Environmental Prevalence of Carbapenem Resistance Enterobacteriaceae (CRE) in a Tropical Ecosystem in India: Human Health Perspectives and Future Directives
Abstract
1. Introduction
2. Carbapenem Resistance Enterobacteriaceae
3. Global Clonal Type of CRE
4. Surface Water and Sediments as Reservoir for CRE
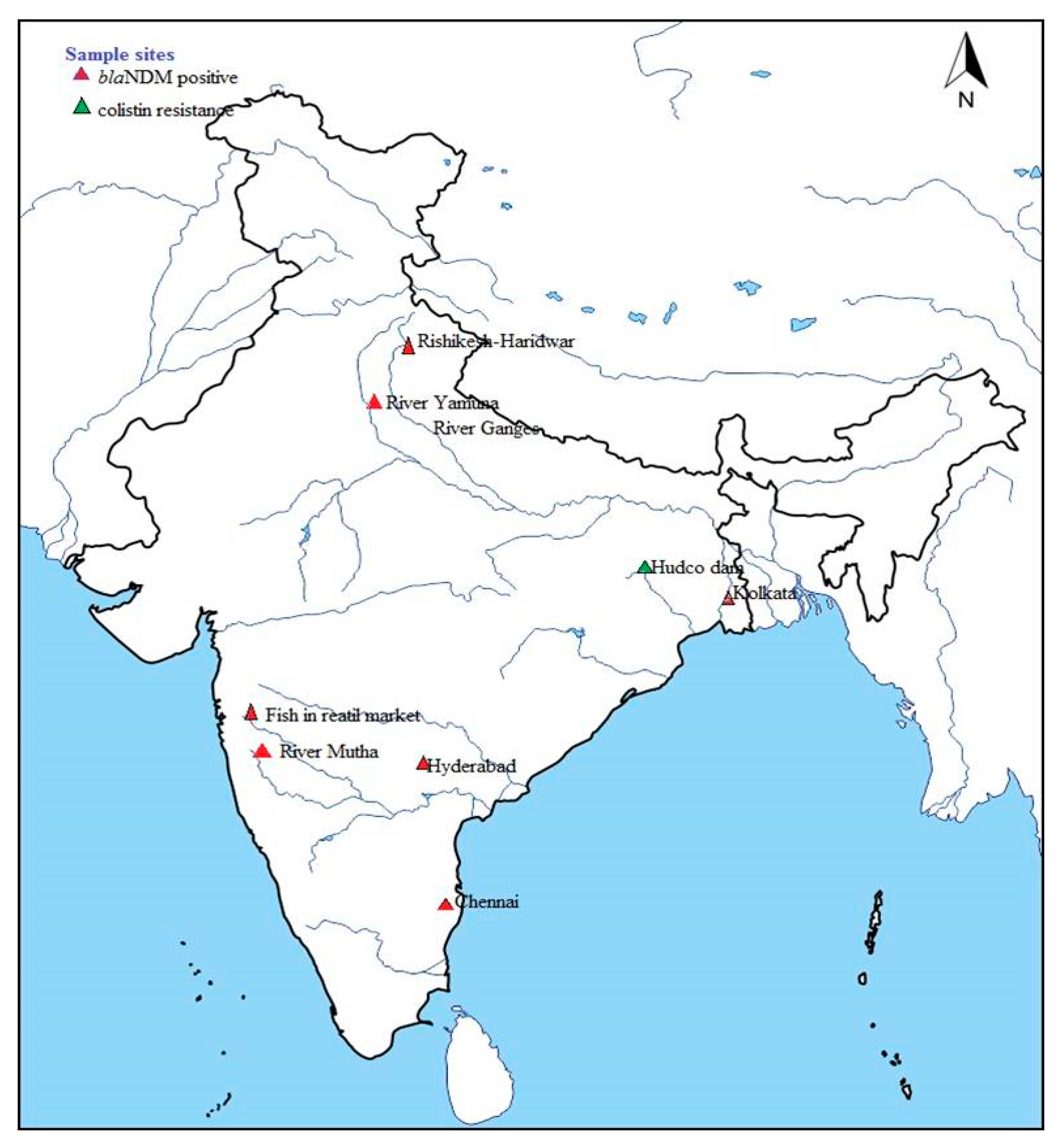
4.1. Situation in India
4.2. Central Region
4.3. Northern Region
4.4. Southern Region
4.5. Western Region
4.6. Eastern Region
5. Future Directions
5.1. Infrastructure Development for Sanitation
5.2. Irrigation Channels Update
5.3. Innovative Changes in Approach in Waste Water Treatment
5.4. Increased Surveillance of Food Supply Chain
5.5. Antimicrobials Prescription Practices/Accessibilities
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. Review on Antimicrobial Resistance; Wellcome Trust and UK Government: London, UK, 2016; Available online: https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf (accessed on 1 August 2019).
