Rates and Correlates of Short Term Virologic Response among Treatment-Naïve HIV-Infected Children Initiating Antiretroviral Therapy in Ethiopia: A Multi-Center Prospective Cohort Study
Abstract
1. Introduction
2. Methods
2.1. Study Participants
2.2. Combination Antiretroviral Therapy
2.3. Data Collection Procedures
2.4. HIV Drug Resistance Testing
2.5. Virological Outcome Definitions
2.6. Statistical Analysis
2.7. Ethics Statement
3. Results
3.1. Baseline Characteristics of Study Participants
3.2. Rates and Correlates of Virological Suppression to Undetectable Levels
3.3. Rates and Correlates of Subsequent Virologic Rebound
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- UNAIDS. Progress towards the 90-90-90 Targets. Available online: http://aidsinfo.unaids.org/ 2018 (accessed on 29 May 2019).
- WHO. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection—Recommendations for a Public Health Approach—Second Edition. Available online: https://www.who.int/hiv/pub/arv/arv-2016/en/ 2016 (accessed on 28 May 2019).
- UNAIDS. 90-90-90: An Ambitious Treatment Target to Help End the AIDS Epidemic. 2017. Available online: https://www.unaids.org/en/resources/909090 (accessed on 29 May 2019).
- Boerma, R.; Schellekens, O.; Rinke de Wit, T.F.; Wit, F.W.; van der Borght, S.; Rijckborst, H.; Chukwumah, P.; Schilthuis, H. Reaching 90-90-90: Outcomes of a 15-year multi-country HIV workplace programme in sub-Saharan Africa. Antivir. Ther. 2019. [Google Scholar] [CrossRef] [PubMed]
- Bain, L.E.; Nkoke, C.; Noubiap, J.J.N. UNAIDS 90-90-90 targets to end the AIDS epidemic by 2020 are not realistic: comment on “Can the UNAIDS 90-90-90 target be achieved? A systematic analysis of national HIV treatment cascades”. BMJ Glob. Health 2017, 2, e000227. [Google Scholar] [CrossRef] [PubMed]
- Levi, J.; Raymond, A.; Pozniak, A.; Vernazza, P.; Kohler, P.; Hill, A. Can the UNAIDS 90-90-90 target be achieved? A systematic analysis of national HIV treatment cascades. BMJ Glob. Health 2016, 1, e000010. [Google Scholar] [CrossRef]
- Chan, M.K.; Goodall, R.; Judd, A.; Klein, N.; Chiappini, E.; Klimkait, T.; Ngo-Giang-Huong, N.; Palma, P.; Rossi, P.; Thorne, C.; et al. Predictors of faster virological suppression in early treated infants with perinatal HIV from Europe and Thailand. AIDS 2019, 33, 1155–1165. [Google Scholar]
- Chandrasekaran, P.; Shet, A.; Srinivasan, R.; Sanjeeva, G.N.; Subramanyan, S.; Sunderesan, S.; Ramesh, K.; Gopalan, B.; Suresh, E.; Poornagangadevi, N.; et al. Long-term virological outcome in children receiving first-line antiretroviral therapy. AIDS Res. Ther. 2018, 15, 23. [Google Scholar] [CrossRef] [PubMed]
- Ateba Ndongo, F.; Texier, G.; Ida Penda, C.; Tejiokem, M.C.; Tetang Ndiang, S.; Ndongo, J.A.; Guemkam, G.; Sofeu, C.L.; Kfutwah, A.; Faye, A.; et al. Virologic Response to Early Antiretroviral Therapy in HIV-infected Infants: Evaluation After 2 Years of Treatment in the Pediacam Study, Cameroon. Pediatr. Infect. Dis. J. 2018, 37, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Raffi, F.; Hanf, M.; Ferry, T.; Khatchatourian, L.; Joly, V.; Pugliese, P.; Katlama, C.; Robineau, O.; Chirouze, C.; Jacomet, C.; et al. Impact of baseline plasma HIV-1 RNA and time to virological suppression on virological rebound according to first-line antiretroviral regimen. J. Antimicrob. Chemother. 2017, 72, 3425–3434. [Google Scholar] [CrossRef] [PubMed]
- Hamers, R.L.; Schuurman, R.; Sigaloff, K.C.; Wallis, C.L.; Kityo, C.; Siwale, M.; Mandaliya, K.; Ive, P.; Botes, M.E.; Wellington, M.; et al. Effect of pretreatment HIV-1 drug resistance on immunological, virological, and drug-resistance outcomes of first-line antiretroviral treatment in sub-Saharan Africa: A multicentre cohort study. Lancet Infect. Dis. 2012, 12, 307–317. [Google Scholar] [CrossRef]
