Prevalence of Various Vaccine Candidate Proteins in Clinical Isolates of Streptococcus pneumoniae: Characterization of the Novel Pht Fusion Proteins PhtA/B and PhtA/D
Abstract
1. Introduction
2. Results
2.1. Serotypes and Sequence Types (STs) of Pneumococcal Isolates
2.2. Detection of Drug Resistance Genes and Antimicrobial Susceptibility
2.3. Detection of the Vaccine Candidate Pneumococcal Protein Genes and the Novel Pht Fusion Proteins
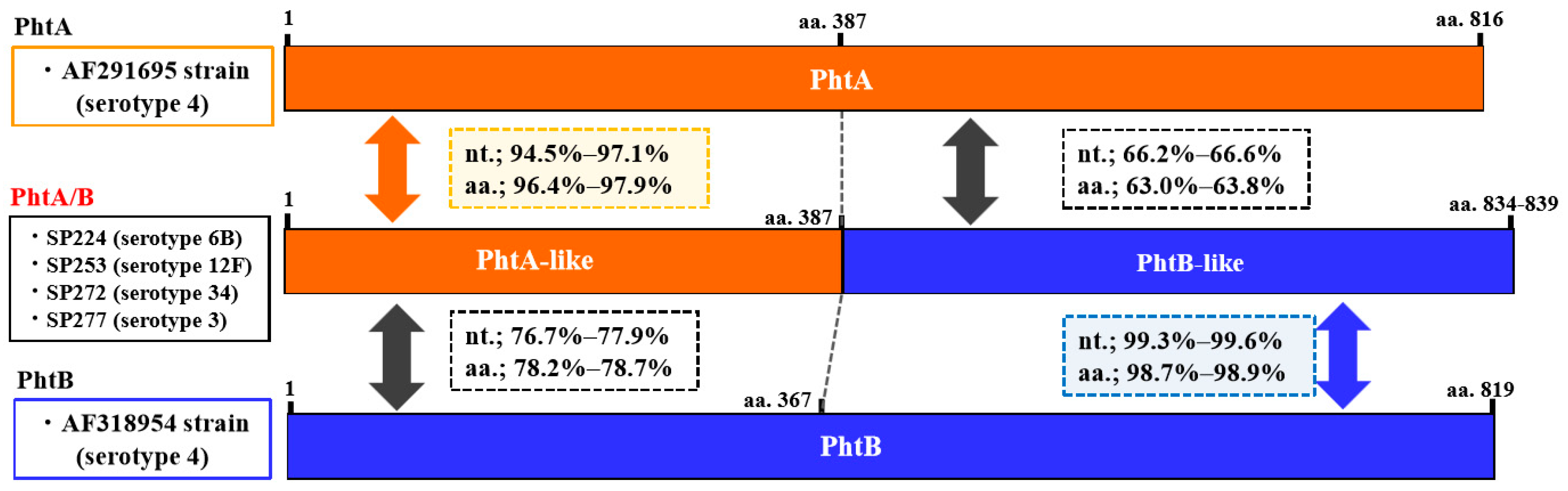
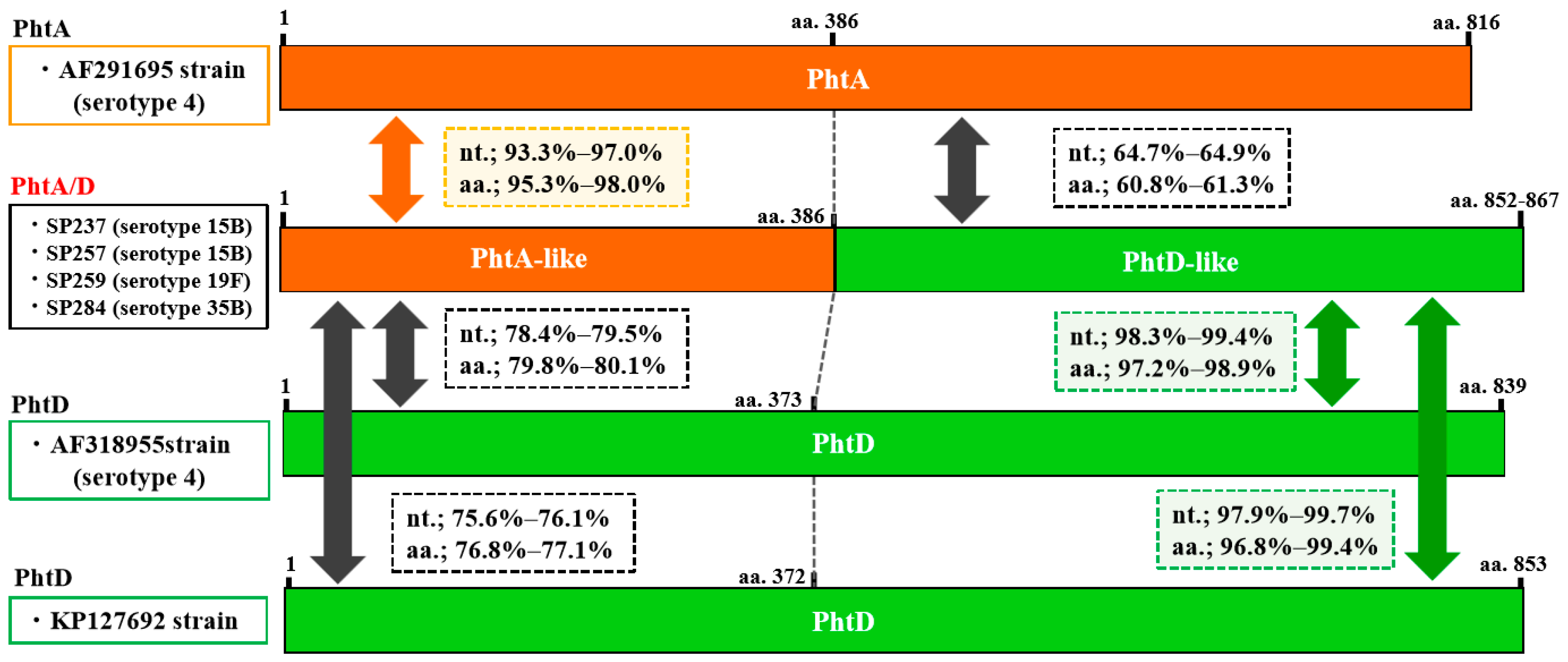
2.4. Prevalence of the Pht Pattern and Sequence Analysis of the Pht Fusion Types
2.5. Sequences of B Cell Epitopes in PhtA, PhtB, PhtD, and Pht Fusion Types
3. Discussion
4. Materials and Methods
4.1. Pneumococcal Isolates
4.2. Total DNA Extraction and Sequencing
4.3. Serotyping, Virulence Gene Detection, and Multilocus Sequence Typing (MLST)
4.4. Antimicrobial Resistance Determinants
4.5. Detection and Sequence Analysis of PhtA/B and PhtA/D Fusion Types
4.6. GenBank Accession Numbers
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Weiser, J.N.; Ferreira, D.M.; Paton, J.C. Streptococcus pneumoniae: Transmission, colonization and invasion. Nat. Rev. Microbiol. 2018, 16, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Kavalari, I.D.; Fuursted, K.; Krogfelt, K.A.; Slotved, H.C. Molecular characterization and epidemiology of Streptococcus pneumoniae serotype 24F in Denmark. Sci. Rep. 2019, 9, 5481. [Google Scholar] [CrossRef] [PubMed]
- Wyllie, A.L.; Rumke, L.W.; Arp, K.; Bosch, A.; Bruin, J.P.; Rots, N.Y.; Wijmenga-Monsuur, A.J.; Sanders, E.A.M.; Trzcinski, K. Molecular surveillance on Streptococcus pneumoniae carriage in non-elderly adults; little evidence for pneumococcal circulation independent from the reservoir in children. Sci. Rep. 2016, 6, 34888. [Google Scholar] [CrossRef] [PubMed]
- Daniels, C.C.; Rogers, P.D.; Shelton, C.M. A Review of Pneumococcal Vaccines: Current Polysaccharide Vaccine Recommendations and Future Protein Antigens. J. Pediatric Pharmacol. Ther. 2016, 21, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Song, J.Y.; Moseley, M.A.; Burton, R.L.; Nahm, M.H. Pneumococcal vaccine and opsonic pneumococcal antibody. J. Infect. Chemother. 2013, 19, 412–425. [Google Scholar] [CrossRef] [PubMed]
- Feikin, D.R.; Kagucia, E.W.; Loo, J.D.; Link-Gelles, R.; Puhan, M.A.; Cherian, T.; Levine, O.S.; Whitney, C.G.; O’Brien, K.L.; Moore, M.R. Serotype-specific changes in invasive pneumococcal disease after pneumococcal conjugate vaccine introduction: A pooled analysis of multiple surveillance sites. PLoS Med. 2013, 10, e1001517. [Google Scholar] [CrossRef] [PubMed]
- Gladstone, R.A.; Devine, V.; Jones, J.; Cleary, D.; Jefferies, J.M.; Bentley, S.D.; Faust, S.N.; Clarke, S.C. Pre-vaccine serotype composition within a lineage signposts its serotype replacement—A carriage study over 7 years following pneumococcal conjugate vaccine use in the UK. Microb. Genom. 2017, 3, e000119. [Google Scholar] [CrossRef] [PubMed]
- Quirk, S.J.; Haraldsson, G.; Erlendsdottir, H.; Hjalmarsdottir, M.A.; van Tonder, A.J.; Hrafnkelsson, B.; Sigurdsson, S.; Bentley, S.D.; Haraldsson, A.; Brueggemann, A.B.; et al. Effect of Vaccination on Pneumococci Isolated from the Nasopharynx of Healthy Children and the Middle Ear of Children with Otitis Media in Iceland. J. Clin. Microbiol. 2018, 56, e01046-18. [Google Scholar] [CrossRef]