- Gelband, H.; Miller-Petrie, M.; Pant, S.; Gandra, S.; Levinson, J.; Barter, D.; White, A.; Laxminarayan, R.; Ganguly, N.; Kariuki, S. The state of the world’s antibiotics. Wound Heal. S. Afr. 2015, 8, 30–34. [Google Scholar]
- Public Health Foundation of India. Antibiotic Use and Resistance in India, Global Antibiotic Resistance Partnership-India National Working Group; Public Health Foundation of India: Gurugram, India, 2011; pp. 1–74. [Google Scholar]
- Lamba, M.; Gupta, S.; Shukla, R.; Graham, D.W.; Sreekrishnan, T.R.; Ahammad, S.Z. Carbapenem resistance exposures via wastewaters across New Delhi. Environ. Int. 2018, 119, 302–308. [Google Scholar] [CrossRef]
- Government of India. National Action Plan on Antimicrobial Resistance (NAP-AMR) 2017–2021. 2017. Available online: http://www.searo.who.int/india/topics/antimicrobial_resistance/nap_amr.pdf (accessed on 1 August 2019).
- World Health Organization. Antimicrobial Resistance. Global Report; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Col, N.F.; O’Connor, R.W. Estimating worldwide current antibiotic usage: Report of task force 1. Rev. Infect. Dis. 1987, 9, S232–S243. [Google Scholar] [CrossRef]
- Batt, A.L.; Snow, D.D.; Aga, D.S. Occurrence of sulphonamide antimicrobials in private water wells in Washington County, Idaho, USA. Chemosphere 2006, 64, 1963–1971. [Google Scholar] [CrossRef]
- Andersson, D.I.; Hughes, D. Microbiological effects of sublethal levels of antibiotics. Nat. Rev. Microbiol. 2014, 12, 465–478. [Google Scholar] [CrossRef]
- Maiques, E.; Ubeda, C.; Campoy, S.; Salvador, N.; Lasa, I.; Novick, R.P.; Barbe, J.; Penades, J.R. β-lactam antibiotics induce the SOS response and horizontal transfer of virulence factors in Staphylococcus aureus. J. Bacteriol. 2006, 188, 2726–2729. [Google Scholar] [CrossRef]
- Andersson, D.I.; Hughes, D. Evolution of antibiotic resistance at non-lethal drug concentrations. Drug Resist. Updates 2012, 15, 162–172. [Google Scholar] [CrossRef]
- Srinivasan, V.; Nam, H.M.; Sawant, A.A.; Headrick, S.I.; Nguyen, L.T.; Oliver, S.P. Distribution of tetracycline and streptomycin resistance genes and class 1 integrons in Enterobacteriaceae isolated from dairy and nondairy farm soils. Microb. Ecol. 2008, 55, 184–193. [Google Scholar] [CrossRef]
- Kristiansson, E.; Fick, J.; Janzon, A.; Grabic, R.; Rutgersson, C.; Weijdegård, B.; Söderström, H.; Larsson, G.D.J. Pyrosequencing of antibiotic-contaminated river sediments reveals high levels of resistance and gene transfer elements. PLoS ONE 2011, 6, e17038. [Google Scholar] [CrossRef]
- Martínez, J.L. Natural antibiotic resistance and contamination by antibiotic resistance determinants: The two ages in the evolution of resistance to antimicrobials. Front. Microbiol. 2012. [Google Scholar] [CrossRef]
- Razavi, M.; Marathe, N.P.; Gillings, M.R.; Flach, C.F.; Kristiansson, E.; Joakim Larsson, D.G. Discovery of the fourth mobile sulphonamide resistance gene. Microbiome 2017, 5, 160. [Google Scholar] [CrossRef] [PubMed]
- Marathe, N.P.; Regina, V.R.; Walujkar, S.A.; Charan, S.S.; Moore, E.R.B.; Larsson, D.G.J.; Shouche, Y.S. A treatment plant receiving waste water from multiple bulk drug manufacturers is a reservoir for highly multi-drug resistant integrons bearing bacteria. PLoS ONE 2013, 8, e77310. [Google Scholar] [CrossRef] [PubMed]
- Song, J.S.; Jeon, J.H.; Lee, J.H.; Jeong, S.H.; Jeong, B.C.; Kim, S.J.; Lee, J.H.; Lee, S.H. Molecular characterization of TEM-type β-lactamases identified in cold-seep sediments of Edison Seamount (south of Lihir Island, Papua New Guinea). J. Microbiol. 2005, 43, 172–178. [Google Scholar] [PubMed]
- Allen, H.K.; Moe, L.A.; Rodbumrer, J.; Gaarder, A.; Handelsman, J. Functional metagenomics reveals diverse β-lactamases in a remote Alaskan soil. ISME J. 2009, 3, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Aminov, R.I. The role of antibiotics and antibiotic resistance in nature. Environ. Microbiol. 2009, 11, 2970–2988. [Google Scholar] [CrossRef]
