The Current Status of Cytomegalovirus (CMV) Prevalence in the MENA Region: A Systematic Review
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction and Analysis
3. Results
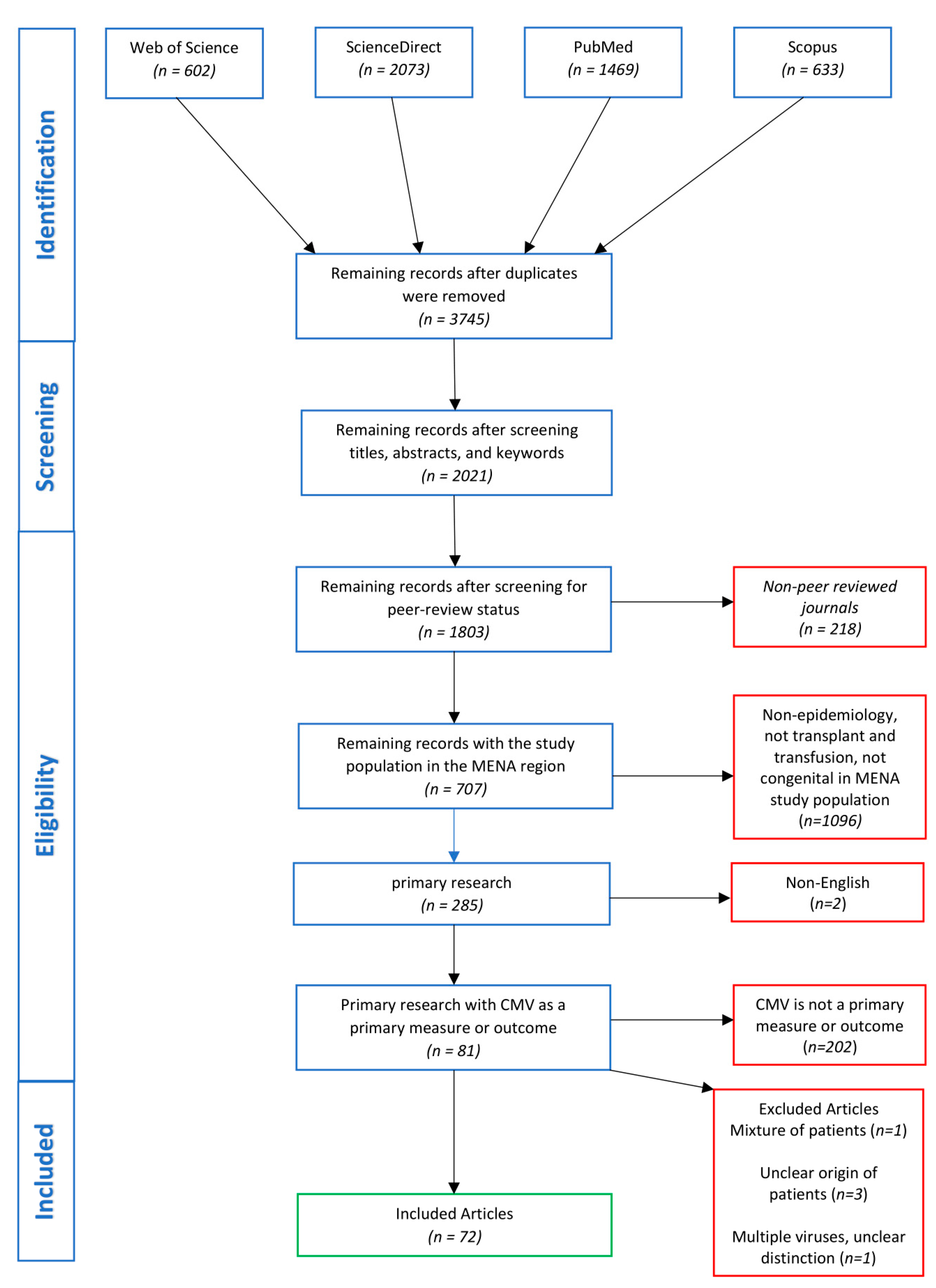
3.1. Search Findings
3.2. Epidemiological Findings
4. Discussion
4.1. CMV in Transplantation Recipients in the MENA Region
4.2. CMV in Blood Transfusion Recipients in the MENA Region
4.3. Congenital CMV (cCMV) in the MENA Region
4.4. Laboratory Diagnosis of CMV in the MENA Region
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cannon, M.J.; Schmid, D.S.; Hyde, T.B. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev. Med. Virol. 2010, 20, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Schottstedt, V.; Blümel, J.; Burger, R.; Drosten, C.; Gröner, A.; Gürtler, L.; Heiden, M.; Hildebrandt, M.; Jansen, B.; Montag-Lessing, T. Human cytomegalovirus (HCMV)–revised. Transfus. Med. Hemotherapy 2010, 37, 365–375. [Google Scholar]
- Nikolich-Žugich, J.; van Lier, R.A. Cytomegalovirus (CMV) research in immune senescence comes of age: Overview of the 6th International Workshop on CMV and Immunosenescence. Geroscience 2017, 39, 245–249. [Google Scholar] [CrossRef] [PubMed]
- CDC. Cytomegalovirus (CMV) and Congenital CMV Infection. Available online: https://www.cdc.gov/cmv/overview.html (accessed on 30 April 2019).
- Sylwester, A.W.; Mitchell, B.L.; Edgar, J.B.; Taormina, C.; Pelte, C.; Ruchti, F.; Sleath, P.R.; Grabstein, K.H.; Hosken, N.A.; Kern, F.; et al. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J. Exp. Med. 2005, 202, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Lancini, D.; Faddy, H.M.; Flower, R.; Hogan, C. Cytomegalovirus disease in immunocompetent adults. Med. J. Aust. 2014, 201, 578–580. [Google Scholar] [CrossRef]
- Miller-Kittrell, M.; Sparer, T.E. Feeling manipulated: Cytomegalovirus immune manipulation. Virol. J. 2009, 6, 4. [Google Scholar] [CrossRef]
- Poole, E.; McGregor Dallas, S.R.; Colston, J.; Joseph, R.S.; Sinclair, J. Virally induced changes in cellular microRNAs maintain latency of human cytomegalovirus in CD34(+) progenitors. J. Gen. Virol. 2011, 92, 1539–1549. [Google Scholar] [CrossRef]
- Pawelec, G.; Akbar, A.; Beverley, P.; Caruso, C.; Derhovanessian, E.; Fulop, T.; Griffiths, P.; Grubeck-Loebenstein, B.; Hamprecht, K.; Jahn, G.; et al. Immunosenescence and Cytomegalovirus: Where do we stand after a decade? Immun. Ageing I A 2010, 7, 13. [Google Scholar] [CrossRef]
- Limaye, A.P.; Bakthavatsalam, R.; Kim, H.W.; Randolph, S.E.; Halldorson, J.B.; Healey, P.J.; Kuhr, C.S.; Levy, A.E.; Perkins, J.D.; Reyes, J.D.; et al. Impact of cytomegalovirus in organ transplant recipients in the era of antiviral prophylaxis. Transplantation 2006, 81, 1645–1652. [Google Scholar] [CrossRef]
- Steininger, C. Clinical relevance of cytomegalovirus infection in patients with disorders of the immune system. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2007, 13, 953–963. [Google Scholar] [CrossRef]
- Limaye, A.P.; Kirby, K.A.; Rubenfeld, G.D.; Leisenring, W.M.; Bulger, E.M.; Neff, M.J.; Gibran, N.S.; Huang, M.L.; Santo Hayes, T.K.; Corey, L.; et al. Cytomegalovirus reactivation in critically ill immunocompetent patients. JAMA 2008, 300, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, P.; Whitley, R.; Snydman, D.R.; Singh, N.; Boeckh, M. Contemporary management of cytomegalovirus infection in transplant recipients: Guidelines from an IHMF workshop, 2007. Herpes J. IHMF 2008, 15, 4–12. [Google Scholar]
- Revello, M.G.; Zavattoni, M.; Furione, M.; Fabbri, E.; Gerna, G. Preconceptional primary human cytomegalovirus infection and risk of congenital infection. J. Infect. Dis. 2006, 193, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Razonable, R.R. Epidemiology of cytomegalovirus disease in solid organ and hematopoietic stem cell transplant recipients. Am. J. Health-Syst. Pharm. Ajhp Off. J. Am. Soc. Health-Syst. Pharm. 2005, 62, S7–S13. [Google Scholar] [CrossRef]
- Manicklal, S.; Emery, V.C.; Lazzarotto, T.; Boppana, S.B.; Gupta, R.K. The “Silent” Global Burden of Congenital Cytomegalovirus. Clin. Microbiol. Rev. 2013, 26, 86–102. [Google Scholar] [CrossRef]
- Azevedo, L.S.; Pierrotti, L.C.; Abdala, E.; Costa, S.F.; Strabelli, T.M.V.; Campos, S.V.; Ramos, J.F.; Latif, A.Z.A.; Litvinov, N.; Maluf, N.Z.; et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo) 2015, 70, 515–523. [Google Scholar] [CrossRef]
- Jacobsohn, D.A.; Vogelsang, G.B. Acute graft versus host disease. Orphanet. J. Rare Dis. 2007, 2, 35. [Google Scholar] [CrossRef]
- Madi, N.; Al-Qaser, M.; Edan, R.; Al-Nakib, W. Clinical Utility of Viral Load in the Management of Cytomegalovirus Infection in Solid Organ Transplant Patients in Kuwait. Transplant. Proc. 2015, 47, 1802–1807. [Google Scholar] [CrossRef]
- Nemati, E.; Taheri, S.; Pourfarziani, V.; Einollahi, B. Cytomegalovirus disease in renal transplant recipients: An Iranian experience. Exp. Clin. Transplant. Off. J. Middle East Soc. Organ Transplant. 2008, 6, 132–136. [Google Scholar]
- Charfeddine, K.; Kharrat, M.; Yaich, S.; Jarraya, F.; Mkawar, K.; Hachicha, J. Infection in kidney transplant recipients in Tunisia. Saudi J. Kidney Dis. Transplant. Off. Publ. Saudi Cent. Organ Transplant. Saudi Arab. 2002, 13, 195–198. [Google Scholar]
- Cohen, L.; Yeshurun, M.; Shpilberg, O.; Ram, R. Risk factors and prognostic scale for cytomegalovirus (CMV) infection in CMV-seropositive patients after allogeneic hematopoietic cell transplantation. Transpl. Infect. Dis. Off. J. Transplant. Soc. 2015, 17, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Valadkhani, B.; Kargar, M.; Ashouri, A.; Hadjibabaie, M.; Gholami, K.; Ghavamzadeh, A. The risk factors for cytomegalovirus reactivation following stem cell transplantation. J. Res. Pharm. Pract. 2016, 5, 63–69. [Google Scholar] [CrossRef]