- Ladhani, S.N.; Collins, S.; Djennad, A.; Sheppard, C.L.; Borrow, R.; Fry, N.K.; Andrews, N.J.; Miller, E.; Ramsay, M.E. Rapid increase in non-vaccine serotypes causing invasive pneumococcal disease in England and Wales, 2000–2017: A prospective national observational cohort study. Lancet Infect. Dis. 2018, 18, 441–451. [Google Scholar] [CrossRef]
- Kawaguchiya, M.; Urushibara, N.; Aung, M.S.; Morimoto, S.; Ito, M.; Kudo, K.; Sumi, A.; Kobayashi, N. Emerging non-PCV13 serotypes of noninvasive Streptococcus pneumoniae with macrolide resistance genes in northern Japan. New Microbes New Infect. 2016, 9, 66–72. [Google Scholar] [CrossRef]
- Kawaguchiya, M.; Urushibara, N.; Ghosh, S.; Kuwahara, O.; Morimoto, S.; Ito, M.; Kudo, K.; Kobayashi, N. Serotype distribution and susceptibility to penicillin and erythromycin among noninvasive or colonization isolates of Streptococcus pneumoniae in northern Japan: A cross-sectional study in the pre-PCV7 routine immunization period. Microb. Drug Resist. 2014, 20, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Southern, J.; Andrews, N.; Sandu, P.; Sheppard, C.L.; Waight, P.A.; Fry, N.K.; Van Hoek, A.J.; Miller, E. Pneumococcal carriage in children and their household contacts six years after introduction of the 13-valent pneumococcal conjugate vaccine in England. PLoS ONE 2018, 13, e0195799. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, E.; Sahin-Toth, J.; Tothpal, A.; Kristof, K.; van der Linden, M.; Tirczka, T.; Dobay, O. Vaccine-driven serotype-rearrangement is seen with latency in clinical isolates: Comparison of carried and clinical pneumococcal isolates from the same time period in Hungary. Vaccine 2019, 37, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Van der Linden, M.; Perniciaro, S.; Imohl, M. Increase of serotypes 15A and 23B in IPD in Germany in the PCV13 vaccination era. BMC Infect. Dis. 2015, 15, 207. [Google Scholar] [CrossRef] [PubMed]
- Golden, A.R.; Adam, H.J.; Gilmour, M.W.; Baxter, M.R.; Martin, I.; Nichol, K.A.; Demczuk, W.H.; Hoban, D.J.; Zhanel, G.G. Assessment of multidrug resistance, clonality and virulence in non-PCV-13 Streptococcus pneumoniae serotypes in Canada, 2011–2013. J. Antimicrob. Chemother. 2015, 70, 1960–1964. [Google Scholar] [PubMed]
- Sheppard, C.; Fry, N.K.; Mushtaq, S.; Woodford, N.; Reynolds, R.; Janes, R.; Pike, R.; Hill, R.; Kimuli, M.; Staves, P.; et al. Rise of multidrug-resistant non-vaccine serotype 15A Streptococcus pneumoniae in the United Kingdom, 2001 to 2014. Eurosurveillance 2016, 21, 30423. [Google Scholar] [CrossRef] [PubMed]
- Briles, D.E.; Tart, R.C.; Swiatlo, E.; Dillard, J.P.; Smith, P.; Benton, K.A.; Ralph, B.A.; Brooks-Walter, A.; Crain, M.J.; Hollingshead, S.K.; et al. Pneumococcal diversity: Considerations for new vaccine strategies with emphasis on pneumococcal surface protein A (PspA). Clin. Microbiol. Rev. 1998, 11, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Miyaji, E.N.; Ferreira, D.M.; Lopes, A.P.; Brandileone, M.C.; Dias, W.O.; Leite, L.C. Analysis of serum cross-reactivity and cross-protection elicited by immunization with DNA vaccines against Streptococcus pneumoniae expressing PspA fragments from different clades. Infect. Immun. 2002, 70, 5086–5090. [Google Scholar] [CrossRef]