- D’Costa, V.M.; King, C.E.; Kalan, L.; Morar, M.; Sung, W.W.L.; Schwarz, C.; Froese, D.; Zazula, G.; Calmels, F.; Debruyne, R.; et al. Antibiotic resistance is ancient. Nature 2011, 477, 457–461. [Google Scholar] [CrossRef]
- Allen, H.K.; Donato, J.; Wang, H.H.; Hansen, K.A.C.; Davies, J.; Handelsman, J. Call of the wild: Antibiotic resistance genes in natural environments. Nat. Rev. Microbiol. 2010, 8, 251–259. [Google Scholar] [CrossRef]
- Ram, S.; Vajpayee, P.; Shanker, R. Prevalence of multi-antimicrobial-agent resistant, shiga toxin and enterotoxin producing Escherichia coli in surface waters of river Ganga. Environ. Sci. Technol. 2007, 41, 7383–7388. [Google Scholar] [CrossRef]
- Knapp, C.W.; McCluskey, S.M.; Singh, B.K.; Campbell, C.D.; Hudson, G.; Graham, D.W. Antibiotic resistance gene abundances correlate with metal and geochemical conditions in archived Scottish soils. PLoS ONE 2011, 6, e27300. [Google Scholar] [CrossRef]
- Cesare, A.D.; Ester, M.; Eckert, G.C. Co-selection of antibiotic and heavy metal resistance in freshwater bacteria. J. Limnol. 2016, 75, 59–66. [Google Scholar] [CrossRef]
- Berger, C.N.; Sodha, S.V.; Shaw, R.K.; Griffin, P.M.; Pink, D.; Hand, P.; Frankel, G. Fresh fruit and vegetables as vehicles for the transmission of human pathogens. Environ. Microbiol. 2010, 12, 2385–2397. [Google Scholar] [CrossRef]
- Rahube, T.O.; Marti, R.; Scott, A.; Tien, Y.C.; Murray, R.; Sabourin, L.; Zhang, Y.; Duenk, P.; Lapen, D.R.; Topp, E. Impact of fertilizing with raw or anaerobically digested sewage sludge on the abundance of antibiotic resistance coliforms, antibiotic resistance genes, and pathogenic bacteria in soil and on vegetables at harvest. Appl. Environ. Microbiol. 2014, 80, 6898–6907. [Google Scholar] [CrossRef]
- Wang, J.; Yao, X.; Luo, J.; Lv LZeng, Z.; Liu, J.H. Emergence of Escherichia coli co-producing NDM-1 and KPC-2 carbapenemases from a retaile vegetable, China. J. Antimicrob. Chemother. 2018, 73, 252–254. [Google Scholar] [CrossRef]
- Monier, J.M.; Demanèche, S.; Delmont, T.O.; Mathieu, A.; Vogel, T.M.; Simonet, P. Metagenomics exploration of antibiotic resistance in soil. Curr. Opin. Microbiol. 2011, 14, 229–235. [Google Scholar] [CrossRef]
- Donato, J.J.; Moe, L.A.; Converse, B.J.; Smart, K.D.; Berklein, F.C.; McManus, P.S.; Handelsman, J. Metagenomic analysis of apple orchard soil reveals antibiotic resistance genes encoding predicted bifunctional proteins. Appl. Environ. Microbiol. 2010, 76, 4396–4401. [Google Scholar] [CrossRef]
- Wright, G.D. The antibiotic resistome. The nexus of chemical and genetic diversity. Nat. Rev. Microbiol. 2007, 5, 175–186. [Google Scholar] [CrossRef]
- Jones, J.G.; Gardener, S.; Simon, B.M.; Pickup, R.W. Antibiotic resistant bacteria in Windermere and two remote upland tarns in the English Lake district. J. Appl. Bacteriol. 1986, 60, 443–453. [Google Scholar] [CrossRef]
- Pote, J.; Haller, L.; Kottelat, R.; Sastre, V.; Arpagaus, P.; Wildi, W. Persistence and growth of faecal culturable bacterial indicators in water column and sediments of Vidy Bay, Lake Geneva, Switzerland. J. Environ. Sci. 2009, 21, 62–69. [Google Scholar] [CrossRef]
- Kwak, Y.K.; Colque, P.; Byfors, S.; Giske, C.G.; Mollby, R.; Kuhn, I. Surveillance of antimicrobial resistance among Escherichia coli in wastewater in Stockholm during 1 year: Does it reflect the resistance trends in the society? Int. J. Antimicrob. Agents 2015, 45, 25–32. [Google Scholar] [CrossRef]
- Larsson, D.G.J.; Andremont, A.; Bengtsson-Palme, J.; Brandt, K.K.; de Roda Husman, A.M.; Fagerstedt, P.; Fick, J.; Flach, C.-F.; Gaze, W.H.; Kuroda, M. Critical knowledge gaps and research needs related to the environmental dimensions of antibiotic resistance. Environ. Int. 2018, 117, 132–138. [Google Scholar] [CrossRef]
- Baquero, F.; Martínez, J.-L.; Cantón, R. Antibiotics and antibiotic resistance in water environments. Curr. Opin. Biotechnol. 2008, 19, 260–265. [Google Scholar] [CrossRef]
- Rizzo, L.; Manaia, C.; Merlin, C.; Schwartz, T.; Dagot, C.; Ploy, M.C.; Michael, I.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: A review. Sci. Total Environ. 2013, 447, 345–360. [Google Scholar] [CrossRef]
