Cross-Reactive Antibodies Binding to the Influenza Virus Subtype H11 Hemagglutinin
Abstract
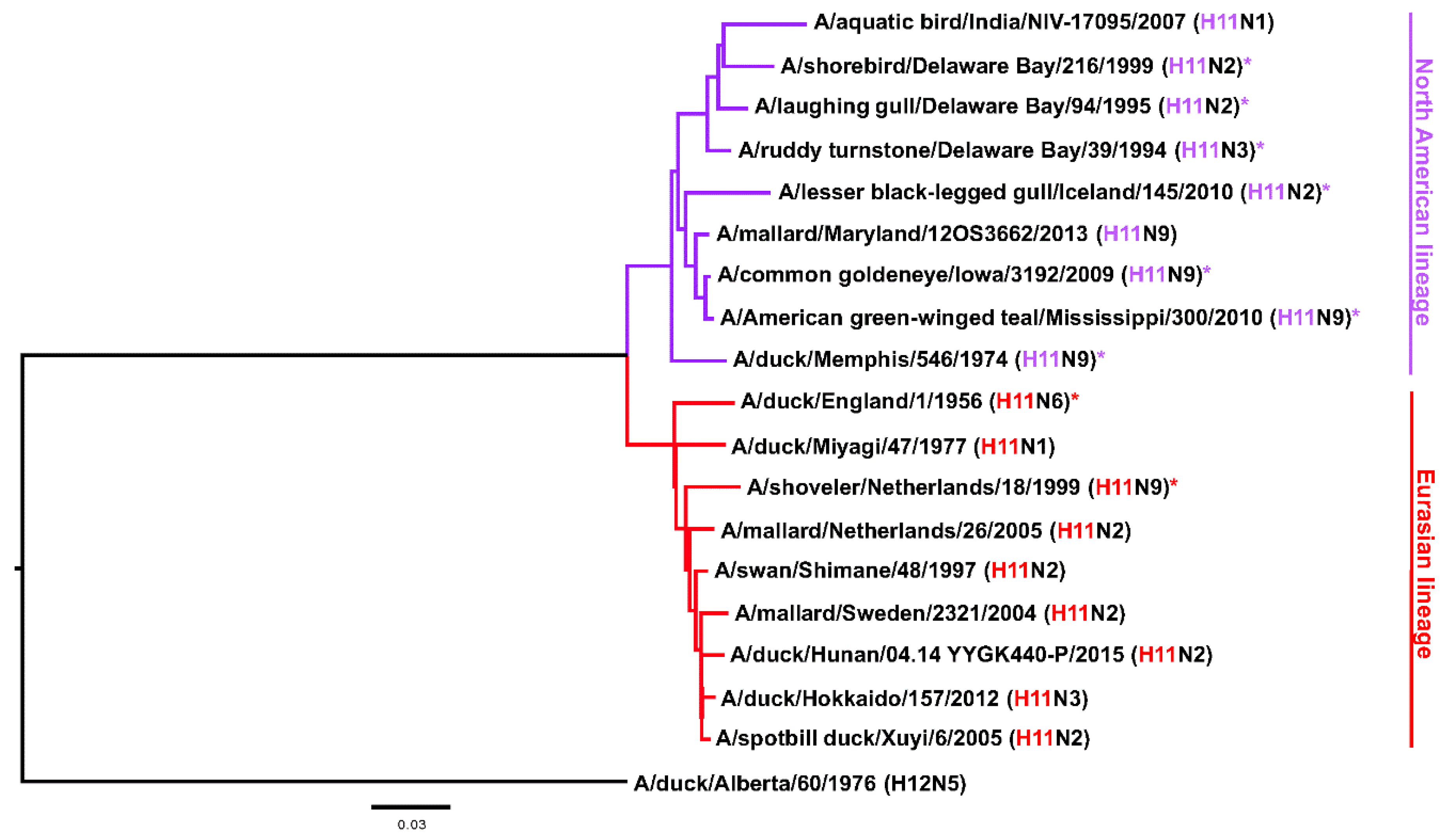
1. Introduction
2. Results
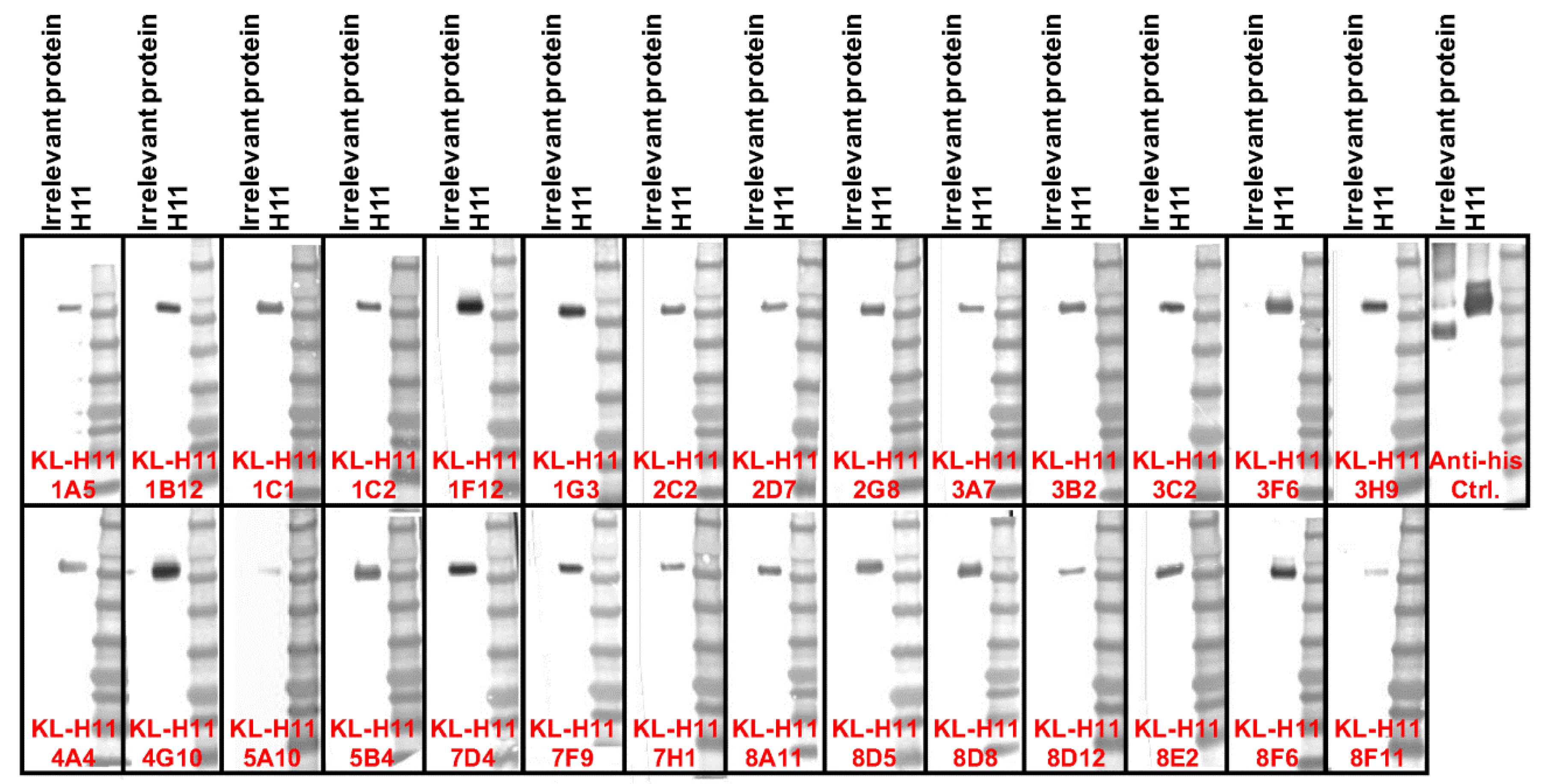
2.1. Generation of mAbs and Intial Characterization
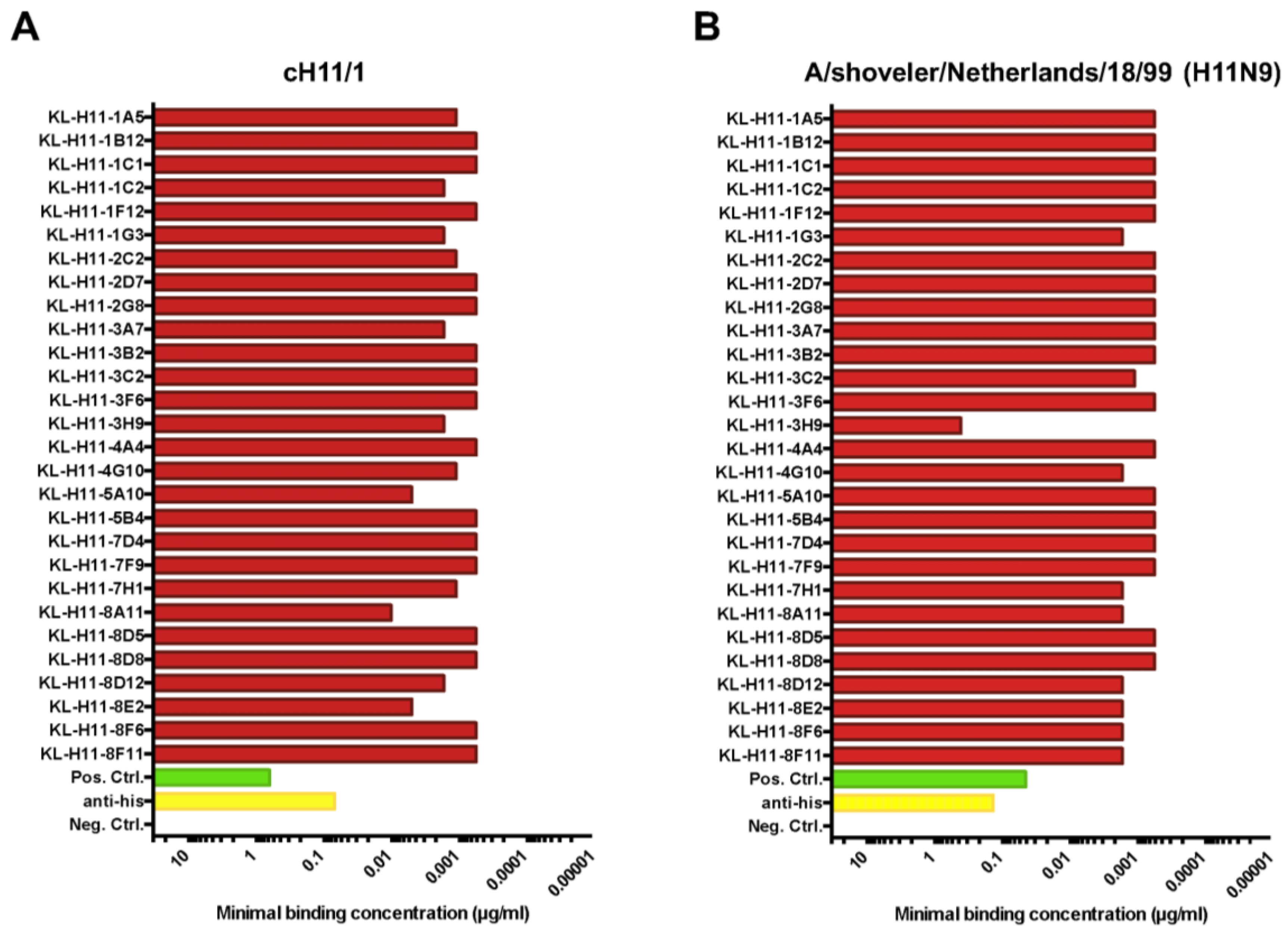
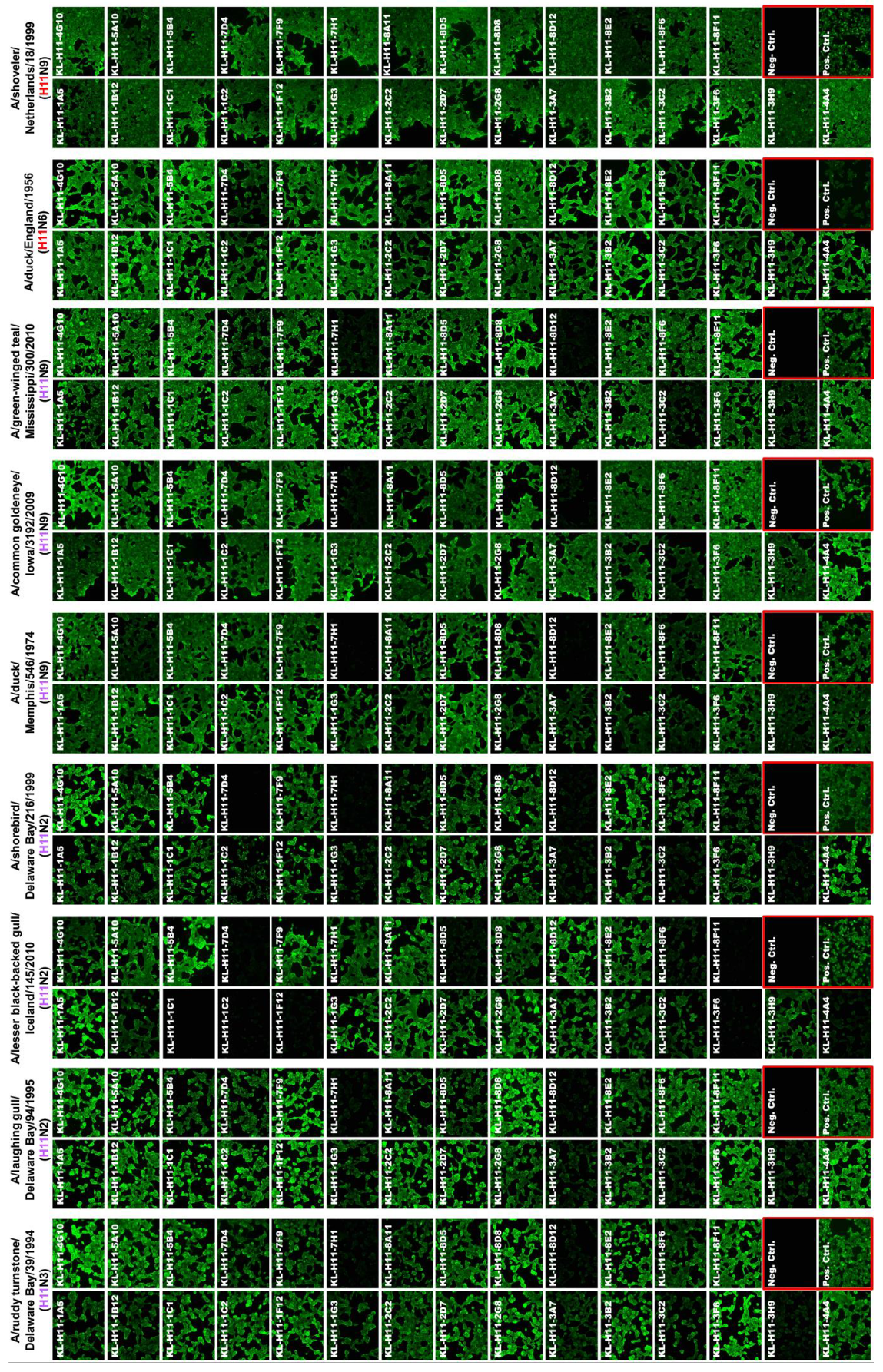
2.2. Most H11 mAbs Are Cross-Reactive
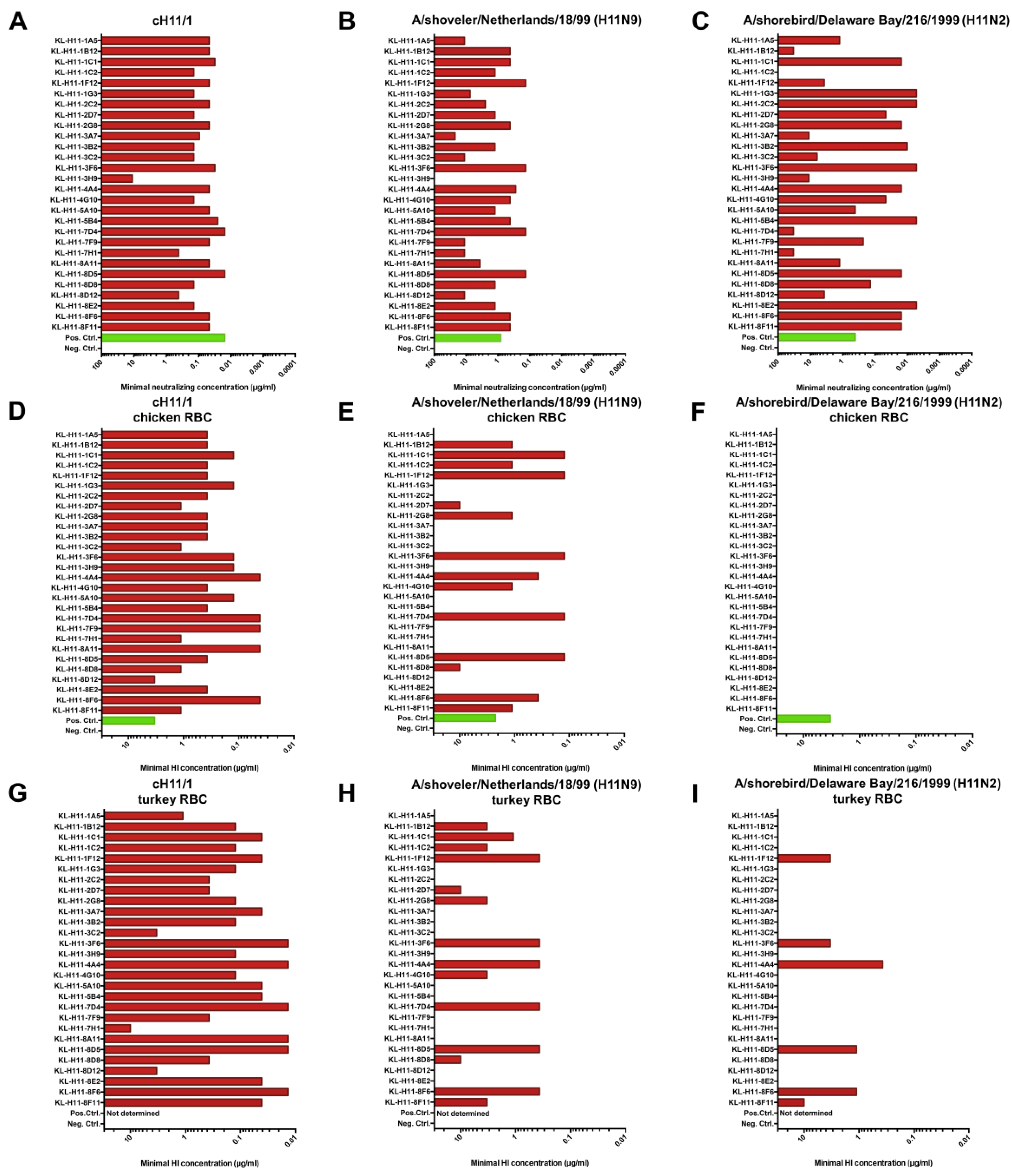
2.3. All H11 mAbs Are HI Active against cH11/1N1 Virus but Not All Are HI Active against HA-Head Homologous Wild Type H11 Virus
2.4. Several H11 mAbs Show HI and Neutralizing Activity against An Eurasian Lineage Virus but Only Show Neutralizing Activity against A North American Lineage Isolate
2.5. H11 mAbs Bind to Linear Epitopes
3. Discussion
4. Materials and Methods
4.1. Cells and Viruses
4.2. Recombinant Proteins
4.3. Enzyme-Linked Immunosorbent Assay
4.4. Generation of Monoclonal H11-Antibodies
4.5. Immunofluorescence Assay
4.6. Hemagglutination Inhibition Assay
4.7. Microneutralization Assay
4.8. Western Blot
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lupiani, B.; Reddy, S.M. The history of avian influenza. Comp. Immunol. Microbiol. Infect. Dis. 2009, 32, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.H. The Isolation of an Influenza a Virus and a Mycoplasma Associated with Duck Sinusitis. Vet. Rec. 1964, 76, 470–473. [Google Scholar]