- Moreno, A.T.; Oliveira, M.L.; Ho, P.L.; Vadesilho, C.F.; Palma, G.M.; Ferreira, J.M., Jr.; Ferreira, D.M.; Santos, S.R.; Martinez, M.B.; Miyaji, E.N. Cross-reactivity of antipneumococcal surface protein C (PspC) antibodies with different strains and evaluation of inhibition of human complement factor H and secretory IgA binding via PspC. Clin. Vaccine Immunol. 2012, 19, 499–507. [Google Scholar] [CrossRef]
- Vadesilho, C.F.; Ferreira, D.M.; Gordon, S.B.; Briles, D.E.; Moreno, A.T.; Oliveira, M.L.; Ho, P.L.; Miyaji, E.N. Mapping of epitopes recognized by antibodies induced by immunization of mice with PspA and PspC. Clin. Vaccine Immunol. 2014, 21, 940–948. [Google Scholar] [CrossRef]
- Visan, L.; Rouleau, N.; Proust, E.; Peyrot, L.; Donadieu, A.; Ochs, M. Antibodies to PcpA and PhtD protect mice against Streptococcus pneumoniae by a macrophage- and complement-dependent mechanism. Hum. Vaccines Immunother. 2018, 14, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Simell, B.; Jaakkola, T.; Lahdenkari, M.; Briles, D.; Hollingshead, S.; Kilpi, T.M.; Kayhty, H. Serum antibodies to pneumococcal neuraminidase NanA in relation to pneumococcal carriage and acute otitis media. Clin. Vaccine Immunol. 2006, 13, 1177–1179. [Google Scholar] [CrossRef] [PubMed]
- Janapatla, R.P.; Chen, C.L.; Hsu, M.H.; Liao, W.T.; Chiu, C.H. Immunization with pneumococcal neuraminidases NanA, NanB and NanC to generate neutralizing antibodies and to increase survival in mice. J. Med. Microbiol. 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Surendran, N.; Ochs, M.; Pichichero, M.E. Human antibodies to PhtD, PcpA, and Ply reduce adherence to human lung epithelial cells and murine nasopharyngeal colonization by Streptococcus pneumoniae. Infect. Immun. 2014, 82, 5069–5075. [Google Scholar] [CrossRef] [PubMed]
- Godfroid, F.; Hermand, P.; Verlant, V.; Denoel, P.; Poolman, J.T. Preclinical evaluation of the Pht proteins as potential cross-protective pneumococcal vaccine antigens. Infect. Immun. 2011, 79, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Melin, M.; Di Paolo, E.; Tikkanen, L.; Jarva, H.; Neyt, C.; Kayhty, H.; Meri, S.; Poolman, J.; Vakevainen, M. Interaction of pneumococcal histidine triad proteins with human complement. Infect. Immun. 2010, 78, 2089–2098. [Google Scholar] [CrossRef] [PubMed]
- Lagousi, T.; Routsias, J.; Piperi, C.; Tsakris, A.; Chrousos, G.; Theodoridou, M.; Spoulou, V. Discovery of Immunodominant B Cell Epitopes within Surface Pneumococcal Virulence Proteins in Pediatric Patients with Invasive Pneumococcal Disease. J. Biol. Chem. 2015, 290, 27500–27510. [Google Scholar] [CrossRef] [PubMed]
- Kallio, A.; Sepponen, K.; Hermand, P.; Denoel, P.; Godfroid, F.; Melin, M. Role of Pht proteins in attachment of Streptococcus pneumoniae to respiratory epithelial cells. Infect. Immun. 2014, 82, 1683–1691. [Google Scholar] [CrossRef]