- Wittkop, L.; Gunthard, H.F.; de Wolf, F.; Dunn, D.; Cozzi-Lepri, A.; de Luca, A.; Kucherer, C.; Obel, N.; von Wyl, V.; Masquelier, B.; et al. Effect of transmitted drug resistance on virological and immunological response to initial combination antiretroviral therapy for HIV (EuroCoord-CHAIN joint project): A European multicohort study. Lancet Infect. Dis. 2011, 11, 363–371. [Google Scholar] [CrossRef]
- Rojas Sanchez, P.; Holguin, A. Drug resistance in the HIV-1-infected paediatric population worldwide: A systematic review. J. Antimicrob. Chemother. 2014, 69, 2032–2042. [Google Scholar] [CrossRef]
- MacLeod, I.J.; Rowley, C.F.; Thior, I.; Wester, C.; Makhema, J.; Essex, M.; Lockman, S. Minor resistant variants in nevirapine-exposed infants may predict virologic failure on nevirapine-containing ART. J. Clin. Virol. 2010, 48, 162–167. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mekuria, L.A.; Nieuwkerk, P.T.; Yalew, A.W.; Sprangers, M.A.; Prins, J.M. High level of virological suppression among HIV-infected adults receiving combination antiretroviral therapy in Addis Ababa, Ethiopia. Antivir. Ther. 2016, 21, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Mulu, A.; Liebert, U.G.; Maier, M. Virological efficacy and immunological recovery among Ethiopian HIV-1 infected adults and children. BMC Infect. Dis. 2014, 14, 28. [Google Scholar] [CrossRef] [PubMed]
- Habtewold, A.; Makonnen, E.; Amogne, W.; Yimer, G.; Aderaye, G.; Bertilsson, L.; Burhenne, J.; Aklillu, E. Is there a need to increase the dose of efavirenz during concomitant rifampicin-based antituberculosis therapy in sub-Saharan Africa? The HIV-TB pharmagene study. Pharmacogenomics 2015, 16, 1047–1064. [Google Scholar] [CrossRef] [PubMed]
- Amogne, W.; Aderaye, G.; Habtewold, A.; Yimer, G.; Makonnen, E.; Worku, A.; Sonnerborg, A.; Aklillu, E.; Lindquist, L. Efficacy and Safety of Antiretroviral Therapy Initiated One Week after Tuberculosis Therapy in Patients with CD4 Counts <200 Cells/muL: TB-HAART Study, a Randomized Clinical Trial. PLoS ONE 2015, 10, e0122587. [Google Scholar]
- Taieb, F.; Madec, Y.; Cournil, A.; Delaporte, E. Virological success after 12 and 24 months of antiretroviral therapy in sub-Saharan Africa: Comparing results of trials, cohorts and cross-sectional studies using a systematic review and meta-analysis. PLoS ONE 2017, 12, e0174767. [Google Scholar] [CrossRef] [PubMed]
- Mulu, A.; Maier, M.; Liebert, U.G. Upward trends of acquired drug resistances in Ethiopian HIV-1C isolates: A decade longitudinal study. PLoS ONE 2017, 12, e0186619. [Google Scholar] [CrossRef]
- Tadesse, B.T.; Kinloch, N.N.; Baraki, B.; Lapointe, H.R.; Cobarrubias, K.D.; Brockman, M.A.; Brumme, C.J.; Foster, B.A.; Jerene, D.; Makonnen, E.; et al. High Levels of Dual-Class Drug Resistance in HIV-Infected Children Failing First-Line Antiretroviral Therapy in Southern Ethiopia. Viruses 2018, 10, 60. [Google Scholar] [CrossRef]
- Telele, N.F.; Kalu, A.W.; Gebre-Selassie, S.; Fekade, D.; Abdurahman, S.; Marrone, G.; Neogi, U.; Tegbaru, B.; Sonnerborg, A. Pretreatment drug resistance in a large countrywide Ethiopian HIV-1C cohort: A comparison of Sanger and high-throughput sequencing. Sci. Rep. 2018, 8, 7556. [Google Scholar] [CrossRef]
- Tadesse, B.T.; Foster, B.A.; Chala, A.; Chaka, T.E.; Bizuayehu, T.; Ayalew, F.; Getahun, H.M.; Tadesse, S.; Jerene, D.; Makonnen, E.; et al. HIV and cART-Associated Dyslipidemia Among HIV-Infected Children. J. Clin. Med. 2019, 8, 430. [Google Scholar] [CrossRef]
- UNAIDS. Ethiopia: Country Factsheets. Available online: https://www.unaids.org/en/regionscountries/countries/ethiopia 2017 (accessed on 20 June 2019).