- Webster, R.G.; Morita, M.; Pridgen, C.; Tumova, B. Ortho- and paramyxoviruses from migrating feral ducks: Characterization of a new group of influenza A viruses. J. Gen. Virol. 1976, 32, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Downie, J.C.; Hinshaw, V.; Laver, W.G. The ecology of influenza. Isolation of type ‘A’ influenza viruses from Australian pelagic birds. Aust. J. Exp. Biol. Med. Sci. 1977, 55, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Wille, M.; Latorre-Margalef, N.; Tolf, C.; Halpin, R.; Wentworth, D.; Fouchier, R.A.M.; Raghwani, J.; Pybus, O.G.; Olsen, B.; Waldenström, J. Where do all the subtypes go? Temporal dynamics of H8–H12 influenza A viruses in waterfowl. Virus Evol. 2018, 4, vey025. [Google Scholar] [CrossRef]
- Kawaoka, Y.; Chambers, T.M.; Sladen, W.L.; Webster, R.G. Is the gene pool of influenza viruses in shorebirds and gulls different from that in wild ducks? Virology 1988, 163, 247–250. [Google Scholar] [CrossRef]
- Zakstel’skaja, L.J.; Isacenko, V.A.; Osidze, N.G.; Timofeeva, C.C.; Slepuskin, A.N.; Sokolova, N.N. Some observations on the circulation of influenzaviruses in domestic and wild birds. Bull. World Health Organ. 1972, 47, 497–501. [Google Scholar]
- Louzis, C.; Andral, B.; Lernoud, J.M. A case of influenza infection of zoo touracos. Avian Pathol. 1985, 14, 163–172. [Google Scholar] [CrossRef]
- Sharp, G.B.; Kawaoka, Y.; Wright, S.M.; Turner, B.; Hinshaw, V.; Webster, R.G. Wild ducks are the reservoir for only a limited number of influenza A subtypes. Epidemiol. Infect. 1993, 110, 161–176. [Google Scholar] [CrossRef]
- Suttie, A.; Karlsson, E.A.; Deng, Y.M.; Hurt, A.C.; Greenhill, A.R.; Barr, I.G.; Dussart, P.; Horwood, P.F. Avian influenza in the Greater Mekong Subregion, 2003–2018. Infect. Genet. Evol. 2019, 74, 103920. [Google Scholar] [CrossRef]