- Behzad-Behbahani, A.; Ehsanipour, F.; Alborzi, A.; Nourani, H.; Ramzi, M.; Rasoli, M. Qualitative detection of human cytomegalovirus DNA in the plasma of bone marrow transplant recipients: Value as a predictor of disease progression. Exp. Clin. Transplant. Off. J. Middle East Soc. Organ Transplant. 2004, 2, 196–200. [Google Scholar]
- Enan, K.A.; Rennert, H.; El-Eragi, A.M.; El Hussein, A.R.M.; Elkhidir, I.M. Comparison of Real-time PCR to ELISA for the detection of human cytomegalovirus infection in renal transplant patients in the Sudan. Virol. J. 2011, 8, 222. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, W.A.; Al Salmi, I.; Jha, A.; Pakkyara, A.; Yasir, M.; Shaheen, F.A.M. Early clinical manifestations and laboratory findings before and after treatment of cytomegalovirus infection in kidney transplant patients. Saudi J. Kidney Dis. Transplant. Off. Publ. Saudi Cent. Organ Transplant. Saudi Arab. 2017, 28, 774–781. [Google Scholar]
- Babazadeh, A.; Javanian, M.; Oliaei, F.; Akbari, R.; Akbarzadepasha, A.; Bijani, A.; Sadeghi, M. Incidence and risk factors for cytomegalovirus in kidney transplant patients in Babol, northern Iran. Casp. J. Intern. Med. 2017, 8, 23–29. [Google Scholar]
- Pour-Reza-Gholi, F.; Labibi, A.; Farrokhi, F.; Nafar, M.; Firouzan, A.; Einollahi, B. Signs and symptoms of cytomegalovirus disease in kidney transplant recipients. Transplant. Proc. 2005, 37, 3056–3058. [Google Scholar] [CrossRef] [PubMed]
- Shibolet, O.; Ilan, Y.; Kalish, Y.; Safadi, R.; Ashur, Y.; Eid, A.; Shouval, D.; Wolf, D. Late cytomegalovirus disease following liver transplantation. Transpl. Int. Off. J. Eur. Soc. Organ Transplant. 2003, 16, 861–865. [Google Scholar] [CrossRef]
- Heybar, H.; Alavi, S.M.; Farashahi Nejad, M.; Latifi, M. Cytomegalovirus infection and atherosclerosis in candidate of coronary artery bypass graft. Jundishapur J. Microbiol. 2015, 8, e15476. [Google Scholar] [CrossRef]
- Boeckh, M.; Geballe, A.P. Cytomegalovirus: Pathogen, paradigm, and puzzle. J. Clin. Investig. 2011, 121, 1673–1680. [Google Scholar] [CrossRef]
- Souza, M.A.; Passos, A.M.; Treitinger, A.; Spada, C. Seroprevalence of cytomegalovirus antibodies in blood donors in southern, Brazil. Rev. Soc. Bras. Med. Trop. 2010, 43, 359–361. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.M.; Levin, S. Infectious complications of solid organ transplantations. Infect. Dis. Clin. North Am. 2001, 15, 521–549. [Google Scholar] [CrossRef]
- Al-Hajjar, S.; Al Seraihi, A.; Al Muhsen, S.; Ayas, M.; Al Jumaah, S.; Al Jefri, A.; Shoukri, M.; El Solh, H. Cytomegalovirus infections in unrelated cord blood transplantation in pediatric patients: Incidence, risk factors, and outcomes. Hematol. Oncol. Stem Cell Ther. 2011, 4, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Alsheikh, R.; Gabardi, S. Post-Renal Transplantation Outcomes in Elderly Patients Compared to Younger Patients in the Setting of Early Steroid Withdrawal. Prog. Transplant. (Aliso Viejo CA) 2018, 28, 322–329. [Google Scholar] [CrossRef]
- Stadler, L.P.; Bernstein, D.I.; Callahan, S.T.; Turley, C.B.; Munoz, F.M.; Ferreira, J.; Acharya, M.; Simone, G.A.G.; Patel, S.M.; Edwards, K.M. Seroprevalence and risk factors for cytomegalovirus infections in adolescent females. J. Pediatric Infect. Dis. Soc. 2012, 2, 7–14. [Google Scholar] [CrossRef][Green Version]
- Staras, S.A.; Dollard, S.C.; Radford, K.W.; Flanders, W.D.; Pass, R.F.; Cannon, M.J. Seroprevalence of cytomegalovirus infection in the United States, 1988–1994. Clin. Infect. Dis. 2006, 43, 1143–1151. [Google Scholar] [CrossRef]
- Boeckh, M.; Nichols, W.G.; Papanicolaou, G.; Rubin, R.; Wingard, J.R.; Zaia, J. Cytomegalovirus in hematopoietic stem cell transplant recipients: Current status, known challenges, and future strategies. Biol. Blood Marrow Transplant. 2003, 9, 543–558. [Google Scholar] [CrossRef]
- Sousa, H.; Boutolleau, D.; Ribeiro, J.; Teixeira, A.L.; Vaz, C.P.; Campilho, F.; Branca, R.; Campos, A., Jr.; Baldaque, I.; Medeiros, R. Cytomegalovirus infection in patients who underwent allogeneic hematopoietic stem cell transplantation in Portugal: A five-year retrospective review. Biol. Blood Marrow Transplant. 2014, 20, 1958–1967. [Google Scholar] [CrossRef]
- Jaskula, E.; Bochenska, J.; Kocwin, E.; Tarnowska, A.; Lange, A. CMV serostatus of donor-recipient pairs influences the risk of CMV infection/reactivation in HSCT patients. Bone Marrow Res. 2012, 2012. [Google Scholar] [CrossRef]
- Taherimahmoudi, M.; Ahmadi, H.; Baradaran, N.; Montaser-Kouhsari, L.; Salem, S.; Mehrsai, A.; Kalantar, E.; Jahani, Y.; Pourmand, G. Cytomegalovirus infection and disease following renal transplantation: Preliminary report of incidence and potential risk factors. Transplant. Proc. 2009, 41, 2841–2844. [Google Scholar] [CrossRef]
- Zekri, A.R.; Mohamed, W.S.; Samra, M.A.; Sherif, G.M.; El-Shehaby, A.M.; El-Sayed, M.H. Risk factors for cytomegalovirus, hepatitis B and C virus reactivation after bone marrow transplantation. Transpl. Immunol. 2004, 13, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Ghods, F.J.; Solgi, G.; Amirzargar, A.A.; Nikbin, B.; Ghods, A.J. High frequency of clinically significant infections and cytomegalovirus disease in kidney transplant recipients with serum mannose-binding lectin deficiency. Iran. J. Kidney Dis. 2009, 3, 28–33. [Google Scholar] [PubMed]
- Almehmadi, M.; Hammad, A.; Heyworth, S.; Moberly, J.; Middleton, D.; Hopkins, M.J.; Hart, I.J.; Christmas, S.E. CD56+ T cells are increased in kidney transplant patients following cytomegalovirus infection. Transpl. Infect. Dis. Off. J. Transplant. Soc. 2015, 17, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Sedky, M.; Mekki, Y.; Mialou, V.; Bleyzac, N.; Girard, S.; Salama, E.; Abdel Rahman, H.; Bertrand, Y. Cytomegalovirus infection in pediatric allogenic hematopoietic stem cell transplantation. A single center experience. Pediatric Hematol. Oncol. 2014, 31, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, R.; Asaad, A.; Hussein, M. Human leukocyte antigen-A genotype as a predictor of cytomegalovirus-pp65 antigenemia and cytomegalovirus disease in solid-organ transplant recipients. Egypt. J. Med Hum. Genet. 2016, 17, 345–352. [Google Scholar] [CrossRef]