- Odutola, A.; Ota, M.O.C.; Antonio, M.; Ogundare, E.O.; Saidu, Y.; Foster-Nyarko, E.; Owiafe, P.K.; Ceesay, F.; Worwui, A.; Idoko, O.T.; et al. Efficacy of a novel, protein-based pneumococcal vaccine against nasopharyngeal carriage of Streptococcus pneumoniae in infants: A phase 2, randomized, controlled, observer-blind study. Vaccine 2017, 35, 2531–2542. [Google Scholar] [CrossRef]
- Odutola, A.; Ota, M.O.C.; Antonio, M.; Ogundare, E.O.; Saidu, Y.; Owiafe, P.K.; Worwui, A.; Idoko, O.T.; Owolabi, O.; Kampmann, B.; et al. Immunogenicity of pneumococcal conjugate vaccine formulations containing pneumococcal proteins, and immunogenicity and reactogenicity of co-administered routine vaccines—A phase II, randomised, observer-blind study in Gambian infants. Vaccine 2019, 37, 2586–2599. [Google Scholar] [CrossRef]
- Odutola, A.; Ota, M.O.; Ogundare, E.O.; Antonio, M.; Owiafe, P.; Worwui, A.; Greenwood, B.; Alderson, M.; Traskine, M.; Verlant, V.; et al. Reactogenicity, safety and immunogenicity of a protein-based pneumococcal vaccine in Gambian children aged 2–4 years: A phase II randomized study. Hum. Vaccines Immunother. 2016, 12, 393–402. [Google Scholar] [CrossRef]
- Leroux-Roels, G.; Maes, C.; De Boever, F.; Traskine, M.; Ruggeberg, J.U.; Borys, D. Safety, reactogenicity and immunogenicity of a novel pneumococcal protein-based vaccine in adults: A phase I/II randomized clinical study. Vaccine 2014, 32, 6838–6846. [Google Scholar] [CrossRef] [PubMed]
- Denoel, P.; Philipp, M.T.; Doyle, L.; Martin, D.; Carletti, G.; Poolman, J.T. A protein-based pneumococcal vaccine protects rhesus macaques from pneumonia after experimental infection with Streptococcus pneumoniae. Vaccine 2011, 29, 5495–5501. [Google Scholar] [CrossRef] [PubMed]
- Adamou, J.E.; Heinrichs, J.H.; Erwin, A.L.; Walsh, W.; Gayle, T.; Dormitzer, M.; Dagan, R.; Brewah, Y.A.; Barren, P.; Lathigra, R.; et al. Identification and characterization of a novel family of pneumococcal proteins that are protective against sepsis. Infect. Immun. 2001, 69, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Rioux, S.; Neyt, C.; Di Paolo, E.; Turpin, L.; Charland, N.; Labbe, S.; Mortier, M.C.; Mitchell, T.J.; Feron, C.; Martin, D.; et al. Transcriptional regulation, occurrence and putative role of the Pht family of Streptococcus pneumoniae. Microbiology 2011, 157, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Holmlund, E.; Quiambao, B.; Ollgren, J.; Jaakkola, T.; Neyt, C.; Poolman, J.; Nohynek, H.; Kayhty, H. Antibodies to pneumococcal proteins PhtD, CbpA, and LytC in Filipino pregnant women and their infants in relation to pneumococcal carriage. Clin. Vaccine Immunol. 2009, 16, 916–923. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Seiberling, M.; Bologa, M.; Brookes, R.; Ochs, M.; Go, K.; Neveu, D.; Kamtchoua, T.; Lashley, P.; Yuan, T.; Gurunathan, S. Safety and immunogenicity of a pneumococcal histidine triad protein D vaccine candidate in adults. Vaccine 2012, 30, 7455–7460. [Google Scholar] [CrossRef]
- Berglund, J.; Vink, P.; Tavares Da Silva, F.; Lestrate, P.; Boutriau, D. Safety, immunogenicity, and antibody persistence following an investigational Streptococcus pneumoniae and Haemophilus influenzae triple-protein vaccine in a phase 1 randomized controlled study in healthy adults. Clin. Vaccine Immunol. 2014, 21, 56–65. [Google Scholar] [CrossRef]