- Narciso-da-Rocha, C.; Manaia, C.M. Multidrug resistance phenotypes are widespread over different bacterial taxonomic groups thriving in surface water. Sci. Total Environ. 2016, 563, 1–9. [Google Scholar] [CrossRef]
- Picão, R.C.; Cardoso, J.P.; Campana, E.H.; Nicoletti, A.G.; Petrolini, F.V.B.; Assis, D.M.; Juliano, L.; Gales, A.C. The route of antimicrobial resistance from the hospital effluent to the environment: Focus on the occurrence of KPC-producing Aeromonas spp. and Enterobacteriaceae in sewage. Diagn. Microbiol. Infect. Dis. 2013, 76, 80–85. [Google Scholar] [CrossRef]
- Cesare, A.D.; Fontaneto, D.; Doppelbauer, J.; Corno, G. Fitness and recovery of bacterial communities and antibiotic resistance genes in urban wastewaters exposed to classical disinfection treatments. Environ. Sci. Technol. 2016, 50, 10153–10161. [Google Scholar] [CrossRef]
- Haller, L.; Chen, H.; Ng, C.; Le, T.H.; Koh, T.H.; Barkham, T.; Sobsey, M.; Gin, K.Y. Occurrence and characteristics of extended-spectrum β-lactamase-and carbapenemase-producing bacteria from hospital effluents in Singapore. Sci. Total Environ. 2018, 615, 1119–1125. [Google Scholar] [CrossRef]
- Cesare, A.D.; Eckert, E.M.; Rogora, M.; Corno, G. Rainfall increases the abundance of antibiotic resistance genes within a riverine microbial community. Environ. Pollut. 2017, 226, 473–478. [Google Scholar] [CrossRef]
- Rowe, W.P.M.; Baker-Austin, C.; Verner-Jeffreys, D.W.; Ryan, J.J.; Micallef, C.; Maskell, D.J.; Pearce, G.P. Overexpression of antibiotic resistance genes in hospital effluents over time. J. Antimicrob. Chemother. 2017, 72, 1617–1623. [Google Scholar] [CrossRef]
- Hrenovic, J.; Ivankovic, T.; Ivekovic, D.; Repec, S.; Stipanicev, D.; Ganjto, M. The fate of carbapenem-resistant bacteria in a wastewater treatment plant. Water Res. 2017, 126, 232–239. [Google Scholar] [CrossRef]
- Bengtsson-Palme, J.; Kristiansson, E.; Larsson, D.G.J. Environmental factors influencing the development and spread of antibiotic resistance. FEMS Microbiol. Rev. 2018, 42. [Google Scholar] [CrossRef]
- Piedra-Carrasco, N.; Fabrega, A.; Calero-Caceres, W.; Cornejo-Sanchez, T.; Brown-Jaque, M.; Mir-Cros, A.; Muniesa, M.; Gonzalez-Lopez, J.J. Carbapenemase-producing enterobacteriaceae recovered from a Spanish river ecosystem. PLoS ONE 2017, 12, e0175246. [Google Scholar] [CrossRef]
- Poirel, L.; Barbosa-Vasconcelos, A.; Simoes, R.R.; da Costa, P.M.; Liu, W.; Nordmann, P. Environmental KPC-producing Escherichia coli isolates in Portugal. Antimicrob. Agents Chemother. 2012, 56, 1662–1663. [Google Scholar] [CrossRef]
- Zurfluh, K.; Hachler, H.; Nuesch-Inderbinen, M.; Stephan, R. Characteristics of extended-spectrum β-lactamase-and carbapenemase-producing Enterobacteriaceae isolates from rivers and lakes in Switzerland. Appl. Environ. Microbiol. 2013, 79, 3021–3026. [Google Scholar] [CrossRef]
- Kittinger, C.; Lipp, M.; Folli, B.; Kirschner, A.; Baumert, R.; Galler, H.; Grisold, A.J.; Luxner, J.; Weissenbacher, M.; Farnleitner, A.H.; et al. Enterobacteriaceae isolated from the river Danube: Antibiotic resistances, with a focus on the presence of ESBL and carbapenemases. PLoS ONE 2016, 11, e0165820. [Google Scholar] [CrossRef]
- Di, D.Y.; Jang, J.; Unno, T.; Hur, H.G. Emergence of Klebsiella variicola positive for NDM-9, a variant of New Delhi metallo-β-lactamase, in an urban river in South Korea. J. Antimicrob. Chemother. 2017, 72, 1063–1067. [Google Scholar] [CrossRef][Green Version]
- Kumarasamy, K.K.; Toleman, M.A.; Walsh, T.R.; Bagaria, J.; Butt, F.; Balakrishnan, R.; Chaudhary, U.; Doumith, M.; Giske, C.G.; Irfan, S.; et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: A molecular, biological, and epidemiological study. Lancet Infect. Dis. 2010, 10, 597–602. [Google Scholar] [CrossRef]
- Government of Western Australia, Department of Public Health, Infection Prevention and Control of Carbapenem- resistant Enterobacteriaceae (CRE) in Western Australian Healthcare Facilities. 2012. Available online: http://www.health.wa.gov.au/circularsnew/attachments/712.pdf (accessed on 2 October 2019).