- Futohi, F.; Saber, A.; Nemati, E.; Einollahi, B.; Rostami, Z. Human Leukocyte Antigen Alleles and Cytomegalovirus Infection After Renal Transplantation. Nephro Urol. Mon. 2015, 7, e31635. [Google Scholar] [CrossRef] [PubMed]
- Niknam, A.; Karimi, M.H.; Yaghobi, R.; Geramizadeh, B.; Roozbeh, J.; Salehipour, M.; Iravani, M. The Association Between Viral Infections and Co-stimulatory Gene Polymorphisms in Kidney Transplant Outcomes. Jundishapur J. Microbiol. 2016, 9, e31338. [Google Scholar] [CrossRef]
- Kotton, C.N.; Kumar, D.; Caliendo, A.M.; Huprikar, S.; Chou, S.; Danziger-Isakov, L.; Humar, A. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation. Transplantation 2018, 102, 900–931. [Google Scholar] [CrossRef]
- Meijer, E.; Boland, G.J.; Verdonck, L.F. Prevention of cytomegalovirus disease in recipients of allogeneic stem cell transplants. Clin. Microbiol. Rev. 2003, 16, 647–657. [Google Scholar] [CrossRef]
- Alsuhaibani, O.; Pereira, W.C.; Tareeqanwar, M.; Khizzi, N.E.; Bakheswain, S.; Shaker, A.; Elyamany, G. Infectious disease screening among stem cell transplant donors: An Institutional experience in Saudi Arabia. Ann. Neurosci. 2015, 22, 81–86. [Google Scholar] [CrossRef]
- Essa, S.; Pacsa, A.; Said, T.; Nampoory, M.R.; Raghupathy, R.; Johny, K.V.; Al-Nakib, W.; Al-Mosawy, M. Is combined pretransplantation seropositivity of kidney transplant recipients for cytomegalovirus antigens (pp150 and pp28) a predictor for protection against infection? Med. Princ. Pract. Int. J. Kuwait Univ. Health Sci. Cent. 2008, 17, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Safabakhsh, H.; Tehranian, F.; Tehranian, B.; Hatami, H.; Karimi, G.; Shahabi, M. Prevalence of anti-CMV antibodies in blood donors in Mashhad, Iran. Iran. J. Epidemiol. 2013, 9, 52–57. [Google Scholar]
- Sepehrvand, N.; Khameneh, Z.R.; Eslamloo, H.R. Survey the seroprevalence of CMV among hemodialysis patients in Urmia, Iran. Saudi J. Kidney Dis. Transplant. Off. Publ. Saudi Cent. Organ Transplant. Saudi Arab. 2010, 21, 363–367. [Google Scholar]
- Mahmoud, R.A.; El-Mazary, A.A.; Khodeary, A. Seroprevalence of Hepatitis C, Hepatitis B, Cytomegalovirus, and Human Immunodeficiency Viruses in Multitransfused Thalassemic Children in Upper Egypt. Adv. Hematol. 2016, 2016, 9032627. [Google Scholar] [CrossRef]
- Gawad, A.A.; Hashish, M.; Abaza, A.; El-Kayal, A. Cytomegalovirus Immunoglobulin G Avidity Index among Blood Donors in Alexandria, Egypt. Cent. Eur. J. Public Health 2016, 24, 314–320. [Google Scholar] [CrossRef]
- Khameneh, Z.R.; Sepehrvand, N.; Aghazadeh, T. Cytomegalovirus infection among Iranian kidney graft recipients. Transplant. Proc. 2013, 45, 178–181. [Google Scholar] [CrossRef]
- Yaghobi, R.; Zamani, S.; Gramizadeh, B.; Rahsaz, M. Etiology of DNA Virus Infections in Liver Transplant Recipients With Neonatal Hepatitis. Transplant. Proc. 2010, 42, 837–838. [Google Scholar] [CrossRef]
- Madi, N.; Al-Nakib, W.; Pacsa, A.; Saeed, T. Cytomegalovirus genotypes gB1 and gH1 are the most predominant genotypes among renal transplant recipients in Kuwait. Transplant. Proc. 2011, 43, 1634–1637. [Google Scholar] [CrossRef]
- Basri, N.; Abdullah, K.A.; Shaheen, F.A. Cytomegalovirus disease in renal transplant recipients: A single-center experience. Exp. Clin. Transplant. Off. J. Middle East Soc. Organ Transplant. 2007, 5, 601–603. [Google Scholar]
- Pourmand, G.; Salem, S.; Mehrsai, A.; Taherimahmoudi, M.; Ebrahimi, R.; Pourmand, M.R. Infectious complications after kidney transplantation: A single-center experience. Transpl. Infect. Dis. Off. J. Transplant. Soc. 2007, 9, 302–309. [Google Scholar] [CrossRef]
- Ramzi, M.; Yaghobi, R.; Etminan, H. The role of clinical, therapeutic and laboratory findings in monitoring of HCMV infection in bone marrow transplant Recipients. Iran. Red. Crescent Med. J. 2009, 11, 46–51. [Google Scholar]
- Saghafi, H.; Qorashi, M.; Heidari, A. Is screening for IgG antibody to cytomegalovirus and Epstein-Barr virus infections mandatory in potential renal transplant recipients and donors in Iran? Transplant. Proc. 2009, 41, 2761–2763. [Google Scholar] [CrossRef] [PubMed]
- Al-Sweedan, S.; Al-Seraihy, A.; Al-Ahmari, A.; Al-Jefri, A.; Mohammed, V.; Jafri, R.; Siddiqui, K.; Ayas, M. Factors Determining the Outcome of Hematopoietic Stem Cell Transplantation in Patients With Acute Lymphoblastic Leukemia at King Faisal Specialist Hospital and Research Center, Riyadh, Saudi Arabia. J. Pediatric Hematol. Oncol. 2017, 39, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Hussein, A.A.; Al-Antary, E.T.; Najjar, R.; Al-Hamdan, D.S.; Al-Zaben, A.; Frangoul, H. Incidence and risk factors for cytomegalovirus (CMV) reactivation following autologous hematopoietic stem cell transplantation in children. Pediatric Blood Cancer 2015, 62, 1099–1101. [Google Scholar] [CrossRef]
- Davoudi, S.; Kasraianfard, A.; Ahmadinejad, Z.; Najafi, A.; Salimi, J.; Makarem, J.; Sohrabpour, A.A.; Jafarian, A. Cytomegalovirus reactivation and preemptive therapy after liver transplant. Exp. Clin. Transplant. Off. J. Middle East Soc. Organ Transplant. 2014, 12 (Suppl. 1), 72–75. [Google Scholar]
- Al-Alousy, B.M.; Abdul-Razak, S.H.; Al-Ajeeli, K.S.; Al-Jashamy, K.A. Anti-HCMV IgG positivity rate among renal transplant recipients in Baghdad. Saudi J. Kidney Dis. Transplant. Off. Publ. Saudi Cent. Organ Transplant. Saudi Arab. 2011, 22, 1269–1274. [Google Scholar]
- Khameneh, Z.R. Occurrence of cytomegalovirus infection and factors causing reactivation of the infection among renal transplant recipients: A single center study. Saudi J. Kidney Dis. Transplant. Off. Publ. Saudi Cent. Organ Transplant. Saudi Arab. 2008, 19, 41–45. [Google Scholar]