- Gill, J.S.; Webby, R.; Gilchrist, M.J.; Gray, G.C. Avian influenza among waterfowl hunters and wildlife professionals. Emerg. Infect. Dis. 2006, 12, 1284–1286. [Google Scholar] [CrossRef] [PubMed]
- Gray, G.C.; Ferguson, D.D.; Lowther, P.E.; Heil, G.L.; Friary, J.A. A national study of US bird banders for evidence of avian influenza virus infections. J. Clin. Virol. 2011, 51, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Kayali, G.; Barbour, E.; Dbaibo, G.; Tabet, C.; Saade, M.; Shaib, H.A.; Debeauchamp, J.; Webby, R.J. Evidence of infection with H4 and H11 avian influenza viruses among Lebanese chicken growers. PLoS ONE 2011, 6, e26818. [Google Scholar] [CrossRef] [PubMed]
- Kayali, G.; Ortiz, E.J.; Chorazy, M.L.; Gray, G.C. Evidence of previous avian influenza infection among US turkey workers. Zoonoses Public Health 2010, 57, 265–272. [Google Scholar] [CrossRef]
- Capuano, A.M.; Miller, M.; Stallknecht, D.E.; Moriarty, M.; Plancarte, M.; Dodd, E.; Batac, F.; Boyce, W.M. Serologic Detection of Subtype-specific Antibodies to Influenza A Viruses in Southern Sea Otters (Enhydra lutris nereis). J. Wildl. Dis. 2017, 53, 906–910. [Google Scholar] [CrossRef]
- Jiménez-Bluhm, P.; Karlsson, E.A.; Ciuoderis, K.A.; Cortez, V.; Marvin, S.A.; Hamilton-West, C.; Schultz-Cherry, S.; Osorio, J.E. Avian H11 influenza virus isolated from domestic poultry in a Colombian live animal market. Emerg. Microbes Infect. 2016, 5, e121. [Google Scholar] [CrossRef]
- Galloway, S.E.; Reed, M.L.; Russell, C.J.; Steinhauer, D.A. Influenza HA subtypes demonstrate divergent phenotypes for cleavage activation and pH of fusion: Implications for host range and adaptation. PLoS Pathog. 2013, 9, e1003151. [Google Scholar] [CrossRef]