- EFMOH: Ministry of Health Federal Democratic Republic of Ethiopia. National Guidelines for Comprehensive HIV Prevention, Care and Treatment; Ethiopian Ministry of Health: Addis Ababa, Ethiopia, 2016.
- Amico, K.R.; Fisher, W.A.; Cornman, D.H.; Shuper, P.A.; Redding, C.G.; Konkle-Parker, D.J.; Barta, W.; Fisher, J.D. Visual analog scale of ART adherence: Association with 3-day self-report and adherence barriers. J. Acquir. Immune Defic. Syndr. 2006, 42, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Tadesse, B.T.; Tsai, O.; Chala, A.; Chaka, T.E.; Eromo, T.; Lapointe, H.R.; Baraki, B.; Shahid, A.; Tadesse, S.; Makonnen, E.; et al. Prevalence and Correlates of Pre-Treatment HIV Drug Resistance among HIV-Infected Children in Ethiopia. Viruses 2019, 11, 877. [Google Scholar] [CrossRef]
- Buckton, A.J.; Prabhu, D.P.; Cane, P.A.; Pillay, D. No evidence for cross-contamination of dried blood spots excised using an office hole-punch for HIV-1 drug resistance genotyping. J. Antimicrob. Chemother. 2009, 63, 615–616. [Google Scholar] [CrossRef] [PubMed]
- Woods, C.K.; Brumme, C.J.; Liu, T.F.; Chui, C.K.; Chu, A.L.; Wynhoven, B.; Hall, T.A.; Trevino, C.; Shafer, R.W.; Harrigan, P.R. Automating HIV drug resistance genotyping with RECall, a freely accessible sequence analysis tool. J. Clin. Microbiol. 2012, 50, 1936–1942. [Google Scholar] [CrossRef] [PubMed]
- Bennett, D.E.; Camacho, R.J.; Otelea, D.; Kuritzkes, D.R.; Fleury, H.; Kiuchi, M.; Heneine, W.; Kantor, R.; Jordan, M.R.; Schapiro, J.M.; et al. Drug resistance mutations for surveillance of transmitted HIV-1 drug-resistance: 2009 update. PLoS ONE 2009, 4, e4724. [Google Scholar] [CrossRef]
- Liu, T.F.; Shafer, R.W. Web resources for HIV type 1 genotypic-resistance test interpretation. Clin. Infect. Dis. 2006, 42, 1608–1618. [Google Scholar] [CrossRef] [PubMed]
- Bulage, L.; Ssewanyana, I.; Nankabirwa, V.; Nsubuga, F.; Kihembo, C.; Pande, G.; Ario, A.R.; Matovu, J.K.; Wanyenze, R.K.; Kiyaga, C. Factors Associated with Virological Non-suppression among HIV-Positive Patients on Antiretroviral Therapy in Uganda, August 2014-July 2015. BMC Infect. Dis. 2017, 17, 326. [Google Scholar] [CrossRef] [PubMed]
- Girum, T.; Wasie, A.; Worku, A. Trend of HIV/AIDS for the last 26 years and predicting achievement of the 90-90-90 HIV prevention targets by 2020 in Ethiopia: A time series analysis. BMC Infect. Dis. 2018, 18, 320. [Google Scholar] [CrossRef]
- UNAIDS. Eastern Southern Africa: Data. 2017. Available online: https://www.unaids.org/en/regionscountries/easternandsouthernafrica (accessed on 2 July 2019).