- Nasiri, S.; Ahmadi, S.F.; Lessan-Pezeshki, M.; Seyfi, S.; Alatab, S. Lack of cytomegalovirus and polyomavirus coexistence in Iranian kidney transplant recipients. Transplant. Proc. 2011, 43, 536–539. [Google Scholar] [CrossRef]
- Saber, A.; Fotuhi, F.; Rostami, Z.; Einollahi, B.; Nemati, E. Vitamin D Levels After Kidney Transplantation and the Risk of Cytomegalovirus Infection. Nephro Urol. Mon. 2015, 7, e29677. [Google Scholar] [CrossRef]
- Amir, J.; Atias, J.; Linder, N.; Pardo, J. Follow-up of infants with congenital cytomegalovirus and normal fetal imaging. Arch. Dis. Child. Fetal Neonatal Ed. 2016, 101, F428–F432. [Google Scholar] [CrossRef]
- Bilavsky, E.; Schwarz, M.; Pardo, J.; Attias, J.; Levy, I.; Haimi-Cohen, Y.; Amir, J. Lenticulostriated vasculopathy is a high-risk marker for hearing loss in congenital cytomegalovirus infections. Acta Paediatr. (Oslo Norway 1992) 2015, 104, e388–e394. [Google Scholar] [CrossRef] [PubMed]
- Hadar, E.; Yogev, Y.; Melamed, N.; Chen, R.; Amir, J.; Pardo, J. Periconceptional cytomegalovirus infection: Pregnancy outcome and rate of vertical transmission. Prenat. Diagn. 2010, 30, 1213–1216. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, C.; Grossman, R.; Bokov, I.; Lipitz, S.; Biegon, A. Effect of cytomegalovirus infection on temporal lobe development in utero: Quantitative MRI studies. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 2010, 20, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Gindes, L.; Teperberg-Oikawa, M.; Sherman, D.; Pardo, J.; Rahav, G. Congenital cytomegalovirus infection following primary maternal infection in the third trimester. BJOG Int. J. Obstet. Gynaecol. 2008, 115, 830–835. [Google Scholar] [CrossRef]
- Lipitz, S.; Yagel, S.; Shalev, E.; Achiron, R.; Mashiach, S.; Schiff, E. Prenatal diagnosis of fetal primary cytomegalovirus infection. Obstet. Gynecol. 1997, 89, 763–767. [Google Scholar] [CrossRef]
- Lipitz, S.; Yinon, Y.; Malinger, G.; Yagel, S.; Levit, L.; Hoffman, C.; Rantzer, R.; Weisz, B. Risk of cytomegalovirus-associated sequelae in relation to time of infection and findings on prenatal imaging. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2013, 41, 508–514. [Google Scholar] [CrossRef]
- Farkas, N.; Hoffmann, C.; Ben-Sira, L.; Lev, D.; Schweiger, A.; Kidron, D.; Lerman-Sagie, T.; Malinger, G. Does normal fetal brain ultrasound predict normal neurodevelopmental outcome in congenital cytomegalovirus infection? Prenat. Diagn. 2011, 31, 360–366. [Google Scholar] [CrossRef]
- Ari-Even Roth, D.; Lubin, D.; Kuint, J.; Teperberg-Oikawa, M.; Mendelson, E.; Strauss, T.; Barkai, G. Contribution of targeted saliva screening for congenital CMV-related hearing loss in newborns who fail hearing screening. Arch. Dis. Child. Fetal Neonatal Ed. 2017, 102, F519–F524. [Google Scholar] [CrossRef]
- Amir, J.; Schwarz, M.; Levy, I.; Haimi-Cohen, Y.; Pardo, J. Is lenticulostriated vasculopathy a sign of central nervous system insult in infants with congenital CMV infection? Arch. Dis. Child. 2011, 96, 846–850. [Google Scholar] [CrossRef]
- Barkai, G.; Ari-Even Roth, D.; Barzilai, A.; Tepperberg-Oikawa, M.; Mendelson, E.; Hildesheimer, M.; Kuint, J. Universal neonatal cytomegalovirus screening using saliva—Report of clinical experience. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2014, 60, 361–366. [Google Scholar] [CrossRef]
- Barkai, G.; Barzilai, A.; Mendelson, E.; Tepperberg-Oikawa, M.; Roth, A.E.D.; Kuint, J. Newborn screening for congenital cytomegalovirus using real-time polymerase chain reaction in umbilical cord blood. Israel Med. Assoc. J. 2013, 15, 279–283. [Google Scholar]
- Arabpour, M.; Kaviyanee, K.; Jankhah, A.; Yaghobi, R. Human cytomegalovirus infection in women of childbearing age, Fars Province: A population-based cohort study. Iran. Red Crescent Med. J. 2008, 10, 100–106. [Google Scholar] [CrossRef][Green Version]
- Karimian, P.; Yaghini, O.; Nasr Azadani, H.; Mohammadizadeh, M.; Arabzadeh, S.A.; Adibi, A.; Rahimi, H. Prevalence, Characteristics, and One-Year Follow-Up of Congenital Cytomegalovirus Infection in Isfahan City, Iran. Interdiscip. Perspect. Infect. Dis. 2016, 2016, 7812106. [Google Scholar] [CrossRef] [PubMed]
- Rasti, S.; Ghasemi, F.S.; Abdoli, A.; Piroozmand, A.; Mousavi, S.G.; Fakhrie-Kashan, Z. ToRCH “co-infections” are associated with increased risk of abortion in pregnant women. Congenit. Anom. 2016, 56, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Al-Ali, H.Y.; Yasseen, S.A.; Raof, T.Y. Follow-up of pregnant women with active cytomegalovirus infection. East. Mediterr. Health J. 1999, 5, 949–954. [Google Scholar] [PubMed]
- Fahimzad, A.; Afgeh, S.A.; Eghbali, E.; Abdinia, B.; Shiva, F.; Rahbar, M. Screening of congenital CMV infection in saliva of neonates by PCR: Report of a pilot screening study in Iran. Clin. Lab. 2013, 59, 1171–1174. [Google Scholar] [CrossRef] [PubMed]
- Bilavsky, E.; Schwarz, M.; Bar-Sever, Z.; Pardo, J.; Amir, J. Hepatic involvement in congenital cytomegalovirus infection—Infrequent yet significant. J. Viral Hepat. 2015, 22, 763–768. [Google Scholar] [CrossRef]
- Hadar, E.; Dorfman, E.; Bardin, R.; Gabbay-Benziv, R.; Amir, J.; Pardo, J. Symptomatic congenital cytomegalovirus disease following non-primary maternal infection: A retrospective cohort study. BMC Infect. Dis. 2017, 17, 31. [Google Scholar] [CrossRef][Green Version]
- Abdelmogheth, A.A.; Al-Nair, A.M.; Balkhair, A.A.; Mahmoud, A.M.; El-Naggari, M. Pattern of Viral Infections among Infants and Children Admitted to the Paediatric Intensive Care Unit at Sultan Qaboos University Hospital, Oman. Sultan Qaboos Univ. Med. J. 2014, 14, e546–e550. [Google Scholar]
- Jahromi. Cytomegalovirus Immunity in Pregnancy in South of Iran. Am. J. Infect. Dis. 2010, 6, 8–12. [Google Scholar] [CrossRef]