- Driskell, E.A.; Jones, C.A.; Stallknecht, D.E.; Howerth, E.W.; Tompkins, S.M. Avian influenza virus isolates from wild birds replicate and cause disease in a mouse model of infection. Virology 2010, 399, 280–289. [Google Scholar] [CrossRef]
- Wu, H.; Peng, X.; Wu, N. Isolation and molecular characterization of reassortant H11N3 subtype avian influenza viruses isolated from domestic ducks in Zhejiang Province in China. Virus Genes 2016, 52, 732–737. [Google Scholar] [CrossRef]
- Runstadler, J.; Hill, N.; Hussein, I.T.; Puryear, W.; Keogh, M. Connecting the study of wild influenza with the potential for pandemic disease. Infect Genet. Evol. 2013, 17, 162–187. [Google Scholar] [CrossRef]
- Pawar, S.; Chakrabarti, A.; Cherian, S.; Pande, S.; Nanaware, M.; Raut, S.; Pal, B.; Jadhav, S.; Kode, S.; Koratkar, S.; et al. An avian influenza A(H11N1) virus from a wild aquatic bird revealing a unique Eurasian-American genetic reassortment. Virus Genes 2010, 41, 14–22. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, J.; Cardona, C.J.; Xing, Z.; Woolcock, P.R. Genetic and phenotypic characterization of a low-pathogenicity avian influenza H11N9 virus. Arch. Virol. 2008, 153, 1899–1908. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Peng, X.; Wu, N. Molecular characterization of a reassortant H11N9 subtype avian influenza virus isolated from a domestic duck in Eastern China. Arch. Virol. 2015, 160, 2595–2601. [Google Scholar] [CrossRef] [PubMed]
