Detection of Second Line Drug Resistance among Drug Resistant Mycobacterium Tuberculosis Isolates in Botswana
Abstract
:1. Background
2. Methods
2.1. Design and Study Population
2.2. Treatment Outcome Definitions
2.3. DNA Extraction
2.4. Genotyping
2.4.1. Spoligotyping
2.4.2. Hain Genotype MTBDRsl Version 2
2.4.3. Data Analysis
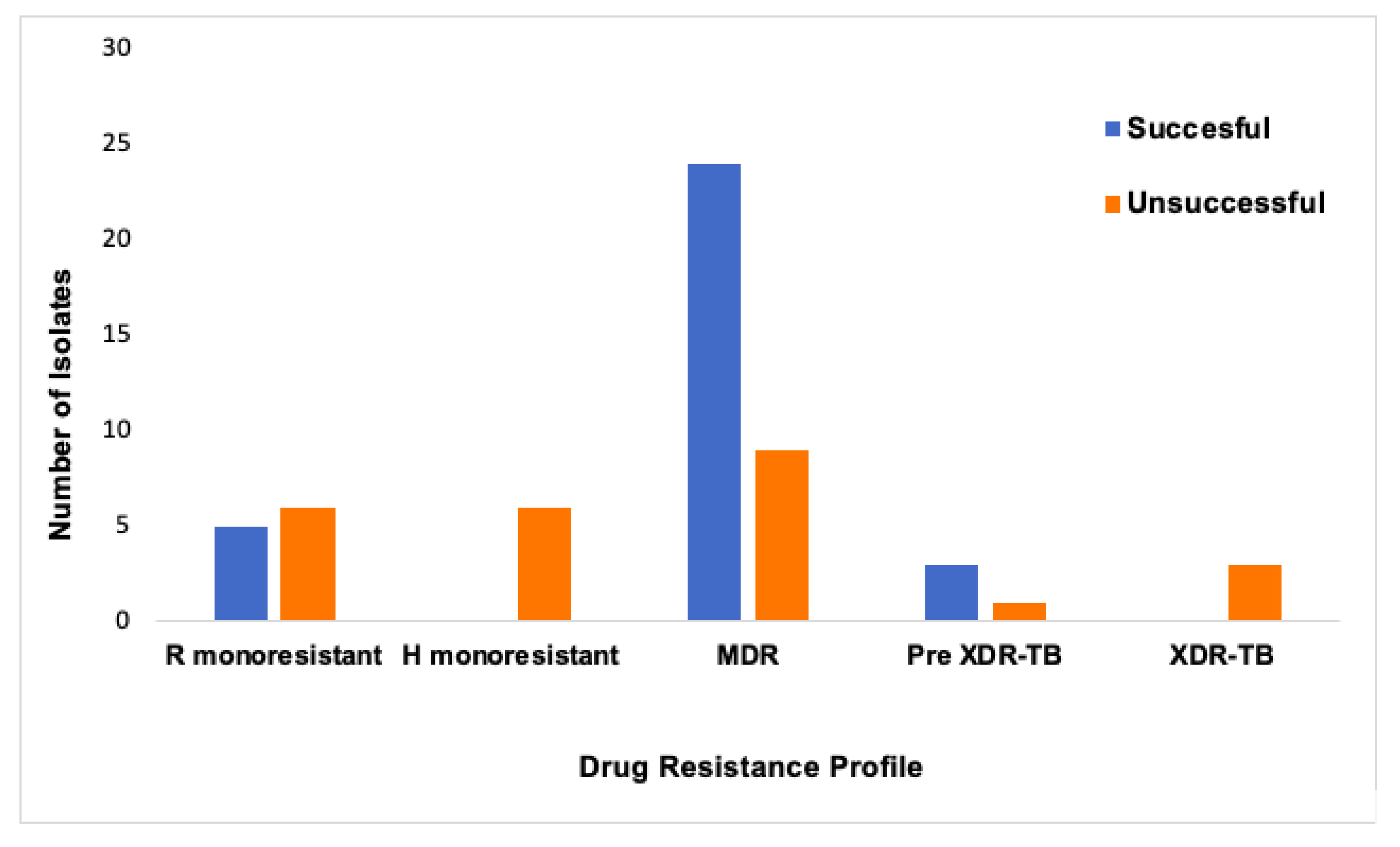
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. Global Tuberculosis Report. Available online: https://www.who.int/tb/publications/global_report/en/ (accessed on 20 July 2019).