- Van Zuylen, W.J.; Hamilton, S.T.; Naing, Z.; Hall, B.; Shand, A.; Rawlinson, W.D. Congenital cytomegalovirus infection: Clinical presentation, epidemiology, diagnosis and prevention. Obstet. Med. 2014, 7, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Dollard, S.C.; Grosse, S.D.; Ross, D.S. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev. Med. Virol. 2007, 17, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Istas, A.S.; Demmler, G.J.; Dobbins, J.G.; Stewart, J.A. Surveillance for congenital cytomegalovirus disease: A report from the National Congenital Cytomegalovirus Disease Registry. Clin. Infect. Dis. 1995, 20, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, A.; Hengel, H. Epidemiological impact and disease burden of congenital cytomegalovirus infection in Europe. Euro. Surveill. 2009, 14, 19140. [Google Scholar] [CrossRef]
- Ebrahimi-Rad, M.; Shakeri, T.S.; Shirvani, F.; Shahrokhi, K.; Shahrokhi, N. Prevalence of congenital cytomegalovirus infection in symptomatic newborns under 3 weeks in Tehran, Iran. BMC Infect. Dis. 2017, 17, 688. [Google Scholar] [CrossRef]
- Schlesinger, Y.; Reich, D.; Eidelman, A.I.; Schimmel, M.S.; Hassanin, J.; Miron, D. Congenital cytomegalovirus infection in Israel: Screening in different subpopulations. Isr. Med Assoc. J. IMAJ 2005, 7, 237–240. [Google Scholar]
- Dietrich, M.L.; Schieffelin, J.S. Congenital Cytomegalovirus Infection. Ochsner J. 2019, 19, 123–130. [Google Scholar] [CrossRef]
- Townsend, C.L.; Forsgren, M.; Ahlfors, K.; Ivarsson, S.A.; Tookey, P.A.; Peckham, C.S. Long-term outcomes of congenital cytomegalovirus infection in Sweden and the United Kingdom. Clin. Infect. Dis. 2013, 56, 1232–1239. [Google Scholar] [CrossRef]
- Vestergaard, H.T.; Thomsen, M.K.; Nielsen, L.; Panum, I. Diagnostics of congenital cytomegalovirus in Denmark. Ugeskr. Laeger 2018, 180, pii:V03180221. [Google Scholar]
- El-Mekki, A.; Deverajan, L.V.; Soufi, S.; Strannegard, O.; al-Nakib, W. Specific and non-specific serological markers in the screening for congenital CMV infection. Epidemiol. Infect. 1988, 101, 495–501. [Google Scholar] [CrossRef]
- Pasternak, Y.; Ziv, L.; Attias, J.; Amir, J.; Bilavsky, E. Valganciclovir Is Beneficial in Children with Congenital Cytomegalovirus and Isolated Hearing Loss. J. Pediatrics 2018, 199, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Miron, D.; Brosilow, S.; Felszer, K.; Reich, D.; Halle, D.; Wachtel, D.; Eidelman, A.I.; Schlesinger, Y. Incidence and clinical manifestations of breast milk-acquired Cytomegalovirus infection in low birth weight infants. J. Perinatol. Off. J. Calif. Perinat. Assoc. 2005, 25, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Bilavsky, E.; Shahar-Nissan, K.; Pardo, J.; Attias, J.; Amir, J. Hearing outcome of infants with congenital cytomegalovirus and hearing impairment. Arch. Dis. Child. 2016, 101, 433–438. [Google Scholar] [CrossRef]
- Neirukh, T.; Qaisi, A.; Saleh, N.; Rmaileh, A.A.; Zahriyeh, E.A.; Qurei, L.; Dajani, F.; Nusseibeh, T.; Khamash, H.; Baraghithi, S.; et al. Seroprevalence of Cytomegalovirus among pregnant women and hospitalized children in Palestine. BMC Infect. Dis. 2013, 13, 528. [Google Scholar] [CrossRef] [PubMed]
- Bilavsky, E.; Pardo, J.; Attias, J.; Levy, I.; Magny, J.F.; Ville, Y.; Leruez-Ville, M.; Amir, J. Clinical Implications for Children Born With Congenital Cytomegalovirus Infection Following a Negative Amniocentesis. Clin. Infect. Dis. 2016, 63, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Ziyaeyan, M.; Alborzi, A.; Abbasian, A.; Kalani, M.; Moravej, A.; Nasiri, J.; Amiri, A.; Hashemi, N.; Sefiddashti, F. Detection of HCMV DNA in placenta, amniotic fluid and fetuses of seropositive women by nested PCR. Euro. J. Pediatrics 2007, 166, 723–726. [Google Scholar] [CrossRef] [PubMed]
- Kanengisser-Pines, B.; Hazan, Y.; Pines, G.; Appelman, Z. High cytomegalovirus IgG avidity is a reliable indicator of past infection in patients with positive IgM detected during the first trimester of pregnancy. J. Perinatal Med. 2009, 37, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Abu Faddan, N.; Eltayeb, A.; Refaiy, A. Cytomegalo virus as a possible risk factor for neonatal gastrointestinal surgical conditions. Fetal Pediatric Pathol. 2011, 30, 124–129. [Google Scholar] [CrossRef]
- Al-Awadhi, R.; Al-Harmi, J.; Alfadhli, S. Prevalence of cytomegalovirus DNA in cord blood and voided urine obtained from pregnant women at the end of pregnancy. Med. Princ. Pract. Int. J. Kuwait Univ. Health Sci. Cent. 2013, 22, 194–199. [Google Scholar] [CrossRef]
- Kamel, N.; Metwally, L.; Gomaa, N.; Sayed Ahmed, W.A.; Lotfi, M.; Younis, S. Primary cytomegalovirus infection in pregnant Egyptian women confirmed by cytomegalovirus IgG avidity testing. Med. Princ. Pract. Int. J. Kuwait Univ. Health Sci. Cent. 2014, 23, 29–33. [Google Scholar] [CrossRef]
- Erfanianahmadpoor, M.; Nasiri, R.; Vakili, R.; Hassannia, T. Seroprevalence, transmission, and associated factors of specific antibodies against cytomegalovirus among pregnant women and their infants in a regional study. Saudi Med. J. 2014, 35, 360–364. [Google Scholar] [PubMed]
- Ebrahim, M.G.; Ali, A.S.; Mustafa, M.O.; Musa, D.F.; El Hussein, A.R.; Elkhidir, I.M.; Enan, K.A. Molecular Detection of Human Cytomegalovirus (HCMV) Among Infants with Congenital Anomalies in Khartoum State, Sudan. Open Virol. J. 2015, 9, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Altayeb, M.A.; Mokhtar, S.I.; Adam, M.E.; Mohammed, S.I.; Musa, H.H. Detection of primary CMV infection in Sudanese pregnant women by IgG avidity test. Asian Pac. J. Trop. Dis. 2016, 6, 816–818. [Google Scholar] [CrossRef]
- Alwan, S.N.; Shamran, H.A.; Ghaib, A.H.; Al-Mayah, Q.S.; Al-Saffar, A.J.; Bayati, A.H.; Arif, H.S.; Fu, J.; Wickes, B.L. Genotyping of Cytomegalovirus from Symptomatic Infected Neonates in Iraq. Am. J. Trop. Med. Hyg. 2019. [Google Scholar] [CrossRef] [PubMed]
- Caliendo, A.M. Overview of Diagnostic Tests for Cytomegalovirus Infection. Available online: https://www.uptodate.com/contents/overview-of-diagnostic-tests-for-cytomegalovirus-infection (accessed on 17 April 2019).