- Karamendin, K.; Kydyrmanov, A.; Zhumatov, K.; Asanova, S.; Ishmukhametova, N.; Sayatov, M. Phylogenetic analysis of avian influenza viruses of H11 subtype isolated in Kazakhstan. Virus Genes 2011, 43, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, M.; Sun, W.; Comella, P.; Nachbagauer, R.; Wohlbold, T.J.; Amanat, F.; Kirkpatrick, E.; Palese, P.; Krammer, F. An immuno-assay to quantify influenza virus hemagglutinin with correctly folded stalk domains in vaccine preparations. PLoS ONE 2018, 13, e0194830. [Google Scholar] [CrossRef]
- Liu, W.C.; Nachbagauer, R.; Stadlbauer, D.; Solórzano, A.; Berlanda-Scorza, F.; García-Sastre, A.; Palese, P.; Krammer, F.; Albrecht, R.A. Sequential Immunization With Live-Attenuated Chimeric Hemagglutinin-Based Vaccines Confers Heterosubtypic Immunity Against Influenza A Viruses in a Preclinical Ferret Model. Front. Immunol. 2019, 10, 756. [Google Scholar] [CrossRef]
- Chen, C.J.; Ermler, M.E.; Tan, G.S.; Krammer, F.; Palese, P.; Hai, R. Influenza A Viruses Expressing Intra- or Intergroup Chimeric Hemagglutinins. J. Virol. 2016, 90, 3789–3793. [Google Scholar] [CrossRef]
- Tran, E.E.; Podolsky, K.A.; Bartesaghi, A.; Kuybeda, O.; Grandinetti, G.; Wohlbold, T.J.; Tan, G.S.; Nachbagauer, R.; Palese, P.; Krammer, F.; et al. Cryo-electron Microscopy Structures of Chimeric Hemagglutinin Displayed on a Universal Influenza Vaccine Candidate. MBio 2016, 7. [Google Scholar] [CrossRef]
- Tan, G.S.; Leon, P.E.; Albrecht, R.A.; Margine, I.; Hirsh, A.; Bahl, J.; Krammer, F. Broadly-Reactive Neutralizing and Non-neutralizing Antibodies Directed against the H7 Influenza Virus Hemagglutinin Reveal Divergent Mechanisms of Protection. PLoS Pathog. 2016, 12, e1005578. [Google Scholar] [CrossRef]
