz, J.; Kolenc, M.; Resman Rus, K.; Vesnaver Vipotnik, T.; Fabjan Vodušek, V.; et al. Zika Virus Associated with Microcephaly. N. Engl. J. Med. 2016, 374, 951–958. [Google Scholar] [CrossRef]
- Cao-Lormeau, V.-M.; Blake, A.; Mons, S.; Lastère, S.; Roche, C.; Vanhomwegen, J.; Dub, T.; Baudouin, L.; Teissier, A.; Larre, P.; et al. Guillain-Barré Syndrome outbreak associated with Zika virus infection in French Polynesia: A case-control study. Lancet 2016, 387, 1531–1539. [Google Scholar] [CrossRef]
- Diamond, M.S.; Ledgerwood, J.E.; Pierson, T.C. Zika Virus Vaccine Development: Progress in the Face of New Challenges. Annu. Rev. Med. 2019, 70, 121–135. [Google Scholar] [CrossRef]
- Jacobs, B.L.; Langland, J.O.; Kibler, K.V.; Denzler, K.L.; White, S.D.; Holechek, S.A.; Wong, S.; Huynh, T.; Baskin, C.R. Vaccinia virus vaccines: Past, present and future. Antivir. Res. 2009, 84, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Dunachie, S.J.; Walther, M.; Vuola, J.M.; Webster, D.P.; Keating, S.M.; Berthoud, T.; Andrews, L.; Bejon, P.; Poulton, I.; Butcher, G.; et al. A clinical trial of prime-boost immunisation with the candidate malaria vaccines RTS,S/AS02A and MVA-CS. Vaccine 2006, 24, 2850–2859. [Google Scholar] [CrossRef] [PubMed]
- Berthoud, T.K.; Hamill, M.; Lillie, P.J.; Hwenda, L.; Collins, K.A.; Ewer, K.J.; Milicic, A.; Poyntz, H.C.; Lambe, T.; Fletcher, H.A.; et al. Potent CD8+ T-cell immunogenicity in humans of a novel heterosubtypic influenza A vaccine, MVA-NP+M1. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2011, 52, 1–7. [Google Scholar] [CrossRef] [PubMed]
- García, F.; Bernaldo de Quirós, J.C.L.; Gómez, C.E.; Perdiguero, B.; Nájera, J.L.; Jiménez, V.; García-Arriaza, J.; Guardo, A.C.; Pérez, I.; Díaz-Brito, V.; et al. Safety and immunogenicity of a modified pox vector-based HIV/AIDS vaccine candidate expressing Env, Gag, Pol and Nef proteins of HIV-1 subtype B (MVA-B) in healthy HIV-1-uninfected volunteers: A phase I clinical trial (RISVAC02). Vaccine 2011, 29, 8309–8316. [Google Scholar] [CrossRef]
- Currier, J.R.; Ngauy, V.; de Souza, M.S.; Ratto-Kim, S.; Cox, J.H.; Polonis, V.R.; Earl, P.; Moss, B.; Peel, S.; Slike, B.; et al. Phase I safety and immunogenicity evaluation of MVA-CMDR, a multigenic, recombinant modified vaccinia Ankara-HIV-1 vaccine candidate. PLoS ONE 2010, 5, e13983. [Google Scholar] [CrossRef][Green Version]
- López-Camacho, C.; Abbink, P.; Larocca, R.A.; Dejnirattisai, W.; Boyd, M.; Badamchi-Zadeh, A.; Wallace, Z.R.; Doig, J.; Velazquez, R.S.; Neto, R.D.L.; et al. Rational Zika vaccine design via the modulation of antigen membrane anchors in chimpanzee adenoviral vectors. Nat. Commun. 2018, 9, 2441. [Google Scholar] [CrossRef]
- Gallego-Gómez, J.C.; Risco, C.; Rodríguez, D.; Cabezas, P.; Guerra, S.; Carrascosa, J.L.; Esteban, M. Differences in Virus-Induced Cell Morphology and in Virus Maturation between MVA and Other Strains (WR, Ankara, and NYCBH) of Vaccinia Virus in Infected Human Cells. J. Virol. 2003, 77, 10606–10622. [Google Scholar] [CrossRef]
- Kim, Y.C.; Lopez-Camacho, C.; Nettleship, J.E.; Rahman, N.; Hill, M.L.; Silva-Reyes, L.; Ortiz-Martinez, G.; Figueroa-Aguilar, G.; Mar, M.A.; Vivanco-Cid, H.; et al. Optimization of Zika virus envelope protein production for ELISA and correlation of antibody titers with virus neutralization in Mexican patients from an arbovirus endemic region. Virol. J. 2018, 15, 193. [Google Scholar] [CrossRef]
- WHO. WHO Target Product Profiles (TPPs). Available online: www.who.int/immunization/research/ppc-tpp/target_product_profiles/en/ (accessed on 25 July 2019).