- Chou, S. Newer methods for diagnosis of cytomegalovirus infection. Rev. Infect. Dis. 1990, 12 (Suppl. 7), S727–S736. [Google Scholar] [CrossRef]
- Engelhard, D.; Weinberg, M.; Or, R.; Shaked, O.; Naparstek, E.; Haikin, H.; Slavin, S.; Sarov, I. Immunoglobulins A, G, and M to cytomegalovirus during recurrent infection in recipients of allogeneic bone marrow transplantation. J. Infect. Dis. 1991, 163, 628–630. [Google Scholar] [CrossRef]
- Abou-Jaoude, M.M.; Ghantous, I.; Almawi, W.Y. Tacrolimus (FK506) versus cyclosporin A microemulsion (Neoral) maintenance immunosuppression: Effects on graft survival and function, infection, and metabolic profile following kidney transplantation (KT). Mol. Immunol. 2003, 39, 1095–1100. [Google Scholar] [CrossRef]
- Essa, S.; Pacsa, A.; Raghupathy, R.; Said, T.; Nampoory, M.R.; Johny, K.V.; Al-Nakib, W. Low levels of Th1-type cytokines and increased levels of Th2-type cytokines in kidney transplant recipients with active cytomegalovirus infection. Transplant. Proc. 2009, 41, 1643–1647. [Google Scholar] [CrossRef]
- Pourmand, G.; Saraji, A.; Salem, S.; Mehrsai, A.; Nikoobakht, M.R.; Taherimahmoudi, M.; Rezaeidanesh, M.; Asadpour, A. Could prophylactic monoclonal antibody improve kidney graft survival? Transplant. Proc. 2009, 41, 2794–2796. [Google Scholar] [CrossRef]
- Kamali, K.; Abbasi, M.A.; Behzadi, A.H.; Mortazavi, A.; Bastani, B. Incidence and risk factors of transplant renal artery stenosis in living unrelated donor renal transplantation. J. Renal Care 2010, 36, 149–152. [Google Scholar] [CrossRef]
- Vaezi, M.; Kasaeian, A.; Souri, M.; Soufiyan, F.; Shokri Boosjin, A.; Setarehdan, S.A.; Alimoghaddam, K.; Ghavamzadeh, A. How Do Donor-Recipient CMV Serostatus and Post-Hematopoietic Stem Cell Transplantation CMV Reactivation Affect Outcomes in Acute Leukemia Patients? Int. J. Hematol. Oncol. Stem Cell Res. 2017, 11, 199–208. [Google Scholar] [PubMed]
- Goldstein, G.; Rutenberg, T.F.; Mendelovich, S.L.; Hutt, D.; Oikawa, M.T.; Toren, A.; Bielorai, B. The role of immunoglobulin prophylaxis for prevention of cytomegalovirus infection in pediatric hematopoietic stem cell transplantation recipients. Pediatric Blood Cancer 2017, 64. [Google Scholar] [CrossRef] [PubMed]
- Lipitz, S.; Hoffmann, C.; Feldman, B.; Tepperberg-Dikawa, M.; Schiff, E.; Weisz, B. Value of prenatal ultrasound and magnetic resonance imaging in assessment of congenital primary cytomegalovirus infection. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2010, 36, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Gabbay-Benziv, R.; Yogev, Y.; Peled, Y.; Amir, J.; Pardo, J. Congenital cytomegalovirus infection following antenatal negative diagnostic amniotic fluid analysis—A single center experience. J. Matern. Fetal Neonatal Med. Off. J. Eur. Assoc. Perinat. Med. Fed. Asia Ocean. Perinat. Soc. Int. Soc. Perinat. Obs. 2012, 25, 1787–1790. [Google Scholar] [CrossRef]
- Rahav, G.; Gabbay, R.; Ornoy, A.; Shechtman, S.; Arnon, J.; Diav-Citrin, O. Primary versus nonprimary cytomegalovirus infection during pregnancy, Israel. Emerg. Infect. Dis. 2007, 13, 1791–1793. [Google Scholar] [CrossRef] [PubMed]
- Kraft, C.S.; Armstrong, W.S.; Caliendo, A.M. Interpreting quantitative cytomegalovirus DNA testing: Understanding the laboratory perspective. Clin. Infect. Dis. 2012, 54, 1793–1797. [Google Scholar] [CrossRef] [PubMed]
- Caliendo, A.M. The long road toward standardization of viral load testing for cytomegalovirus. Clin. Infect. Dis. 2013, 56, 374–375. [Google Scholar] [CrossRef][Green Version]
- Tong, Y.; Pang, X.L.; Mabilangan, C.; Preiksaitis, J.K. Determination of the Biological Form of Human Cytomegalovirus DNA in the Plasma of Solid-Organ Transplant Recipients. J. Infect. Dis. 2017, 215, 1094–1101. [Google Scholar] [CrossRef]
- Preiksaitis, J.K.; Hayden, R.T.; Tong, Y.; Pang, X.L.; Fryer, J.F.; Heath, A.B.; Cook, L.; Petrich, A.K.; Yu, B.; Caliendo, A.M. Are We There Yet? Impact of the First International Standard for Cytomegalovirus DNA on the Harmonization of Results Reported on Plasma Samples. Clin. Infect. Dis. 2016, 63, 583–589. [Google Scholar] [CrossRef]
- Cook, L.; Starr, K.; Boonyaratanakornkit, J.; Hayden, R.; Sam, S.S.; Caliendo, A.M. Does Size Matter? Comparison of Extraction Yields for Different-Sized DNA Fragments by Seven Different Routine and Four New Circulating Cell-Free Extraction Methods. J. Clin. Microbiol. 2018, 56. [Google Scholar] [CrossRef]
- Al-Amro, A.A.; al-Jafari, A.A.; al-Fagih, M.R.; Tajeldin, M.; Qavi, H.B. Frequency of occurrence of cytomegalovirus and Chlamydia pneumoniae in lymphocytes of atherosclerotic patients. Cent. Eur. J. Public Health 2001, 9, 106–108. [Google Scholar] [PubMed]
- Behzad-Behbahani, A.; Entezam, M.; Mojiri, A.; Pouransari, R.; Rahsaz, M.; Banihashemi, M.; Heidari, T.; Farhadi, A.; Azarpira, N.; Yaghobi, R.; et al. Incidence of human herpes virus-6 and human cytomegalovirus infections in donated bone marrow and umbilical cord blood hematopoietic stem cells. Indian J. Med. Microbiol. 2008, 26, 252–255. [Google Scholar] [CrossRef][Green Version]
- Peled, O.; Berkovitch, M.; Rom, E.; Bilavsky, E.; Bernfeld, Y.; Dorfman, L.; Pappo, A.; Ziv-Baran, T.; Brandriss, N.; Bar-Haim, A.; et al. Valganciclovir Dosing for Cytomegalovirus Prophylaxis in Pediatric Solid-organ Transplant Recipients: A Prospective Pharmacokinetic Study. Pediatric Infect. Dis. J. 2017, 36, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Madi, N.; Al-Nakib, W.; Pacsa, A. Does Cytomegalovirus Develop Resistance following Antiviral Prophylaxis and Treatment in Renal Transplant Patients in Kuwait? Adv. Virol. 2011, 2011, 260561. [Google Scholar] [CrossRef][Green Version]
- Shoeibi, N.; Abrishami, M.; Mohammad Esmaeil, E.; Hosseini, S.M. Visual prognosis, clinical features, and predisposing factors in non-HIV patients with cytomegalovirus retinitis. Int. Ophthalmol. 2018. [Google Scholar] [CrossRef]
- Kotton, C.N.; Kumar, D.; Caliendo, A.M.; Asberg, A.; Chou, S.; Danziger-Isakov, L.; Humar, A. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation 2013, 96, 333–360. [Google Scholar] [CrossRef]
- Shamran, H.A.; Kadhim, H.S.; Hussain, A.R.; Kareem, A.; Taub, D.D.; Price, R.L.; Nagarkatti, M.; Nagarkatti, P.S.; Singh, U.P. Detection of human cytomegalovirus in different histopathological types of glioma in Iraqi patients. BioMed Res. Int. 2015, 2015, 642652. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.S.; Abdalhamid, B.A.; El-Badawy, S.A.; Sorour, Y.M.; Almsned, F.M.; Al-Abbadi, M.A. Expression of cytomegalovirus in glioblastoma multiforme: Myth or reality? Br. J. Neurosurg. 2016, 30, 307–312. [Google Scholar] [CrossRef]
- Mohammadizadeh, F.; Mahmudi, F. Evaluation of human cytomegalovirus antigen expression in invasive breast carcinoma in a population of Iranian patients. Infect. Agents Cancer 2017, 12, 39. [Google Scholar] [CrossRef]
- El Shazly, D.F.; Bahnassey, A.A.; Omar, O.S.; Elsayed, E.T.; Al-Hindawi, A.; El-Desouky, E.; Youssef, H.; Zekri, A.N. Detection of Human Cytomegalovirus in Malignant and Benign Breast Tumors in Egyptian Women. Clin. Breast Cancer 2018, 18, e629–e642. [Google Scholar] [CrossRef]
- Shmueli, E.; Hadar, E.; Pardo, J.; Attias, J.; Amir, J.; Bilavsky, E. Congenital Cytomegalovirus Infection After a Multiple Birth Pregnancy. Pediatric Infect. Dis. J. 2017, 36, e298–e302. [Google Scholar] [CrossRef] [PubMed]