- Stadlbauer, D.; Amanat, F.; Strohmeier, S.; Nachbagauer, R.; Krammer, F. Cross-reactive mouse monoclonal antibodies raised against the hemagglutinin of A/Shanghai/1/2013 (H7N9) protect against novel H7 virus isolates in the mouse model. Emerg. Microbes Infect. 2018, 7, 110. [Google Scholar] [CrossRef]
- Amanat, F.; Meade, P.; Strohmeier, S.; Krammer, F. Cross-reactive antibodies binding to H4 hemagglutinin protect against a lethal H4N6 influenza virus challenge in the mouse model. Emerg. Microbes Infect. 2019, 8, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Isakova-Sivak, I.; Chen, L.M.; Matsuoka, Y.; Voeten, J.T.; Kiseleva, I.; Heldens, J.G.; den Bosch, H.; Klimov, A.; Rudenko, L.; Cox, N.J.; et al. Genetic bases of the temperature-sensitive phenotype of a master donor virus used in live attenuated influenza vaccines: A/Leningrad/134/17/57 (H2N2). Virology 2011, 412, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Klausberger, M.; Wilde, M.; Palmberger, D.; Hai, R.; Albrecht, R.A.; Margine, I.; Hirsh, A.; García-Sastre, A.; Grabherr, R.; Krammer, F. One-shot vaccination with an insect cell-derived low-dose influenza A H7 virus-like particle preparation protects mice against H7N9 challenge. Vaccine 2014, 32, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F.; Margine, I.; Tan, G.S.; Pica, N.; Krause, J.C.; Palese, P. A carboxy-terminal trimerization domain stabilizes conformational epitopes on the stalk domain of soluble recombinant hemagglutinin substrates. PLoS ONE 2012, 7, e43603. [Google Scholar] [CrossRef]