- Teasdale, C.A.; Sogaula, N.; Yuengling, K.A.; Wang, C.; Mutiti, A.; Arpadi, S.; Nxele, M.; Pepeta, L.; Mogashoa, M.; Rivadeneira, E.D.; et al. HIV viral suppression and longevity among a cohort of children initiating antiretroviral therapy in Eastern Cape, South Africa. J. Int. AIDS Soc. 2018, 21, e25168. [Google Scholar] [CrossRef]
- Boender, T.S.; Hoenderboom, B.M.; Sigaloff, K.C.; Hamers, R.L.; Wellington, M.; Shamu, T.; Siwale, M.; Labib Maksimos, E.E.; Nankya, I.; Kityo, C.M.; et al. Pretreatment HIV drug resistance increases regimen switches in sub-Saharan Africa. Clin. Infect. Dis. 2015, 61, 1749–1758. [Google Scholar] [CrossRef]
- Kantor, R.; Smeaton, L.; Vardhanabhuti, S.; Hudelson, S.E.; Wallis, C.L.; Tripathy, S.; Morgado, M.G.; Saravanan, S.; Balakrishnan, P.; Reitsma, M.; et al. Pretreatment HIV Drug Resistance and HIV-1 Subtype C Are Independently Associated With Virologic Failure: Results From the Multinational PEARLS (ACTG A5175) Clinical Trial. Clin. Infect. Dis. 2015, 60, 1541–1549. [Google Scholar] [CrossRef] [PubMed]
- Kityo, C.; Boerma, R.S.; Sigaloff, K.C.E.; Kaudha, E.; Calis, J.C.J.; Musiime, V.; Balinda, S.; Nakanjako, R.; Boender, T.S.; Mugyenyi, P.N.; et al. Pretreatment HIV drug resistance results in virological failure and accumulation of additional resistance mutations in Ugandan children. J. Antimicrob. Chemother. 2017, 72, 2587–2595. [Google Scholar] [CrossRef] [PubMed]
- Ngo-Giang-Huong, N.; Wittkop, L.; Judd, A.; Reiss, P.; Goetghebuer, T.; Duiculescu, D.; Noguera-Julian, A.; Marczynska, M.; Giacquinto, C.; Ene, L.; et al. Prevalence and effect of pre-treatment drug resistance on the virological response to antiretroviral treatment initiated in HIV-infected children-A EuroCoord-CHAIN-EPPICC joint project. BMC Infect. Dis. 2016, 16, 654. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Zheng, X.; Liu, Y.; Liu, L.; Xin, R.; Lu, H.; Huang, C.; Bai, L.; Mammano, F.; Zhang, T.; et al. Detection of pretreatment minority HIV-1 reverse transcriptase inhibitor-resistant variants by ultra-deep sequencing has a limited impact on virological outcomes. J. Antimicrob. Chemother. 2019, 74, 1408–1416. [Google Scholar] [CrossRef] [PubMed]