- Nabors, G.S.; Braun, P.A.; Herrmann, D.J.; Heise, M.L.; Pyle, D.J.; Gravenstein, S.; Schilling, M.; Ferguson, L.M.; Hollingshead, S.K.; Briles, D.E.; et al. Immunization of healthy adults with a single recombinant pneumococcal surface protein A (PspA) variant stimulates broadly cross-reactive antibodies to heterologous PspA molecules. Vaccine 2000, 18, 1743–1754. [Google Scholar] [CrossRef]
- Bologa, M.; Kamtchoua, T.; Hopfer, R.; Sheng, X.; Hicks, B.; Bixler, G.; Hou, V.; Pehlic, V.; Yuan, T.; Gurunathan, S. Safety and immunogenicity of pneumococcal protein vaccine candidates: Monovalent choline-binding protein A (PcpA) vaccine and bivalent PcpA-pneumococcal histidine triad protein D vaccine. Vaccine 2012, 30, 7461–7468. [Google Scholar] [CrossRef]
- Blumental, S.; Granger-Farbos, A.; Moisi, J.C.; Soullie, B.; Leroy, P.; Njanpop-Lafourcade, B.M.; Yaro, S.; Nacro, B.; Hallin, M.; Koeck, J.L. Virulence Factors of Streptococcus pneumoniae. Comparison between African and French Invasive Isolates and Implication for Future Vaccines. PLoS ONE 2015, 10, e0133885. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dopazo, J.; Mendoza, A.; Herrero, J.; Caldara, F.; Humbert, Y.; Friedli, L.; Guerrier, M.; Grand-Schenk, E.; Gandin, C.; de Francesco, M.; et al. Annotated draft genomic sequence from a Streptococcus pneumoniae type 19F clinical isolate. Microb. Drug Resist. 2001, 7, 99–125. [Google Scholar] [CrossRef] [PubMed]
- Donati, C.; Hiller, N.L.; Tettelin, H.; Muzzi, A.; Croucher, N.J.; Angiuoli, S.V.; Oggioni, M.; Dunning Hotopp, J.C.; Hu, F.Z.; Riley, D.R.; et al. Structure and dynamics of the pan-genome of Streptococcus pneumoniae and closely related species. Genome Biol. 2010, 11, R107. [Google Scholar] [CrossRef] [PubMed]
- Adam, H.J.; Golden, A.R.; Karlowsky, J.A.; Baxter, M.R.; Nichol, K.A.; Martin, I.; Demczuk, W.; Mulvey, M.R.; Gilmour, M.W.; Hoban, D.J.; et al. Analysis of multidrug resistance in the predominant Streptococcus pneumoniae serotypes in Canada: The SAVE study, 2011–2015. J. Antimicrob. Chemother. 2018, 73, vii12–vii19. [Google Scholar] [CrossRef] [PubMed]
- Richter, S.S.; Diekema, D.J.; Heilmann, K.P.; Dohrn, C.L.; Riahi, F.; Doern, G.V. Changes in pneumococcal serotypes and antimicrobial resistance after introduction of the 13-valent conjugate vaccine in the United States. Antimicrob. Agents Chemother. 2014, 58, 6484–6489. [Google Scholar] [CrossRef] [PubMed]
- Nagai, K.; Shibasaki, Y.; Hasegawa, K.; Davies, T.A.; Jacobs, M.R.; Ubukata, K.; Appelbaum, P.C. Evaluation of PCR primers to screen for Streptococcus pneumoniae isolates and beta-lactam resistance, and to detect common macrolide resistance determinants. J. Antimicrob. Chemother. 2001, 48, 915–918. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Wu, H.; Kojima, K.; Taniguchi, K.; Urasawa, S.; Uehara, N.; Omizu, Y.; Kishi, Y.; Yagihashi, A.; Kurokawa, I. Detection of mecA, femA, and femB genes in clinical strains of staphylococci using polymerase chain reaction. Epidemiol. Infect. 1994, 113, 259–266. [Google Scholar] [CrossRef]
- Pai, R.; Gertz, R.E.; Beall, B. Sequential multiplex PCR approach for determining capsular serotypes of Streptococcus pneumoniae isolates. J. Clin. Microbiol. 2006, 44, 124–131. [Google Scholar] [CrossRef]