- Parvez, S.; Khan, A.U. Hospital sewage water: A reservoir for variants of New Delhi metallo-β-lactamase (NDM)-and extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae. Int. J. Antimicrob. Agents 2018, 51, 82–88. [Google Scholar] [CrossRef]
- Khan, A.U.; Nordmann, P. Spread of carbapenemase NDM-1 producers: The situation in India and what may be proposed. Scand. J. Infect. Dis. 2012, 44, 531–535. [Google Scholar] [CrossRef]
- Marathe, N.P.; Pal, C.; Gaikwad, S.S.; Jonsson, V.; Kristiansson, E.; Larsson, D.G.J. Untreated urban waste contaminates Indian river sediments with resistance genes to last resort antibiotics. Water Res. 2017, 124, 388–397. [Google Scholar] [CrossRef]
- Khan, A.U.; Maryam, L.; Zarrilli, R. Structure, genetics and worldwide spread of New Delhi metallo-β-lactamase (NDM): A threat to public health. BMC Microbiol. 2017, 17, 101. [Google Scholar] [CrossRef]
- Mollenkopf, D.F.; Stull, J.W.; Mathys, D.A.; Bowman, A.S.; Feicht, S.M.; Grooters, S.V.; Daniels, J.B.; Wittum, T.E. Carbapenemase-producing Enterobacteriaceae recovered from the environment of a swine farrow-to-finish operation in the United States. Antimicrob. Agents Chemother. 2017, 61, e01298-16. [Google Scholar] [CrossRef] [PubMed]
- Nordmann, P.; Dortet, L.; Poirel, L. Carbapenem resistance in Enterobacteriaceae: Here is the storm! Trends Mol. Med. 2012, 18, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Nordmann, P. Carbapenemase-producing Enterobacteriaceae: Overview of a major public health challenge. Med. Et Mal. Infect. 2014, 44, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Palzkill, T. Metallo-β-lactamase structure and function. Ann. N. Y. Acad. Sci. 2013, 1277, 91–104. [Google Scholar] [CrossRef]
- Lee, C.R.; Lee, J.H.; Park, K.S.; Kim, Y.B.; Jeong, B.C.; Lee, S.H. Global dissemination of carbapenemase-producing Klebsiella pneumoniae: Epidemiology, genetic context, treatment options, and detection methods. Front. Microbiol. 2016, 7, 895. [Google Scholar] [CrossRef]
- Chen, L.; Todd, R.; Kiehlbauch, J.; Walters, M.; Kallen, A. Notes from the field: Pan-resistant New Delhi metallo-β-lactamase-producing Klebsiella pneumoniae—Washoe County, Nevada, 2016. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 33. [Google Scholar] [CrossRef]
- Yong, D.; Toleman, M.A.; Giske, C.G.; Cho, H.S.; Sundman, K.; Lee, K.; Walsh, T.R. Characterization of a New metallo-β-lactamase gene, blaNDM-1, and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob. Agents Chemother. 2009, 53, 5046–5054. [Google Scholar] [CrossRef]
- Zarfel, G.; Hoenigl, M.; Würstl, B.; Leitner, E.; Salzer, H.J.; Valentin, T.; Posch, J.; Krause, R.; Grisold, A.J. Emergence of carbapenem-resistant Enterobacteriaceae in Austria, 2001–2010. Clin. Microbiol. Infect. 2011, 17, E5–E8. [Google Scholar] [CrossRef][Green Version]
- Hornsey, M.; Phee, L.; Wareham, D.W. A novel variant, NDM-5, of the New Delhi metallo-β-lactamase in a multidrug-resistant Escherichia coli ST648 isolate recovered from a patient in the United Kingdom. Antimicrob. Agents Chemother. 2011, 55, 5952–5954. [Google Scholar] [CrossRef]
- Kus, J.V.; Tadros, M.; Simor, A.; Low, D.E.; McGeer, A.J.; Willey, B.M.; Larocque, C.; Pike, K.; Edwards, I.-A.; Dedier, H.; et al. New Delhi metallo-β-lactamase-1: Local acquisition in Ontario, Canada, and challenges in detection. CMAJ 2011, 183, 1257–1261. [Google Scholar] [CrossRef]
- Fu, Y.; Du, X.; Ji, J.; Chen, Y.; Jiang, Y.; Yu, Y. Epidemiological characteristics and genetic structure of blaNDM-1 in non-baumannii Acinetobacter spp. in China. J. Antimicrob. Chemother. 2012, 67, 2114–2122. [Google Scholar] [CrossRef] [PubMed]
- Arpin, C.; Noury, P.; Boraud, D.; Coulange, L.; Manetti, A.; André, C.; M’Zali, F.; Quentin, C. NDM-1-producing Klebsiella pneumoniae resistant to colistin in a French community patient without history of foreign travel. Antimicrob. Agents Chemother. 2012, 56, 3432–3434. [Google Scholar] [CrossRef] [PubMed]