- Di Biagio, A.; Rusconi, S.; Marzocchetti, A.; Signori, A.; Schiavetti, I.; Bruzzone, B.; Monno, L.; Punzi, G.; Colao, M.G.; Penco, G.; et al. The role of baseline HIV-1 RNA, drug resistance, and regimen type as determinants of response to first-line antiretroviral therapy. J. Med. Virol. 2014, 86, 1648–1655. [Google Scholar] [CrossRef] [PubMed]
- Paredes, R.; Mocroft, A.; Kirk, O.; Lazzarin, A.; Barton, S.E.; van Lunzen, J.; Katzenstein, T.L.; Antunes, F.; Lundgren, J.D.; Clotet, B. Predictors of virological success and ensuing failure in HIV-positive patients starting highly active antiretroviral therapy in Europe: Results from the EuroSIDA study. Arch. Intern. Med. 2000, 160, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Derache, A.; Iwuji, C.C.; Danaviah, S.; Giandhari, J.; Marcelin, A.G.; Calvez, V.; de Oliveira, T.; Dabis, F.; Pillay, D.; Gupta, R.K. Predicted antiviral activity of tenofovir versus abacavir in combination with a cytosine analogue and the integrase inhibitor dolutegravir in HIV-1-infected South African patients initiating or failing first-line ART. J. Antimicrob. Chemother. 2019, 74, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Cruciani, M.; Mengoli, C.; Malena, M.; Serpelloni, G.; Parisi, S.G.; Moyle, G.; Bosco, O. Virological efficacy of abacavir: Systematic review and meta-analysis. J. Antimicrob. Chemother. 2014, 69, 3169–3180. [Google Scholar] [CrossRef] [PubMed]
- Sax, P.E.; Tierney, C.; Collier, A.C.; Fischl, M.A.; Mollan, K.; Peeples, L.; Godfrey, C.; Jahed, N.C.; Myers, L.; Katzenstein, D.; et al. Abacavir-lamivudine versus tenofovir-emtricitabine for initial HIV-1 therapy. N. Engl. J. Med. 2009, 361, 2230–2240. [Google Scholar] [CrossRef]


| Variable | Frequency (%) | N with Data |
|---|---|---|
| Age at enrollment, median (Q1–Q3), years | 9 (5–12) | 111 |
| Sex, n (%) Male | 63 (56.8) | 111 |
| Any PDR, n (%) Yes | 6 (11.8) | 51 |
| Tuberculosis, n (%) Yes | 16 (16.4) | 111 |
| Isoniazid preventive therapy, n (%) Yes | 34 (30.6) | 111 |