- Kawaguchiya, M.; Urushibara, N.; Aung, M.S.; Habadera, S.; Ito, M.; Kudo, K.; Kobayashi, N. Association Between Pneumococcal Surface Protein A Family and Genetic/Antimicrobial Resistance Traits of Non-Invasive Pneumococcal Isolates from Adults in Northern Japan. Microb. Drug Resist. 2019, 25, 744–751. [Google Scholar] [CrossRef]
- Kawaguchiya, M.; Urushibara, N.; Aung, M.S.; Morimoto, S.; Ito, M.; Kudo, K.; Kobayashi, N. Genetic diversity of pneumococcal surface protein A (PspA) in paediatric isolates of non-conjugate vaccine serotypes in Japan. J. Med. Microbiol. 2018, 67, 1130–1138. [Google Scholar] [CrossRef]
- Kawaguchiya, M.; Urushibara, N.; Aung, M.S.; Shinagawa, M.; Takahashi, S.; Kobayashi, N. Serotype distribution, antimicrobial resistance and prevalence of pilus islets in pneumococci following the use of conjugate vaccines. J. Med. Microbiol. 2017, 66, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchiya, M.; Urushibara, N.; Kobayashi, N. High prevalence of genotype 6E (putative serotype 6E) among noninvasive/colonization isolates of Streptococcus pneumoniae in northern Japan. Microb. Drug Resist. 2015, 21, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Golden, A.R.; Rosenthal, M.; Fultz, B.; Nichol, K.A.; Adam, H.J.; Gilmour, M.W.; Baxter, M.R.; Hoban, D.J.; Karlowsky, J.A.; Zhanel, G.G. Characterization of MDR and XDR Streptococcus pneumoniae in Canada, 2007–2013. J. Antimicrob. Chemother. 2015, 70, 2199–2202. [Google Scholar] [CrossRef] [PubMed]
- Choe, Y.J.; Lee, H.J.; Lee, H.; Oh, C.E.; Cho, E.Y.; Choi, J.H.; Kang, H.M.; Yoon, I.A.; Jung, H.J.; Choi, E.H. Emergence of antibiotic-resistant non-vaccine serotype pneumococci in nasopharyngeal carriage in children after the use of extended-valency pneumococcal conjugate vaccines in Korea. Vaccine 2016, 34, 4771–4776. [Google Scholar] [CrossRef] [PubMed]
- Klaassen, C.H.; Mouton, J.W. Molecular detection of the macrolide efflux gene: To discriminate or not to discriminate between mef(A) and mef(E). Antimicrob. Agents Chemother. 2005, 49, 1271–1278. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Doherty, N.; Trzcinski, K.; Pickerill, P.; Zawadzki, P.; Dowson, C.G. Genetic diversity of the tet(M) gene in tetracycline-resistant clonal lineages of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 2000, 44, 2979–2984. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.S.; Ambler, J.; Mehtar, S.; Fisher, L.M. Involvement of topoisomerase IV and DNA gyrase as ciprofloxacin targets in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 1996, 40, 2321–2326. [Google Scholar] [CrossRef]
| Vaccine Type | Serotype | Children | Adults | Total | ST c (No. of Isolates) | MLST Allelic Profile d | Remarks |
|---|---|---|---|---|---|---|---|
| n = 10 | n = 47 | n = 57 (%) | (PMEN Clone/Related ST) e | ||||
| PCV13 a type | 3 | 0 | 5 | 5 (8.8) | 180 (4) | 7-15-2-10-6-1-22 | Netherlands3-31 |
| 2331 (1) | 10-16-150-1-17-1-29 | ||||||
| 6A | 0 | 2 | 2 (3.5) | 3113 (1) | 8-8-4-16-77-1-68 | ||
| 7836 (1) | 15-29-4-21-6-1-14 | ||||||
| 6B | 0 | 2 | 2 (3.5) | 14,601 (1) | 2-29-4-1-6-121-11 | SLV of ST5232 | |
| 1092 (1) | 2-13-2-1-3-19-14 | ||||||
| 19F | 0 | 2 | 2 (3.5) | 257 (1) | 22-16-19-15-6-20-14 | DLV of ST236/Taiwan19F-14 | |