- Pasteran, F.; Albornoz, E.; Faccone, D.; Gomez, S.; Valenzuela, C.; Morales, M.; Estrada, P.; Valenzuela, L.; Matheu, J.; Guerriero, L.; et al. Emergence of NDM-1-producing Klebsiella pneumoniae in Guatemala. J. Antimicrob. Chemother. 2012, 67, 1795–1797. [Google Scholar] [CrossRef] [PubMed]
- Espinal, P.; Fugazza, G.; López, Y.; Kasma, M.; Lerman, Y.; Malhotra-Kumar, S.; Goossens, H.; Carmeli, Y.; Vila, J. Dissemination of an NDM-2-producing Acinetobacter baumannii clone in an Israeli rehabilitation center. Antimicrob. Agents Chemother. 2011, 55, 5396–5398. [Google Scholar] [CrossRef]
- Poirel, L.; Al Maskari, Z.; Al Rashdi, F.; Bernabeu, S.; Nordmann, P. NDM-1-producing Klebsiella pneumoniae isolated in the Sultanate of Oman. J. Antimicrob. Chemother. 2011, 66, 304–306. [Google Scholar] [CrossRef]
- Poirel, L.; Revathi, G.; Bernabeu, S.; Nordmann, P. Detection of NDM-1-producing Klebsiella pneumoniae in Kenya. Antimicrob. Agents Chemother. 2011, 55, 934–936. [Google Scholar] [CrossRef]
- Jamal, W.; Rotimi, V.O.; Albert, M.J.; Khodakhast, F.; Udo, E.E.; Poirel, L. Emergence of nosocomial New Delhi metallo-β-lactamase-1 (NDM-1)-producing Klebsiella pneumoniae in patients admitted to a tertiary care hospital in Kuwait. Int. J. Antimicrob. Agents 2012, 39, 183–184. [Google Scholar] [CrossRef]
- Brink, A.J.; Coetzee, J.; Clay, C.G.; Sithole, S.; Richards, G.A.; Poirel, L.; Nordmann, P. Emergence of New Delhi metallo-β-lactamase (NDM-1) and Klebsiella pneumoniae carbapenemase (KPC-2) in South Africa. J. Clin. Microbiol. 2012, 50, 525–527. [Google Scholar] [CrossRef]
- Kim, M.-N.; Yong, D.; An, D.; Chung, H.-S.; Woo, J.H.; Lee, K.; Chong, Y. Nosocomial clustering of NDM-1-producing Klebsiella pneumoniae sequence type 340 strains in four patients at a South Korean tertiary care hospital. J. Clin. Microbiol. 2012, 50, 1433–1436. [Google Scholar] [CrossRef]
- Jovcic, B.; Lepsanovic, Z.; Suljagic, V.; Rackov, G.; Begovic, J.; Topisirovic, L.; Kojic, M. Emergence of NDM-1 metallo-β-lactamase in Pseudomonas aeruginosa clinical isolates from Serbia. Antimicrob. Agents Chemother. 2011, 55, 3929–3931. [Google Scholar] [CrossRef]
- Rimrang, B.; Chanawong, A.; Lulitanond, A.; Wilailuckana, C.; Charoensri, N.; Sribenjalux, P.; Phumsrikaew, W.; Wonglakorn, L.; Kerdsin, A.; Chetchotisakd, P. Emergence of NDM-1-and IMP-14a-producing Enterobacteriaceae in Thailand. J. Antimicrob. Chemother. 2012, 67, 2626–2630. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, P.; Singh, N.S.; Kanaujia, P.K.; Virdi, J.S. Distribution and molecular characterization of genes encoding CTX-M and AmpC β-lactamases in Escherichia coli isolated from an Indian urban aquatic environment. Sci. Total Environ. 2015, 505, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Giani, T.; Pini, B.; Arena, F.; Conte, V.; Bracco, S.; Migliavacca, R.; Pantosti, A.; Pagani, L.; Luzzaro, F.; Rossolini, G.M. Epidemic diffusion of KPC carbapenemase-producing Klebsiella pneumoniae in Italy: Results of the first countrywide survey, 15 May to 30 June 2011. Eurosurveillance 2013, 18, 20489. [Google Scholar] [PubMed]
- Chen, L.; Mathema, B.; Pitout, J.D.D.; DeLeo, F.R.; Kreiswirth, B.N. Epidemic Klebsiella pneumoniae ST258 is a hybrid strain. MBio 2014, 5, e01355-14. [Google Scholar] [CrossRef]
- Bowers, J.R.; Kitchel, B.; Driebe, E.M.; MacCannell, D.R.; Roe, C.; Lemmer, D.; de Man, T.; Rasheed, J.K.; Engelthaler, D.M.; Keim, P.; et al. Genomic analysis of the emergence and rapid global dissemination of the clonal group 258 Klebsiella pneumoniae pandemic. PLoS ONE 2015, 10, e0133727. [Google Scholar] [CrossRef]
- Lascols, C.; Peirano, G.; Hackel, M.; Laupland, K.B.; Pitout, J.D.D. Surveillance and molecular epidemiology of Klebsiella pneumoniae isolates that produce carbapenemases: First report of OXA-48-like enzymes in North America. Antimicrob. Agents Chemother. 2013, 57, 130–136. [Google Scholar] [CrossRef]
- Nordmann, P.; Poirel, L. The difficult to control spread of carbapenemase producers among Enterobacteriaceae worldwide. Clin. Microbiol. Infect. 2014, 20, 821–830. [Google Scholar] [CrossRef]