- Margine, I.; Palese, P.; Krammer, F. Expression of Functional Recombinant Hemagglutinin and Neuraminidase Proteins from the Novel H7N9 Influenza Virus Using the Baculovirus Expression System. J. Vis. Exp. 2013. [Google Scholar] [CrossRef]
- Dreyfus, C.; Laursen, N.S.; Kwaks, T.; Zuijdgeest, D.; Khayat, R.; Ekiert, D.C.; Lee, J.H.; Metlagel, Z.; Bujny, M.V.; Jongeneelen, M.; et al. Highly conserved protective epitopes on influenza B viruses. Science 2012, 337, 1343–1348. [Google Scholar] [CrossRef]
- Amanat, F.; Duehr, J.; Oestereich, L.; Hastie, K.M.; Ollmann Saphire, E.; Krammer, F. Antibodies to the Glycoprotein GP2 Subunit Cross-React between Old and New World Arenaviruses. mSphere 2018, 3. [Google Scholar] [CrossRef]
- Tan, G.S.; Lee, P.S.; Hoffman, R.M.; Mazel-Sanchez, B.; Krammer, F.; Leon, P.E.; Ward, A.B.; Wilson, I.A.; Palese, P. Characterization of a broadly neutralizing monoclonal antibody that targets the fusion domain of group 2 influenza a virus hemagglutinin. J. Virol. 2014, 88, 13580–13592. [Google Scholar] [CrossRef]
- Wohlbold, T.J.; Chromikova, V.; Tan, G.S.; Meade, P.; Amanat, F.; Comella, P.; Hirsh, A.; Krammer, F. Hemagglutinin Stalk- and Neuraminidase-Specific Monoclonal Antibodies Protect against Lethal H10N8 Influenza Virus Infection in Mice. J. Virol. 2015, 90, 851–861. [Google Scholar] [CrossRef]
- Wohlbold, T.J.; Podolsky, K.A.; Chromikova, V.; Kirkpatrick, E.; Falconieri, V.; Meade, P.; Amanat, F.; Tan, J.; tenOever, B.R.; Tan, G.S.; et al. Broadly protective murine monoclonal antibodies against influenza B virus target highly conserved neuraminidase epitopes. Nat. Microbiol. 2017, 2, 1415–1424. [Google Scholar] [CrossRef]