| 236 (1) | 15-16-19-15-6-20-26 | Taiwan19F-14 | |||||
| PPSV23 b Type (Except PCV13 Type) | 10A | 1 | 1 | 2 (3.5) | 5236 * (2) | 7-12-1-1-10-1-11 | DLV of ST113/Netherlands18C-36 |
| 11A/11D | 1 | 1 | 2 (3.5) | 99 (3) | 5-8-4-16-6-1-31 | ||
| 12F | 0 | 1 | 1 (1.8) | 4846 (1) | 12-32-111-1-13-48-6 | ||
| 14 | 0 | 2 | 2 (3.5) | 2922 (2) | 1-5-4-5-5-20-8 | SLV of ST9/England14-9 | |
| 15B | 2 | 2 | 4 (7.0) | 199 (4) | 8-13-14-4-17-4-14 | Netherlands15B-37 | |
| 22F/22A | 0 | 4 | 4 (7.0) | 433 * (4) | 1-1-4-1-18-58-17 | ||
| 33F | 0 | 1 | 1 (1.8) | 717 (1) | 5-35-29-1-45-39-18 | ||
| Non-Vaccine Type | 6C | 0 | 5 | 5 (8.8) | 282 (1) | 30-4-2-4-4-1-1 | SLV of ST81/Spain23F-1 |
| 5832 (4) | 7-9-4-16-1-6-384 | ||||||
| 6E | 0 | 1 | 1 (1.8) | 90 (1) | 5-6-1-2-6-3-4 | Spain6B-2 | |
| 15A | 0 | 8 | 8 (14.0) | 63 * (5) | 2-5-36-12-17-21-14 | Sweden15A-25 | |
| 13,065 * (1) | 2-5-36-12-17-21-384 | SLV of ST63/Sweden15A-25 | |||||
| 13,068 (1) | 2-5-36-12-17-777-14 | SLV of ST63/Sweden15A-25 | |||||
| 14,602 (1) | 7-8-8-8-6-28-664 | SLV of ST292 | |||||
| 15C | 0 | 2 | 2 (3.5) | 199 (2) | 8-13-14-4-17-4-14 | Netherlands15B-37 | |
| 23A | 2 | 0 | 2 (3.5) | 338 (1) | 7-13-8-6-1-6-8 | Colombia23F-26 | |
| 8340 (1) | 7-367-8-6-1-337-8 | DLV of ST338 | |||||
| 24F | 2 | 0 | 2 (3.5) | 2572 (1) | 7-75-9-6-25-6-14 | ||
| 5496 (1) | 7-257-9-6-25-6-14 | ||||||
| 31 | 0 | 1 | 1 (1.8) | 11,184 (1) | 1-2-461-16-15-155-18 | ||
| 34 | 0 | 2 | 2 (3.5) | 3116 (3) | 10-8-6-1-9-1-279 | ||
| 35B | 2 | 3 | 5 (8.8) | 558 (3) | 18-12-4-44-14-77-97 | SLV of ST377/Utah35B-24 | |
| 2755 (2) | 10-12-2-1-152-28-14 | ||||||
| 37 | 0 | 2 | 2 (3.5) | 447 (1) | 29-33-19-1-36-22-31 | ||
| 7970 (1) | 29-33-19-1-36-482-31 | SLV of ST447 |
| No. of Isolates with Pneumococcal Protein Gene | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serotype | pspAa | pspC | pht | nan | Others | |||||||||||||||
| (No. of Isolates) | fam1/fam2/fam3 | pspC | pspC.4 | phtA | phtB | phtD | phtE | phtA/Bb | phtA/Db | nanA | nanB | nanC | pcpA | psrp | ply | pavA | ||||
| PCV13 Serotype | ||||||||||||||||||||
| 3 (5) | 4/1/0 | 5 | 0 | 1 | 0 | 1 | 5 | 4 | 0 | 5 | 5 | 1 | 1 | 1 | 5 | 5 | ||||
| 6A (2) | 1/1/0 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 0 | 2 | 2 | 0 | 2 | 1 | 2 | 2 | ||||
| 19F (2) | 1/2/0 | 2 | 2 | 0 | 1 | 0 | 2 | 1 | 1 | 2 | 2 | 0 | 2 | 0 | 2 | 2 | ||||
| 6B (2) | 1/1/0 | 0 | 0 | 0 | 0 | 2 | 2 | 2 | 0 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | ||||
| PPSV23 Serotype (Except PCV13 Type) | ||||||||||||||||||||
| 10A (2) | 2/0/0 | 2 | 0 | 2 | 0 | 2 | 2 | 0 | 0 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | ||||
| 11A/11D (2) | 0/2/0 | 2 | 0 | 2 | 0 | 2 | 2 | 0 | 0 | 2 | 2 | 1 | 2 | 0 | 2 | 2 | ||||
| 12F (1) | 0/1/0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | ||||
| 14 (2) | 2/0/0 | 2 | 0 | 0 | 0 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | ||||
| 15B (4) | 0/4/0 | 3 | 2 | 0 | 0 | 2 | 4 | 0 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | ||||
| 22F/22A (4) | 4/0/0 | 4 | 4 | 0 | 0 | 0 | 4 | 4 | 0 | 4 | 4 | 0 | 4 | 0 | 4 | 4 | ||||
| 33F (1) | 1/0/0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||
| Non-Vaccine Serotype | ||||||||||||||||||||