- Pitart, C.; Sole, M.; Roca, L.; Fabrega, A.; Vila, J.; Marco, F. First outbreak of a plasmid-mediated carbapenem-hydrolyzing OXA-48 β-lactamase in Klebsiella pneumoniae in Spain. Antimicrob. Agents Chemother. 2011, 55, 4398–4401. [Google Scholar] [CrossRef]
- Seiffert, S.N.; Marschall, J.; Perreten, V.; Carattoli, A.; Furrer, H.; Endimiani, A. Emergence of Klebsiella pneumoniae co-producing NDM-1, OXA-48, CTX-M-15, CMY-16, QnrA and ArmA in Switzerland. Int. J. Antimicrob. Agents 2014, 44, 260–262. [Google Scholar] [CrossRef]
- Tafoukt, R.; Touati, A.; Leangapichart, T.; Bakour, S.; Rolain, J.M. Characterization of OXA-48-like-producing Enterobacteriaceae isolated from river water in Algeria. Water Res. 2017, 120, 185–189. [Google Scholar] [CrossRef]
- Oliveira, S.; Moura, R.A.; Silva, K.C.; Pavez, M.; McCulloch, J.A.; Dropa, M.; Matté, M.H.; Mamizuka, E.M.; Sato, M.I.; de Castro, A.F.P.; et al. Isolation of KPC-2-producing Klebsiella pneumoniae strains belonging to the high-risk multiresistant clonal complex 11 (ST437 and ST340) in urban rivers. J. Antimicrob. Chemother. 2014, 69, 849–852. [Google Scholar] [CrossRef] [PubMed]
- Zurfluh, K.; Nüesch-Inderbinen, M.; Morach, M.; Zihler Berner, A.; Hächler, H.; Stephan, R. Extended-spectrum-β-lactamase-producing Enterobacteriaceae isolated from vegetables imported from the Dominican Republic, India, Thailand, and Vietnam. Appl. Environ. Microbiol. 2015, 81, 3115–3120. [Google Scholar] [CrossRef] [PubMed]
- van der Bij, A.K.; Pitout, J.D. The role of international travel in the worldwide spread of multiresistant Enterobacteriaceae. J. Antimicrob. Chemother. 2012, 67, 2090–2100. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, D.; van Essen-Zandbergen, A.; Veldman, K.T.; Tafro, N.; Haenen, O.; Mevius, D.J. Chromosome-based blaOXA-48-like variants in Shewanella species isolates from food-producing animals, fish, and the aquatic environment. Antimicrob. Agents Chemother. 2017, 61, e01013-16. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, M. Water technologies and the environment: Ramping up by scaling down. Technol. Soc. 2008, 30, 415–422. [Google Scholar] [CrossRef]
- United Nations (UN). Health, Water and Sanitation; UN: New York, NY, USA, 2011. [Google Scholar]
- The Economic Times India has 60.4 per Cent People without Access to Toilet: Study 2015. Available online: https://economictimes.indiatimes.com/article.india/india-news-india/india-has-60-4-per-cent-people-withoutaccess-to-toilet-study/ (accessed on 1 August 2019).
- Ghafur, A.K. An obituary—On the death of antibiotics! J. Assoc. Physician India 2010, 58, 143–144. [Google Scholar]
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.; Wertheim, H.F.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H.; et al. Antibiotic resistance—The need for global solutions. Lancet Infect. Dis. 2013, 13, 1057–1098. [Google Scholar] [CrossRef]
- Galler, H.; Feierl, G.; Petternel, C.; Reinthaler, F.F.; Haas, D.; Grisold, A.J.; Luxner, J.; Zarfel, G. KPC-2 and OXA-48 carbapenemase-harbouring Enterobacteriaceae detected in an Austrian wastewater treatment plant. Clin. Microbiol. Infect. 2014, 20, O132–O134. [Google Scholar] [CrossRef]
- Walsh, T.R.; Weeks, J.; Livermore, D.M.; Toleman, M.A. Dissemination of NDM-1 positive bacteria in the New Delhi environment and its implications for human health: An environmental point prevalence study. Lancet Infect. Dis. 2011, 11, 355–362. [Google Scholar] [CrossRef]
- Chandran, S.P.; Diwan, V.; Tamhankar, A.J.; Joseph, B.V.; Rosales-Klintz, S.; Mundayoor, S.; Lundborg, C.S.; Macaden, R. Detection of carbapenem resistance genes and cephalosporin, and quinolone resistance genes along with oqxAB gene in Escherichia coli in hospital wastewater: A matter of concern. J. Appl. Microbiol. 2014, 117, 984–995. [Google Scholar] [CrossRef]
- Ahammad, Z.S.; Sreekrishnan, T.R.; Hands, C.L.; Knapp, C.W.; Graham, D.W. Increased waterborne blaNDM-1 resistance gene abundances associated with seasonal human pilgrimages to the Upper Ganges River. Environ. Sci. Technol. 2014, 48, 3014–3020. [Google Scholar] [CrossRef]
- Dortet, L.; Poirel, L.; Anguel, N.; Nordmann, P. New Delhi metallo-β-lactamase 4-producing Escherichia coli in Cameroon. Emerg. Infect. Dis. 2012, 18, 1540–1542. [Google Scholar] [CrossRef]