- Oudghiri, A.; Karimi, H.; Chetioui, F.; Zakham, F.; Bourkadi, J.E.; Elmessaoudi, M.D.; Laglaoui, A.; Chaoui, I.; El Mzibri, M. Molecular characterization of mutations associated with resistance to second-line tuberculosis drug among multidrug-resistant tuberculosis patients from high prevalence tuberculosis city in Morocco. BMC Infect. Dis. 2018, 18, 98. [Google Scholar] [CrossRef] [PubMed]
- Villegas, L.; Otero, L.; Sterling, T.R.; Huaman, M.A.; Van der Stuyft, P.; Gotuzzo, E.; Seas, C. Prevalence, risk factors, and treatment outcomes of isoniazid-and rifampicin-mono-resistant pulmonary tuberculosis in lima, peru. PLoS ONE 2016, 11, e0152933. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, M. Isoniazid monoresistance: A precursor to multidrug-resistant tuberculosis? Ann. Am. Thorac. Soc. 2018, 15, 306–307. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.B.; Nguyen, N.V.; Tran, H.T.; Nguyen, H.V.; Bui, Q.T. Prevalence of resistance to second-line tuberculosis drug among multidrug-resistant tuberculosis patients in Viet Nam, 2011. Western Pac. Surveill. Response J. 2016, 7, 35–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Y.; Hoffner, S.; Wu, L.; Zhao, Q.; Jiang, W.; Xu, B. Prevalence and genetic characterization of second-line drug-resistant and extensively drug-resistant Mycobacterium tuberculosis in Rural China. Antimicrob. Agents Chemother. 2013, 57, 3857–3863. [Google Scholar] [CrossRef]
- Gardee, Y.; Dreyer, A.W.; Koornhof, H.J.; Omar, S.V.; da Silva, P.; Bhyat, Z.; Ismail, N.A. Evaluation of the GenoType MTBDRsl version 2.0 assay for second-line drug resistance detection of mycobacterium tuberculosis isolates in South Africa. J. Clin. Microbiol. 2017, 55, 791–800. [Google Scholar] [CrossRef]
- Farhat, M.R.; Jacobson, K.R.; Franke, M.F.; Kaur, D.; Sloutsky, A.; Mitnick, C.D.; Murray, M. Gyrase mutations are associated with variable levels of fluoroquinolone resistance in mycobacterium tuberculosis. J. Clin. Microbiol. 2016, 54, 727–733. [Google Scholar] [CrossRef]
- Hooper, D.C.; Jacoby, G.A. Mechanisms of drug resistance: Quinolone resistance. Ann. N.Y. Acad. Sci. 2015, 1354, 12–31. [Google Scholar] [CrossRef]
- Jabeen, K.; Shakoor, S.; Malik, F.; Hasan, R. Fluoroquinolone resistance in Mycobacterium tuberculosis isolates from Pakistan 2010–2014: Implications for disease control. Int. J. Mycobacteriol. 2015, 4, 47–48. [Google Scholar] [CrossRef]
- WHO. The Use of Molecular Line Probe Assays for the Detection of Resistance to Second-Line Anti-Tuberculosis Drugs: Policy Guidance; WHO: Geneva, Switzerland, 2016; Available online: https://www.who.int/tb/publications/lpa-mdr-diagnostics/en/ (accessed on 23 July 2019).
- HainLifescience. Instructions for Use for Hain GenoType MTBDRsl, Version 2.0; HainLifescience: Nehren, Germany; Available online: https://www.hain-lifescience.de/en/instructions-for-use.html (accessed on 20 July 2019).