- Ziv, L.; Yacobovich, J.; Pardo, J.; Yarden-Bilavsky, H.; Amir, J.; Osovsky, M.; Bilavsky, E. Hematologic Adverse Events Associated With Prolonged Valganciclovir Treatment in Congenital Cytomegalovirus Infection. Pediatric Infect. Dis. J. 2019, 38, 127–130. [Google Scholar] [CrossRef] [PubMed]
| Country | Study Type | Study Period | Transplant Type | No. of Patients | Seroprevalence | Median Time of Detection after TX | Symptoms and Complications | Reference |
|---|---|---|---|---|---|---|---|---|
| Iran | Retrospective study | 1984–2002 | Kidney | 1925 | 5.2% | 1 to 9 months | Elevated serum creatinine, fever, thrombocytopenia, nausea, vomiting, elevated alkaline phosphatase, leukocytosis, leukopenia, rarely pneumonia, conjunctivitis, vascular dermatitis. | Pour-Reza-Gholi et al., 2005 [28] |
| Iran | Retrospective study | 1984–2007 | Kidney | 2211 | 2.1% | NM | 1 patient died, 3 lost their allograft function | Nemati et al., 2008 [20] |
| Iran | Cross-sectional study | 1991–2010 | Kidney | 96 | 37.5% | NM | NM | Khameneh et al., 2013 [57] |
| Tunisia | Cohort study | 1994–1998 | Kidney | 18 | 72% | First 30 days | Six patients had acute rejection | Charfeddine et al., 2002 [21] |
| Iran | Retrospective study | 1996–2007 | Liver | 22 | 4.5% | NM | NM | Yaghobi et al., 2010 [58] |
| Saudi Arabia | Cross-sectional | 1996–2014 | Donors | 263 | 13.2% | NM | NM | Alsuhaibani et al., 2015 [51] |
| Iran | Retrospective study | 1998–2014 | Kidney | 725 | 24.6% | First 5 months | Weakness, fever, respiratory symptoms | Babazadeh et al., 2017 [27] |
| Kuwait | Cohort Study | 2000–2005 | Kidney | 54 | 11.11% | NM | NM | Madi et al., 2011 [59] |
| Saudi Arabia | Retrospective study | 2000–2006 | Kidney | 689 | 3.6% | NM | Fever, malaise, leukopenia | Basri et al., 2007 [60] |
| Iran | cohort study | 2001–2002 | BMT | 15 | 53.5% | 4 weeks | Fever; gastrointestinal; skin lesion; retinitis, pneumonia, UTI | Behzad-Behbahani et al., 2004 [24] |
| Egypt | Retrospective study | 2001–2003 | BMT | 28 | 39% | NM | NM | Zekri et al., 2004 [42] |
| Iran | Cross-sectional | 2002–2004 | Kidney | 179 | 17.6% | NA | Fever, malaise, arthralgias, myalgia, leukopenia and/or thrombocytopenia, or tissue-invasive disease | Pourmand et al., 2007 [61] |
| Iran | Retrospective study | 2002–2006 | BMT | 104 | IgG seroprevalence: 8.7% IgM Seroprevalence: 9.6% | 1st–10th weeks | NA | Ramzi et al., 2009 [62] |
| Saudi Arabia | Retrospective study | 2002–2007 | Cord blood | 73 | 58.9% | NM | NM | Al-Hajjar et al., 2011 [34] |
| Israel | Cohort study | 2003 | Liver | 81 | 8.5% | NA | Fever, disturbed liver functions in all patients, one patient had concurrent CMV pneumonitis and one CMV retinitis | Shibolet et al., 2003 [29] |
| Iran | Retrospective study | 2005–2008 | Kidney | 925 | IgG seroprevalence: 100% | NA | NA | Saghafi et al., 2009 [63] |
| Saudi Arabia | Retrospective study | 2005–2011 | HSCT | 82 | 1.22% | NA | NA | Al-Sweedan et al., 2017 [64] |
| Jordan | Retrospective study | 2005–2013 | HSCT | 72 | 31% | 23 days (12–31) post-transplantation | None of the patients developed CMV disease | Hussain et al., 2015 [65] |
| Sudan | Retrospective study | 2006 | Kidney | 98 | 32.7% | 2–3 months after kidney transplantation | Fever, diarrhea, hepatitis, neutropenia and/or thrombocytopenia. | Enan et al., 2011 [25] |
| Iran | Prospective study | 2006–2008 | Kidney | 40 | Infection a: 82.5% Disease b: 25% | Infection: 4.7 weeks Disease: 11 weeks | CMV disease, nine patients manifested with elevated serum creatinine values and one, elevated liver enzymes | Taherimahmoudi et al., 2009 [41] |
| Iran | Retrospective study | 2006–2013 | Liver | 145 | 32% | 12 to 445 days post transplantation | Only 1 patient (2%) developed CMV disease | Davoudi et al., 2014 [66] |
| Oman | Retrospective study | 2006–2015 | Kidney | 703 | 14.5% | 21 months (15 days–84 months) | Fever, diarrhea, pneumonitis, lymphopenia, anemia, thrombocytopenia | Siddiqui et al., 2017 [26] |
| Iraq | Cross-sectional study | 2007–2008 | Kidney | 43 | 97.7% | NA | NA | Al-Alousy et al., 2011 [67] |
| Iran | Cross-sectional | 2007–2010 | Transfusion | 96 | IgG Seroprevalence: 77.4% IgM seroprevalence: 7.1% | NA | NA | Sepehrvand et al., 2010 [54] |
| Israel | Retrospective study | 2007–2012 | HSCT | 121 | 61% | NA | First CMV infection with myeloablative conditioning and acute GVHD | Cohen et al., 2015 [22] |
| Iran | Prospective study | 2008 | Kidney | 68 | 70.6% | NA | 19 cases of acute rejection | Khameneh et al., 2008 [68] |
| Iran | Cross section | 2009–2010 | Kidney | 91 | 34.4% | 30 days | NA | Nasiri et al., 2011 [69] |
| Iran | Retrospective study | 2011–2013 | HSCT | 126 | 34% | 40 days (3–77) after transplantation | GI, dermal symptoms with hepatic involvement * 9 cases develop GVHD | Valadkhani et al., 2016 [23] |
| Iran | Case-control study | 2012–2013 | coronary artery bypass graft (CABG) | 110 | CMV DNA in Cases: 14.5% CMV DNA in Controls: 4% | NM | CMV in aortic plaques associated with increased risk of atherosclerosis | Heydar et al., 2015 [30] |
| Iran | Cross-sectional | 2012–2013 | Kidney Graft | 96 | 19.8% | NM | NM | Khameneh et al., 2013 [57] |
| Iran | Cross-sectional | 2012–2013 | Donors | 1008 | IgG seroprevalence: 99.2% IgM seroprevalence: 1.6% | NA | NA | Safabakhsh et al., 2013 [53] |
| Kuwait | Retrospective study | 2012–2014 | Kidney | 1168 | 15.4% | NA | 41% have graft rejection, 34.4% develop systemic CMV disease, 24.5% develop CMV syndrome, 1.6% died | Madi et al., 2015 [19] |
| Iran | Cohort study | 2013 | Kidney | 82 | 49% | Four months post-transplantation | The study aimed to correlate CMV infection with decreasing in vitamin D level. | Saber et al., 2015 [70] |
| Egypt | Cross-sectional | 2016 | Donors | 88 | IgG Seroprevalence: 96.6% | NA | NA | Gawad et al., 2016 [56] |
| Country | Study Type | Study Period | No. of Patients | CMV Results | Symptoms and Complications | Reference |
|---|---|---|---|---|---|---|
| Kuwait | Experimental study | 1988 | 575 infants | 2.6% positive IgM | NM | El-Mekki et al., 1988 [101] |
| Israel | Retrospective study | 1993–1997 | 63 pregnant women | 34.8% showed vertical transmission | Abnormal ultrasound, neurologic sequelae | Lipitz et al., 1997 [76] |
| Iraq | Prospective-follow up until delivery | 1999 | 60 pregnant women | 10% CMV IgM in cord blood | Congenital malformations, microcephaly | Al-Ali et al., 1999 [86] |
| Israel | Retrospective study | 1999–2008 | 59 primary Periconceptional CMV infection | 18.6% CMV infections | NM | Hadar et al., 2010 [73] |
| Israel | Retrospective study | 2001–2012 | 9845 infants | 0.57% CMV infection | Abnormal hearing | Barkai et al., 2014 [81] |
| Iran | case-control study | 2002–2003 | 95 with sensory hearing loss | 34.6% CMV infection | Sensorineural hearing loss | Pasternak et al., 2018 [102] |
| Iran | This case-control study | 2003–2004 | 250 women with a history of abortion and 200 matched with no abortion | 5% positive for CMV | Abortion | Jahromi et al., 2010 [91] |
| Israel | A prospective study | 2005 | 70 infants who received breast milk from seropositive mothers | 5.7% acquired CMV by the second or third week of pregnancy | NM | Miron et al., 2005 [103] |