- Pérez, P.; Marín, M.Q.; Lázaro-Frías, A.; Jiménez de Oya, N.; Blázquez, A.-B.; Escribano-Romero, E.; Sorzano, C.Ó.S.; Ortego, J.; Saiz, J.-C.; Esteban, M.; et al. A Vaccine Based on a Modified Vaccinia Virus Ankara Vector Expressing Zika Virus Structural Proteins Controls Zika Virus Replication in Mice. Sci. Rep. 2018, 8, 17385. [Google Scholar] [CrossRef]
- Brault, A.C.; Domi, A.; McDonald, E.M.; Talmi-Frank, D.; McCurley, N.; Basu, R.; Robinson, H.L.; Hellerstein, M.; Duggal, N.K.; Bowen, R.A.; et al. A Zika Vaccine Targeting NS1 Protein Protects Immunocompetent Adult Mice in a Lethal Challenge Model. Sci. Rep. 2017, 7, 14769. [Google Scholar] [CrossRef]
- Reyes-Sandoval, A.; Berthoud, T.; Alder, N.; Siani, L.; Gilbert, S.C.; Nicosia, A.; Colloca, S.; Cortese, R.; Hill, A.V.S. Prime-boost immunization with adenoviral and modified vaccinia virus Ankara vectors enhances the durability and polyfunctionality of protective malaria CD8+ T-cell responses. Infect. Immun. 2010, 78, 145–153. [Google Scholar] [CrossRef] [PubMed]
- López-Gil, E.; Lorenzo, G.; Hevia, E.; Borrego, B.; Eiden, M.; Groschup, M.; Gilbert, S.C.; Brun, A. A Single Immunization with MVA Expressing GnGc Glycoproteins Promotes Epitope-specific CD8+-T Cell Activation and Protects Immune-competent Mice against a Lethal RVFV Infection. PLoS Negl. Trop. Dis. 2013, 7, e2309. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, N.K.; Padron-Regalado, E.; Thompson, C.P.; Kupke, A.; Wells, D.; Sloan, M.A.; Grehan, K.; Temperton, N.; Lambe, T.; Warimwe, G.; et al. ChAdOx1 and MVA based vaccine candidates against MERS-CoV elicit neutralising antibodies and cellular immune responses in mice. Vaccine 2017, 35, 3780–3788. [Google Scholar] [CrossRef] [PubMed]
- Larocca, R.A.; Abbink, P.; Peron, J.P.S.; de Zanotto, P.M.A.; Iampietro, M.J.; Badamchi-Zadeh, A.; Boyd, M.; Ng’ang’a, D.; Kirilova, M.; Nityanandam, R.; et al. Vaccine protection against Zika virus from Brazil. Nature 2016, 536, 474–478. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Camacho, C.; Kim, Y.C.; Abbink, P.; Larocca, R.A.; Huiskonen, J.T.; Barouch, D.H.; Reyes-Sandoval, A. Assessment of Immunogenicity and Efficacy of a Zika Vaccine Using Modified Vaccinia Ankara Virus as Carriers. Pathogens 2019, 8, 216. https://doi.org/10.3390/pathogens8040216
López-Camacho C, Kim YC, Abbink P, Larocca RA, Huiskonen JT, Barouch DH, Reyes-Sandoval A. Assessment of Immunogenicity and Efficacy of a Zika Vaccine Using Modified Vaccinia Ankara Virus as Carriers. Pathogens. 2019; 8(4):216. https://doi.org/10.3390/pathogens8040216
Chicago/Turabian StyleLópez-Camacho, César, Young Chan Kim, Peter Abbink, Rafael A. Larocca, Juha T. Huiskonen, Dan H. Barouch, and Arturo Reyes-Sandoval. 2019. "Assessment of Immunogenicity and Efficacy of a Zika Vaccine Using Modified Vaccinia Ankara Virus as Carriers" Pathogens 8, no. 4: 216. https://doi.org/10.3390/pathogens8040216
APA StyleLópez-Camacho, C., Kim, Y. C., Abbink, P., Larocca, R. A., Huiskonen, J. T., Barouch, D. H., & Reyes-Sandoval, A. (2019). Assessment of Immunogenicity and Efficacy of a Zika Vaccine Using Modified Vaccinia Ankara Virus as Carriers. Pathogens, 8(4), 216. https://doi.org/10.3390/pathogens8040216