- Tagliani, E.; Cabibbe, A.M.; Miotto, P.; Borroni, E.; Toro, J.C.; Mansjo, M.; Hoffner, S.; Hillemann, D.; Zalutskaya, A.; Skrahina, A.; et al. Diagnostic performance of the new version (v2.0) of genotype mtbdrsl assay for detection of resistance to fluoroquinolones and second-line injectable drugs: A multicenter study. J. Clin. Microbiol. 2015, 53, 2961–2969. [Google Scholar] [CrossRef]
- Ismail, N.; Ismail, F.; Omar, S.V.; Blows, L.; Gardee, Y.; Koornhof, H.; Onyebujoh, P.C. Drug resistant tuberculosis in Africa: Current status, gaps and opportunities. Afr. J. Lab. Med. 2018, 7, 781. [Google Scholar] [CrossRef] [PubMed]
- Nanzaluka, F.H.; Chibuye, S.; Kasapo, C.C.; Langa, N.; Nyimbili, S.; Moonga, G.; Kapata, N.; Kumar, R.; Chongwe, G. Factors associated with unfavourable tuberculosis treatment outcomes in Lusaka, Zambia, 2015: A secondary analysis of routine surveillance data. Pan. Afr. Med. J. 2019, 32, 159. [Google Scholar] [CrossRef]
- Tanue, E.A.; Nsagha, D.S.; Njamen, T.N.; Assob, N.J.C. Tuberculosis treatment outcome and its associated factors among people living with HIV and AIDS in Fako Division of Cameroon. PLoS ONE 2019, 14, e0218800. [Google Scholar] [CrossRef] [PubMed]
- Mogashoa, T.; Melamu, P.; Ley, S.D.; Streicher, E.M.; Iketleng, T.; Kelentse, N.; Mupfumi, L.; Mokomane, M.; Kgwaadira, B.; Novitsky, V.; et al. Genetic diversity of Mycobacterium tuberculosis strains circulating in Botswana. PLoS ONE 2019, 14, e0216306. [Google Scholar] [CrossRef]
- National Tuberculosis Programme Manual 2011. Available online: https://www.who.int/hiv/pub/guidelines/botswana_tb.pdf (accessed on 5 January 2019).
- HainLifescience. Instructions for Use for Hain GenoLyse Kit for Isolation of Genomic Bacterial DNA; HainLifescience: Nehren, Germany; Available online: https://www.hain-lifescience.de/en/instructions-for-use.html (accessed on 20 July 2019).
- Kamerbeek, J.; Schouls, L.; Fau-Kolk, A.; Kolk, A.; Fau-van Agterveld, M.; van Agterveld, M.; Fau-van Soolingen, D.; van Soolingen, D.; Fau-Kuijper, S.; Kuijper, S.; et al. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J. Clin. Microbiol. 1997, 35, 907–914. [Google Scholar] [PubMed]
- Cox, H.; Kebede, Y.; Allamuratova, S.; Ismailov, G.; Davletmuratova, Z.; Byrnes, G.; Stone, C.; Niemann, S.; Rusch-Gerdes, S.; Blok, L.; et al. Tuberculosis recurrence and mortality after successful treatment: Impact of drug resistance. PLoS Med. 2006, 3, e384. [Google Scholar] [CrossRef]
- Munang, M.L.; Kariuki, M.; Dedicoat, M. Isoniazid-resistant tuberculosis in Birmingham, United Kingdom, 1999–2010. QJM 2015, 108, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Kendall, E.A.; Malhotra, S.; Cook-Scalise, S.; Denkinger, C.M.; Dowdy, D.W. Estimating the impact of a novel drug regimen for treatment of tuberculosis: A modeling analysis of projected patient outcomes and epidemiological considerations. BMC Infect. Dis. 2019, 19, 794. [Google Scholar] [CrossRef]
- Saravu, K.; Pai, M. Drug-resistant tuberculosis: Progress towards shorter and safer regimens. Lung India 2019, 36, 373–375. [Google Scholar] [CrossRef]
- GLI. Line Probe Assays for Drug Resistant Tuberculosis Detection. Interpretation and Reporting Guide for Laboratory Staff and Clinicians. Available online: http://www.stoptb.org/wg/gli/assets/documents/LPA_test_web_ready.pdf (accessed on 20 July 2019).