| Israel | Experimental study | 2005 | 5000 Newborns | 81.5–85% serum IgM 0.7% had cCMV infection | NM | Ziyaeyan et al., 2007 [97] |
| Israel | Retrospective study | 2005–2013 | 149 | 36% CMV infection | Severe hearing loss | Bilavsky et al., 2016 [104] |
| Israel | Retrospective-cohort study | 2005–2012 | 210 infants with cCMV | 75% symptomatic 25% asymptomatic | Prematurity, abnormal hearing, lenticulostriate vasculopathy | Bilavsky et al., 2015 [72] |
| Israel | Retrospective study | 2005–2013 | 284 infants with cCMV | 69.7% symptomatic 30.3% asymptomatic | Hepatitis, cholestatic disease | Bliavsky et al., 2015 [88] |
| Palestine | Retrospective study | 2006–2012 | 249 newborns | 4 out of 249 newborns with cCMV born to mothers with positive CMV DNA in urine | NM | Neirukh et al., 2013 [105] |
| Israel | Retrospective case-control study | 2006–2015 | 138 infants with cCMV | 66.67% positive with amniocentesis | Abnormal hearing, developmental delay | Bilavsky et al., 2016 [106] |
| Iran | Experimental study | 2007 | 92 pregnant women with caesarian section | 98% of women had positive serum IgG 5.4% of women had positive serum IgM Neonates from IgG positive mothers had positive IgM | NM | Townsend et al., 2013 [107] |
| Iran | Experimental study | 2008 | 844 pregnant women | 93% had positive serum IgG 5% had positive serum IgM | Congenital disorders | Arapour et al., 2008 [83] |
| Israel | Observational study | 2008 | Twenty-eight pregnant mothers primary CMV infection acquired after 25 weeks of gestation | 21 neonates had a vertical transmission with no symptoms One pregnancy was terminated in 36 weeks with apparent symptoms | All 21 infected neonate showed clinical symptoms of CMV infection | Gindes et al., 2008 [75] |
| Israel | Retrospective study | 2009 | All pregnant mothers with positive IgM & high IgG Avidity | 79 women with CMV IgM-high IgG avidity combination (indicate past infection) | NM | Kanengisser -Pines et al., 2009 [108] |
| Israel | Retrospective study | 2009–2010 | 8105 infants | 0.28% prevalence | CNS involvement, abnormal hearing | Barkai et al., 2013 [82] |
| Israel | Cohort study | 2010 | 27 cCMV infected fetuses | Temporal lobe volumes were significantly smaller in fetuses infected with CMV compared to uninfected fetuses | Severe brain dysmorphology in first and second trimesters. | Hoffman et al., 2010 [74] |
| Egypt | Experimental study | 2011 | 33 neonate and mothers | Four neonates with positive IgM, two of which had mothers with positive IgM | Gastrointestinal complications | Abu Faddan et al., 2011 [109] |
| Israel | Prospective study behavioral studies of LSV symptoms | 2011 | 92 infants with congenital CMV | 50 cases had lenticulostriate vasculopathy and hearing loss. | CNS impairment, abnormal hearing | Amir et al., 2011 [80] |
| Israel | Retrospective study | 2011 | Infected infants (CMV DNA positive) | NM | Abnormal white matter | Farkas et al., 2011 [78] |
| Oman | Retrospective review | 2011–2012 | 373 infants | 34 positives cases | Death, prolonged PICU stay, respiratory complication | Abdelmogheth et al., 2014 [90] |
| Kuwait | Prevalence study-follow up until pregnancy | 2013 | 983 pregnant mothers | 9% positive cord blood IgM 0.9% positive urine PCR. Seven of the nine cases had a high viral load | NA | Al-Awadhi et al., 2013 [110] |
| Egypt | Cross-sectional study | 2013 | 546 pregnant women | 100% positive serum IgG 7.3% positive serum IgM with an intermediate IgG avidity index. Of these, 50% had higher avidity indices after the 3rd trimester. | NM | Kamel et al., 2014 [111] |
| Israel | Prospective cohort study | 2013 | 142 pregnant women with primary CMV infection and vertical transmission in the 1st and second trimesters | The primary infection occurred in the 1st (50%) and second (50%) trimester Seven pregnancies terminated with neurologic sequelae one neonate died due to neurologic complications | Auditory damage or neurodevelopmental disabilities | Lipitz et al., 2013 [77] |
| Iran | Prevalence study | 2013–2014 | 100 symptomatic infants less than 3-weeks old | 58% with cCMV | Hearing loss | Ebrahimi-Rad et al., 2017 [96] |
| Iran | Cross-sectional study | 2014 | 225 pregnant women and their newborns | 100% of mothers had positive IgG, of which 2.7% had positive IgM Women with normal deliveries showed low IgG compared to caesarian section | CMV infection by radiological evaluation (CT scan) | Erfanianahmadpoor et al., 2014 [112] |
| Israel | Retrospective study | 2014–2015 | 178 infants with hearing disability | 2.2% with cCMV | CNS symptoms | Ari-Even Roith et al., 2017 [79] |
| Iran | Prospective study | 2014–2016 | 1617 neonate | 0.49% with cCMV | Short-term growth impairment | Karimian et al., 2016 [84] |
| Sudan | Experimental study | 2015 | 50 infants | 8% with cCMV | Congenital anomalies | Ebrahimet al., 2015 [113] |
| Sudan | Experimental study | 2016 | 90 pregnant women | 98.9% had positive serum IgG 1.1% had positive serum IgM | NA | Altayeb et al., 2016 [114] |
| Israel | Retrospective cohort study | 2016 | 98 infants from infected mothers | 52 received antiviral upon delivery | Lenticulostriate vasculopathy on postnatal US, Sensorineural hearing loss | Amir et al., 2016 [71] |
| Iran | Case control study | 2016 | 81 pregnant women who aborted | Anti-CMV IgM was higher compared to controls (25.9% compared to 12.2%; OR = 12.2, p = 0.019) | Early abortion | Rasti et al., 2016 [85] |
| Israel | Retrospective cohort study | 2017 | 107 infants with cCMV 95 of which are from mothers with primary infection, 12 from mothers with non-primary infection | Incidence of abnormal brain sonographic findings high in infants of mothers with primary infection was 67%, compared to non-primary infection 8.3% | Infant’s Mothers acquired gestational hypertensive disorder and GDM | Hadar et al., 2010 [89] |
| Iraq | This prospective study | 2019 | 24 neonates | 96% with CMV infection | Jaundice-, hepatosplenomegaly | Alwan et al., 2019 [115] |
| Iran | Pilot study | January 2012 to March 2012 | 620 infants | 0.32% positive for CMV DNA | Infected infants showed no symptoms | Fahimzad et al., 2013 [87] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Mana, H.; Yassine, H.M.; Younes, N.N.; Al-Mohannadi, A.; Al-Sadeq, D.W.; Alhababi, D.; Nasser, E.A.; Nasrallah, G.K. The Current Status of Cytomegalovirus (CMV) Prevalence in the MENA Region: A Systematic Review. Pathogens 2019, 8, 213. https://doi.org/10.3390/pathogens8040213
Al Mana H, Yassine HM, Younes NN, Al-Mohannadi A, Al-Sadeq DW, Alhababi D, Nasser EA, Nasrallah GK. The Current Status of Cytomegalovirus (CMV) Prevalence in the MENA Region: A Systematic Review. Pathogens. 2019; 8(4):213. https://doi.org/10.3390/pathogens8040213
Chicago/Turabian StyleAl Mana, Hassan, Hadi M. Yassine, Nadin N. Younes, Anjud Al-Mohannadi, Duaa W. Al-Sadeq, Dalal Alhababi, Elham A. Nasser, and Gheyath K. Nasrallah. 2019. "The Current Status of Cytomegalovirus (CMV) Prevalence in the MENA Region: A Systematic Review" Pathogens 8, no. 4: 213. https://doi.org/10.3390/pathogens8040213
APA StyleAl Mana, H., Yassine, H. M., Younes, N. N., Al-Mohannadi, A., Al-Sadeq, D. W., Alhababi, D., Nasser, E. A., & Nasrallah, G. K. (2019). The Current Status of Cytomegalovirus (CMV) Prevalence in the MENA Region: A Systematic Review. Pathogens, 8(4), 213. https://doi.org/10.3390/pathogens8040213