| 6C (5) | 4/1/0 | 4 | 0 | 4 | 4 | 5 | 5 | 1 | 0 | 5 | 5 | 5 | 5 | 1 | 5 | 5 | ||||
| 6E (1) | 1/0/0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | ||||
| 15A (8) | 0/6/0 | 2 | 0 | 7 | 0 | 8 | 8 | 1 | 0 | 8 | 8 | 7 | 7 | 7 | 8 | 8 | ||||
| 15C (2) | 0/2/0 | 2 | 2 | 0 | 0 | 0 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | ||||
| 23A (2) | 2/0/0 | 2 | 0 | 2 | 2 | 2 | 2 | 0 | 0 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | ||||
| 24F (2) | 2/0/0 | 2 | 0 | 2 | 0 | 2 | 2 | 0 | 0 | 2 | 2 | 0 | 2 | 0 | 2 | 2 | ||||
| 31 (1) | 1/0/0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | ||||
| 34 (2) | 2/0/0 | 2 | 0 | 1 | 1 | 0 | 2 | 1 | 0 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | ||||
| 35B (5) | 5/0/0 | 5 | 3 | 1 | 1 | 4 | 5 | 3 | 1 | 5 | 5 | 0 | 5 | 1 | 5 | 5 | ||||
| 37 (2) | 0/0/2 | 2 | 0 | 2 | 2 | 1 | 2 | 0 | 0 | 2 | 2 | 0 | 2 | 0 | 2 | 2 | ||||
| Total (57) | 33/20/2 | 45 | 15 | 28 | 15 | 35 | 57 | 21 | 8 | 57 | 57 | 30 | 52 | 24 | 57 | 57 | ||||
| Positive Rate; % | 57.9/35.1/3.5 | 78.9 | 26.3 | 49.1 | 26.3 | 61.4 | 100 | 36.9 | 14.0 | 100 | 100 | 52.6 | 91.2 | 42.1 | 100 | 100 | ||||
| Profile of pht Genes | No. of Isolates (%) | Serotypes (No. of Isolates) |
|---|---|---|
| phtA + phtB + phtE | 5 (8.8) | 6E (1), 31 (1), 33F (1), 34 (1), 37 (1) |
| phtA + phtD + phtE | 15 (26.3) | 3 (1), 10A (2), 11A/11D (2), 15A (7), 24F (2), 35B (1) |
| phtA + phtB + phtD + phtE | 8 (14.0) | 6A (1), 6C (4), 23A (2), 37 (1) |
| phtA/Ba + phtE | 11 (19.3) | 19F (1), 6A (1), 3 (4), 34 (1), 22F (4) |
| phtA/Ba + phtD + phtE | 10 (17.5) | 6B (2), 6C (1), 12F (1), 14 (2), 15A (1), 35B (3) |
| phtA/Db + phtE | 4 (7.0) | 15B (2), 15C (2) |
| phtA/Db + phtB + phtE | 2 (3.5) | 19F (1), 35B (1) |
| phtA/Db + phtD + phtE | 2 (3.5) | 15B (2) |
| Profile of the pht Fusion Type | 29 (50.9) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawaguchiya, M.; Urushibara, N.; Aung, M.S.; Shinagawa, M.; Takahashi, S.; Kobayashi, N. Prevalence of Various Vaccine Candidate Proteins in Clinical Isolates of Streptococcus pneumoniae: Characterization of the Novel Pht Fusion Proteins PhtA/B and PhtA/D. Pathogens 2019, 8, 162. https://doi.org/10.3390/pathogens8040162
Kawaguchiya M, Urushibara N, Aung MS, Shinagawa M, Takahashi S, Kobayashi N. Prevalence of Various Vaccine Candidate Proteins in Clinical Isolates of Streptococcus pneumoniae: Characterization of the Novel Pht Fusion Proteins PhtA/B and PhtA/D. Pathogens. 2019; 8(4):162. https://doi.org/10.3390/pathogens8040162
Chicago/Turabian StyleKawaguchiya, Mitsuyo, Noriko Urushibara, Meiji Soe Aung, Masaaki Shinagawa, Satoshi Takahashi, and Nobumichi Kobayashi. 2019. "Prevalence of Various Vaccine Candidate Proteins in Clinical Isolates of Streptococcus pneumoniae: Characterization of the Novel Pht Fusion Proteins PhtA/B and PhtA/D" Pathogens 8, no. 4: 162. https://doi.org/10.3390/pathogens8040162
APA StyleKawaguchiya, M., Urushibara, N., Aung, M. S., Shinagawa, M., Takahashi, S., & Kobayashi, N. (2019). Prevalence of Various Vaccine Candidate Proteins in Clinical Isolates of Streptococcus pneumoniae: Characterization of the Novel Pht Fusion Proteins PhtA/B and PhtA/D. Pathogens, 8(4), 162. https://doi.org/10.3390/pathogens8040162