- Maitre, T.; Petitjean, G.; Chauffour, A.; Bernard, C.; El Helali, N.; Jarlier, V.; Reibel, F.; Chavanet, P.; Aubry, A.; Veziris, N. Are moxifloxacin and levofloxacin equally effective to treat XDR tuberculosis? J. Antimicrob. Chemother. 2017, 72, 2326–2333. [Google Scholar] [CrossRef] [Green Version]
- Chien, J.Y.; Chen, Y.T.; Wu, S.G.; Lee, J.J.; Wang, J.Y.; Yu, C.J. Treatment outcome of patients with isoniazid mono-resistant tuberculosis. Clin. Microbiol. Infect. 2015, 21, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Yimer, S.A.; Agonafir, M.; Derese, Y.; Sani, Y.; Bjune, G.A.; Holm-Hansen, C. Primary drug resistance to anti-tuberculosis drugs in major towns of Amhara region, Ethiopia. APMIS 2012, 120, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Asmamaw, D.; Seyoum, B.; Makonnen, E.; Atsebeha, H.; Woldemeskel, D.; Yamuah, L.; Addus, H.; Aseffa, A. Primary drug resistance in newly diagnosed smear positive tuberculosis patients in Addis Ababa, Ethiopia. Ethiop. Med. J. 2008, 46, 367–374. [Google Scholar] [PubMed]
- Vidyaraj, C.K.; Chitra, A.; Smita, S.; Muthuraj, M.; Govindarajan, S.; Usharani, B.; Anbazhagi, S. Prevalence of rifampicin-resistant Mycobacterium tuberculosis among human-immunodeficiency-virus-seropositive patients and their treatment outcomes. J. Epidemiol. Glob. Health 2017, 7, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Haar, C.H.; Cobelens, F.G.; Kalisvaart, N.A.; van der Have, J.J.; van Gerven, P.J.; van Soolingen, D. Tuberculosis drug resistance and HIV infection, the Netherlands. Emerg. Infect. Dis. 2007, 13, 776–778. [Google Scholar] [CrossRef]
- Fenner, L.; Atkinson, A.; Boulle, A.; Fox, M.P.; Prozesky, H.; Zurcher, K.; Ballif, M.; Furrer, H.; Zwahlen, M.; Davies, M.A.; et al. HIV viral load as an independent risk factor for tuberculosis in South Africa: Collaborative analysis of cohort studies. J. Int. AIDS Soc. 2017, 20, 21327. [Google Scholar] [CrossRef]
- Ajileye, A.; Alvarez, N.; Merker, M.; Walker, T.M.; Akter, S.; Brown, K.; Moradigaravand, D.; Schon, T.; Andres, S.; Schleusener, V.; et al. Some synonymous and nonsynonymous gyra mutations in mycobacterium tuberculosis lead to systematic false-positive fluoroquinolone resistance results with the hain genotype mtbdrsl assays. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef]
- Disratthakit, A.; Prammananan, T.; Tribuddharat, C.; Thaipisuttikul, I.; Doi, N.; Leechawengwongs, M.; Chaiprasert, A. Role of gyrB mutations in pre-extensively and extensively drug-resistant tuberculosis in thai clinical isolates. Antimicrob. Agents Chemother. 2016, 60, 5189–5197. [Google Scholar] [CrossRef]
| n | % | |
|---|---|---|
| Sex | ||
| Male | 29 | 50.9 |
| Female | 28 | 49.1 |
| Age in years | ||
| <20 years | 9 | 16.1 |
| 20–39 years | 28 | 50.0 |
| 40–59 years | 16 | 28.6 |
| >60 years | 3 | 5.4 |
| HIV status | ||
| Negative | 15 | 26.3 |
| Positive | 31 | 54.4 |
| Unknown | 11 | 19.3 |
| Specimen type | ||
| Extra-pulmonary | 1 | 1.8 |
| Pulmonary | 55 | 96.5 |
| Unknown | 1 | 1.8 |
| Smear results | ||
| Negative | 12 | 21.1 |
| Positive | 45 | 79 |
| Drug resistance profile | ||
| Rifampicin monoresistant | 11 | 19.3 |
| Isoniazid monoresistant | 6 | 10.5 |
| Multi-drug resistant (MDR) | 33 | 57.9 |
| Pre-XDR* | 4 | 7.0 |
| XDR** | 3 | 5.3 |
| Region | ||
| Central | 24 | 42.1 |
| South West | 1 | 1.8 |
| North West | 5 | 8.8 |
| Southern | 27 | 47.4 |
| Lineage | ||
| Lineage 1 | 7 | 12.3 |
| Lineage 2 | 11 | 19.3 |
| Lineage 4 | 38 | 66.7 |
| Unknown | 1 | 1.8 |