| Cotrimozazole preventive therapy, n (%) Yes | 81 (83.5) | 97 |
| Hematocrit, median (Q1–Q3) in % | 37.3 (35.1–40.5) | 84 |
| CD4+ T cell count, median (Q1–Q3) | 330 (178–741) | 59 |
| Aspartate aminotransferase, median (Q1–Q3) units/L | 38 (30–48) | 108 |
| Alanine aminotransferase, median (Q1–Q3), units/L | 27 (20–38) | 106 |
| Total Bilirubin, median (Q1–Q3) mg/dL | 0.8 (0.4–1.1) | 105 |
| Albumin, median (Q1–Q3) g/dL | 3.8 (3.2–4.2) | 104 |
| Blood urea nitrogen, median (Q1–Q3) mg/dL | 18 (12.1–26.5) | 108 |
| Creatinine, median (Q1–Q3) mg/dL | 0.6 (0.4–0.7) | 107 |
| Total cholesterol, median (Q1–Q3) mg/dL | 120 (96–150) | 106 |
| cART Regimen | 111 | |
| Abacavir-based | 37 (33.3) | |
| Zidovudine-based | 15 (13.5) | |
| Tenofovir-based | 59 (53.2) | |
| WHO clinical stage at enrolment | 110 | |
| Stage 1 | 44 (40) | |
| Stage 2 | 23 (20.9) | |
| Stage 3 | 33 (30) | |
| Stage 4 | 10 (9.1) | |
| Low density lipoprotein, median (Q1–Q3) mg/dL | 45 (32–63) | 108 |
| High density lipoprotein, median (Q1–Q3) mg/dL | 47 (38–65) | 105 |
| Triglyceride, median (Q1–Q3) mg/dL | 103 (87–151) | 106 |
| Weight for age Z score, median (Q1–Q3) mg/dL | –1.2 (–2.5–(–0.6)) | 73 |
| Height for age Z score, median (Q1–Q3) mg/dL | –1.4 (–2.5–(–0.6)) | 109 |
| Body mass index Z score, median (Q1–Q3) mg/dL | –1.2 (–2.4–(–0.3)) | 109 |
| Log10 plasma viral load, median (Q1–Q3) copies/mL | 4.2 (3.3–4.9) | 110 |
| Variable | Virological Suppression | Crude HR (95% CI) | P Value | aHR (95%CI) | P Value | |
|---|---|---|---|---|---|---|
| Undetectable | Detectable | |||||
| Age in years: median (Q1–Q3) | 9 (5–12.5) | 9 (5.5–12.5) | 1.0 (0.9–1.1) | 0.82 | ||
| Sex: N (%) | ||||||
| Male | 57 (60.6) | 6 (37.5) | Ref | Ref | ||
| Female | 37 (39.4) | 10 (62.5) | 1.1 (0.7–1.6) | 0.76 | ||
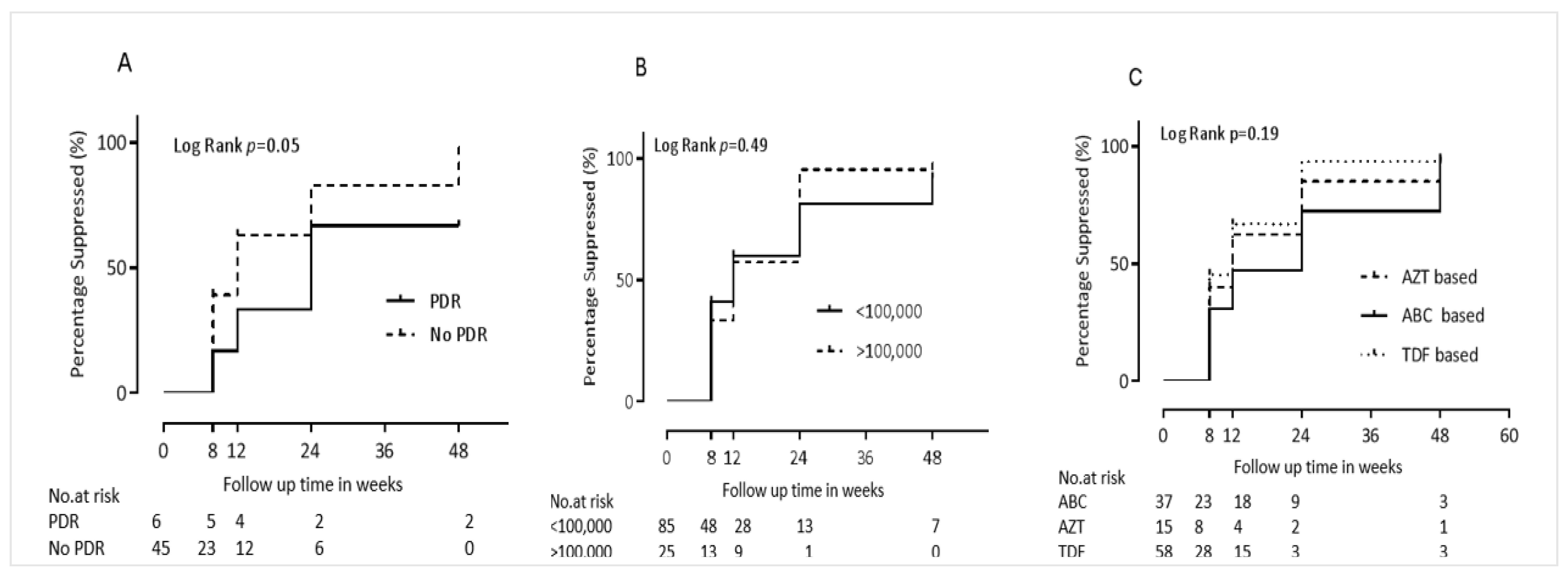
| PDR: N Yes (%) | ||||||
| Yes | 3 (7.3) | 3 (30) | Ref | Ref | Ref | Ref |
| No | 38 (92.7) | 7 (70) | 2.6 (0.8–8.3) | 0.12 | 11.7 (1.3–104.2) | 0.028 |
| TB: N (%) | ||||||
| Yes | 13 (14) | 3 (18.8) | Ref | Ref | ||
| No | 80 (86) | 13 (81.2) | 1.0 (0.5–1.8) | 0.96 | ||
| IPT: N (%) | ||||||