| Name | Subtype |
|---|---|
| KL-H11-1A5 | IgG2a |
| KL-H11-1B12 | IgG2a |
| KL-H11-1C1 | IgG2b |
| KL-H11-1C2 | IgG2a |
| KL-H11-1F12 | IgG2b |
| KL-H11-1G3 | IgG2a |
| KL-H11-2C2 | IgG2a |
| KL-H11-2D7 | IgG2a |
| KL-H11-2G8 | IgG2a |
| KL-H11-3A7 | IgG2b |
| KL-H11-3B2 | IgG2b |
| KL-H11-3C2 | IgG2b |
| KL-H11-3F6 | IgG2b |
| KL-H11-3H9 | IgG2a |
| KL-H11-4A4 | IgG2a |
| KL-H11-4G10 | IgG2a |
| KL-H11-5A10 | IgG2a |
| KL-H11-5B4 | IgG2b |
| KL-H11-7D4 | IgG2a |
| KL-H11-7F9 | IgG2a |
| KL-H11-7H1 | IgG2a |
| KL-H11-8A11 | IgG2a |
| KL-H11-8D5 | IgG2b |
| KL-H11-8D8 | IgG1 |
| KL-H11-8D12 | IgG2a |
| KL-H11-8E2 | IgG2a |
| KL-H11-8F6 | IgG3 |
| KL-H11-8F11 | IgG2a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strohmeier, S.; Amanat, F.; Krammer, F. Cross-Reactive Antibodies Binding to the Influenza Virus Subtype H11 Hemagglutinin. Pathogens 2019, 8, 199. https://doi.org/10.3390/pathogens8040199
Strohmeier S, Amanat F, Krammer F. Cross-Reactive Antibodies Binding to the Influenza Virus Subtype H11 Hemagglutinin. Pathogens. 2019; 8(4):199. https://doi.org/10.3390/pathogens8040199
Chicago/Turabian StyleStrohmeier, Shirin, Fatima Amanat, and Florian Krammer. 2019. "Cross-Reactive Antibodies Binding to the Influenza Virus Subtype H11 Hemagglutinin" Pathogens 8, no. 4: 199. https://doi.org/10.3390/pathogens8040199
APA StyleStrohmeier, S., Amanat, F., & Krammer, F. (2019). Cross-Reactive Antibodies Binding to the Influenza Virus Subtype H11 Hemagglutinin. Pathogens, 8(4), 199. https://doi.org/10.3390/pathogens8040199