- Lamba, M.; Graham, D.W.; Ahammad, S.Z. Hospital wastewater releases of carbapenem-resistance pathogens and genes in urban India. Environ. Sci. Technol. 2017, 51, 13906–13912. [Google Scholar] [CrossRef]
- Rutgersson, C.; Fick, J.; Marathe, N.; Kristiansson, E.; Janzon, A.; Angelin, M.; Johansson, A.; Shouche, Y.; Flach, C.-F.; Larsson, D.G.J. Fluoroquinolones and qnr genes in sediment, water, soil, and human fecal flora in an environment polluted by manufacturing discharges. Environ. Sci. Technol. 2014, 48, 7825–7832. [Google Scholar] [CrossRef]
- Lübbert, C.; Baars, C.; Dayakar, A.; Lippmann, N.; Rodloff, A.C.; Kinzig, M.; Sörgel, F. Environmental pollution with antimicrobial agents from bulk drug manufacturing industries in Hyderabad, South India, is associated with dissemination of extended-spectrum β-lactamase and carbapenemase-producing pathogens. Infection 2017, 45, 479–491. [Google Scholar] [CrossRef]
- Akiba, M.; Sekizuka, T.; Yamashita, A.; Kuroda, M.; Fujii, Y.; Murata, M.; Lee, K.-I.; Joshua, D.I.; Balakrishna, K.; Bairy, I.; et al. Distribution and relationships of antimicrobial resistance determinants among extended-spectrum-cephalosporin-resistant or carbapenem-resistant Escherichia coli isolates from rivers and sewage treatment plants in India. Antimicrob. Agents Chemother. 2016, 60, 2972–2980. [Google Scholar] [CrossRef]
- Singh, A.S.; Lekshmi, M.; Nayak, B.B.; Kumar, S.H. Isolation of Escherichia coli harboring blaNDM-5 from fresh fish in India. J. Microbiol. Immunol. Infect. 2016, 49, 822–823. [Google Scholar] [CrossRef]
- Das, U.N.; Singh, A.S.; Lekshmi, M.; Nayak, B.B.; Kumar, S. Characterization of blaNDM-harboring, multidrug-resistant Enterobacteriaceae isolated from seafood. Environ. Sci. Pollut. Res. 2019, 26, 2455–2463. [Google Scholar] [CrossRef]
- Singh, S.K.; Mishra, M.; Sahoo, M.; Patole, S.; Mohapatra, H. Efflux mediated colistin resistance in diverse clones of Klebsiella pneumoniae from aquatic environment. Microb. Pathog. 2017, 102, 109–112. [Google Scholar] [CrossRef]
- Ghatak, S.; Singha, A.; Sen, A.; Guha, C.; Ahuja, A.; Bhattacharjee, U.; Das, S.; Pradhan, N.R.; Puro, K.; Jana, C.; et al. Detection of New Delhi metallo-β-lactamase and extended-spectrum β-lactamase genes in Escherichia coli isolated from mastitic milk samples. Transbound. Emerg. Dis. 2013, 60, 385–389. [Google Scholar] [CrossRef]
- Shahid, M.; Malik, A.; Adil, M.; Jahan, N.; Malik, R. Comparison of β-lactamase genes in clinical and food bacterial isolates in India. J. Infect. Dev. Ctries. 2009, 3, 593–598. [Google Scholar] [CrossRef]
- Ghafur, A.; Mathai, D.; Muruganathan, A.; Jayalal, J.A.; Kant, R.; Chaudhary, D.; Prabhash, K.; Abraham, O.C.; Gopalakrishnan, R.; Ramasubramanian, V.; et al. The Chennai Declaration: A roadmap to tackle the challenge of antimicrobial resistance. Indian J. Cancer 2013, 50, 71–73. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sivalingam, P.; Poté, J.; Prabakar, K. Environmental Prevalence of Carbapenem Resistance Enterobacteriaceae (CRE) in a Tropical Ecosystem in India: Human Health Perspectives and Future Directives. Pathogens 2019, 8, 174. https://doi.org/10.3390/pathogens8040174
Sivalingam P, Poté J, Prabakar K. Environmental Prevalence of Carbapenem Resistance Enterobacteriaceae (CRE) in a Tropical Ecosystem in India: Human Health Perspectives and Future Directives. Pathogens. 2019; 8(4):174. https://doi.org/10.3390/pathogens8040174
Chicago/Turabian StyleSivalingam, Periyasamy, John Poté, and Kandasamy Prabakar. 2019. "Environmental Prevalence of Carbapenem Resistance Enterobacteriaceae (CRE) in a Tropical Ecosystem in India: Human Health Perspectives and Future Directives" Pathogens 8, no. 4: 174. https://doi.org/10.3390/pathogens8040174
APA StyleSivalingam, P., Poté, J., & Prabakar, K. (2019). Environmental Prevalence of Carbapenem Resistance Enterobacteriaceae (CRE) in a Tropical Ecosystem in India: Human Health Perspectives and Future Directives. Pathogens, 8(4), 174. https://doi.org/10.3390/pathogens8040174