| MDR N = 50 | 2nd Line Drug Resistance* N = 7 | p-value | |
|---|---|---|---|
| Sex | n (%) | n (%) | 0.253 |
| Male | 27 (54) | 2 (29) | |
| Female | 23 (46) | 5 (71) | |
| Age in years | 0.833 | ||
| <20 years | 8 (16) | 1 (14) | |
| 20–39 years | 23 (47) | 5 (71) | |
| 40–59 years | 15 (31) | 1 (14) | |
| >60 years | 3 (6) | 0 (0) | |
| HIV status | 0.226 | ||
| Negative | 15 (30) | 0 (0) | |
| Positive | 26 (52) | 5 (71) | |
| Unknown | 9 (18) | 2 (29) | |
| Smear results | 0.630 | ||
| Negative | 10 (20) | 2 (29) | |
| Positive | 40 (80) | 5 (71) | |
| Region | 0.866 | ||
| Central | 20 (40) | 4 (57) | |
| South West | 1 (2) | 0 (0) | |
| North West | 24 (48) | 0 (0) | |
| Southern | 5 (10) | 3 (43) | |
| Lineage | 0.066 | ||
| Lineage 1 | 4 (8) | 3 (43) | |
| Lineage 2 | 11 (22) | 0 (0) | |
| Lineage 4 | 34 (68) | 4 (57) | |
| Unknown | 1 (2) | 0 (0) | |
| Case | Age | Sex | Region | HIV Status | FLDs Drug Resistance Pattern | Hybridization Pattern (s) | Codon Mutations | SLDs Drug Resistance Pattern | M.tb Lineage, Spoligo Family | Treatment Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 29 | F | Central | Positive | H; R; S; E | gyrA ΔWT3 + rrs ΔWT1 + rrs MUT1 | Undefined mutation, A1401G | OFL; LFX; KAN; AM; CAP | L4, X3 | Failed |
| 2 | 28 | F | North West | Unknown | H; R; S; E | gyrA ΔWT3 + rrs ΔWT1 + rrs MUT1 | Undefined mutation, A1401G | OFL; LFX; KAN; AM; CAP | L4, X3 | Failed |
| 3 | 32 | M | North West | Positive | H; R; E | gyrA ΔWT2 + gyrA MUT1+ rrs ΔWT1 + rrs MUT1 | A90V, A1401G | OFL; LFX; KAN; AM; CAP | L4, LAM4 | Deceased |
| 4 | 37 | F | Central | Positive | H; R; E | gyrA ΔWT3 + gyrA MUT1 | A90V | OFL; LFX | L1, EAI1_SOM | Completed |
| 5 | 44 | M | Central | Unknown | H; R; S; E | gyrA ΔWT2 + gyrA MUT1 | A90V | OFL; LFX | L1, EAI1_SOM | *Not evaluated |
| 6 | 34 | F | Central | Positive | H; R; S; E | gyrA ΔWT2 + gyrA MUT1 | A90V | OFL; LFX | L1, EAI1_SOM | Completed |
| 7 | 16 | F | South | Positive | H; R; S; E | gyrA ΔWT1 | G88A/G88C | OFL; LFX | L4, LAM3 | Cured |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mogashoa, T.; Melamu, P.; Derendinger, B.; Ley, S.D.; Streicher, E.M.; Iketleng, T.; Mupfumi, L.; Mokomane, M.; Kgwaadira, B.; Rankgoane-Pono, G.; et al. Detection of Second Line Drug Resistance among Drug Resistant Mycobacterium Tuberculosis Isolates in Botswana. Pathogens 2019, 8, 208. https://doi.org/10.3390/pathogens8040208
Mogashoa T, Melamu P, Derendinger B, Ley SD, Streicher EM, Iketleng T, Mupfumi L, Mokomane M, Kgwaadira B, Rankgoane-Pono G, et al. Detection of Second Line Drug Resistance among Drug Resistant Mycobacterium Tuberculosis Isolates in Botswana. Pathogens. 2019; 8(4):208. https://doi.org/10.3390/pathogens8040208
Chicago/Turabian StyleMogashoa, Tuelo, Pinkie Melamu, Brigitta Derendinger, Serej D. Ley, Elizabeth M. Streicher, Thato Iketleng, Lucy Mupfumi, Margaret Mokomane, Botshelo Kgwaadira, Goabaone Rankgoane-Pono, and et al. 2019. "Detection of Second Line Drug Resistance among Drug Resistant Mycobacterium Tuberculosis Isolates in Botswana" Pathogens 8, no. 4: 208. https://doi.org/10.3390/pathogens8040208
APA StyleMogashoa, T., Melamu, P., Derendinger, B., Ley, S. D., Streicher, E. M., Iketleng, T., Mupfumi, L., Mokomane, M., Kgwaadira, B., Rankgoane-Pono, G., Tsholofelo, T. T., Kasvosve, I., Moyo, S., Warren, R. M., & Gaseitsiwe, S. (2019). Detection of Second Line Drug Resistance among Drug Resistant Mycobacterium Tuberculosis Isolates in Botswana. Pathogens, 8(4), 208. https://doi.org/10.3390/pathogens8040208