| Yes | 29 (30.9) | 4 (25) | Ref | Ref | Ref | Ref |
| No | 65 (69.2) | 12 (75) | 0.7 (0.5–1.2) | 0.19 | 0.6 (0.1–4.5) | 0.64 |
| CPT: N (%) | ||||||
| Yes | 65 (82.3) | 12 (85.7) | Ref | Ref | ||
| No | 14 (17.7) | 2 (14.3) | 1.0 (0.5–1.7) | 0.92 | ||
| HCT in %: median (Q1–Q3) | 37.7 (35.1–41.2) | 36.7 (35.5–39) | 1.0 (1.0–1.1) | 0.55 | ||
| CD4 cells/mm3 median (Q1–Q3) | 381 (223–741) | 217 (178–317) | 1.0 (0.9–1.0) | 0.95 | ||
| AST in units/dL: median (Q1–Q3) | 37 (30–46) | 45 (37–60) | 1.0 (0.9–1.0) | 0.31 | ||
| ALT in units/dL: median (Q1–Q3) | 26 (18–36) | 39 (33–42) | 1.0 (0.9–1.0) | 0.47 | ||
| Bil-T in mg/dL: median (Q1–Q3) | 0.7 (0.4–1.1) | 0.9 (0.5–1.1) | 1.06 (0.8–1.4) | 0.68 | ||
| ALB in mg/dL: median (Q1–Q3) | 3.8 (3.2–4.3) | 3.8 (3.5–4.1) | 1.0 (0.8–1.3) | 0.95 | ||
| BUN in mg/dL: median (Q1–Q3) | 17 (12–24) | 20.5 (15–36) | 1.0 (0.9–1.0) | 0.27 | ||
| Cr in mg/dL: median (Q1–Q3) | 0.6 (0.4–0.7) | 0.6 (0.4–0.7) | 1.3 (0.6–2.7) | 0.54 | ||
| TChol in mg/dL: median (Q1–Q3) | 122 (98–152) | 103 (65–142) | 1.0 (0.9–1.0) | 0.70 | ||
| cART Regimen, N (%) | ||||||
| ABC | 34 (91.9) | 3 (8.1) | Ref | Ref | Ref | Ref |
| AZT | 13 (86.7) | 2 (13.3) | 1.2 (0.6–2.3) | 0.57 | 4.6 (0.1–25.8) | 0.08 |
| TDF | 47 (81) | 11 (19) | 1.4 (0.9–2.1) | 0.18 | 3.1 (1.0–9.6) | 0.049 |
| WHO Stage, N (%) | ||||||
| 1 | 38 (88.4) | 5 (11.6) | Ref | Ref | Ref | Ref |
| 2 | 21 (91.1) | 2 (9.9) | 0.7 (0.4–1.1) | 0.14 | 0.6 (0.1–6.0) | 0.64 |
| 3 | 29 (87.9) | 4 (12.1) | 0.7 (0.4–1.1) | 0.09 | 1.0 (0.1–8.1) | 0.99 |
| 4 | 5 (50) | 5 (50) | 0.6 (0.2–1.5) | 0.28 | 1.1 (0.1–9.0) | 0.93 |
| LDL in mg/dL: median (Q1–Q3) | 45 (32–63) | 43 (35–56) | 0.9 (0.9–1) | 0.17 | 1.0 (0.9–1.0) | 0.45 |
| HDL in mg/dL: median (Q1–Q3) | 47 (38–65) | 45 (33–59) | 0.9 (0.9–1) | 0.42 | ||
| TG in mg/dL: median (Q1–Q3) | 106 (89–157) | 91 (69–144) | 1.0 (0.9–1) | 0.95 | ||
| WAZ in ZS: median (Q1–Q3) | −1.1 (−2.2– (−0.5)) | −2.6 (−3.4– (−1.1)) | 1.0 (0.9–1.1) | 0.11 | 1.0 (0.6–1.6) | 0.92 |
| HAZ in ZS: median (Q1–Q3) | −1.4 (−2.3– (−0.6)) | −1.7 (−3.0– (−0.6)) | 1.0 (0.9–1.1) | 0.13 | 7.4 (0.2–223) | 0.25 |
| BAZ in ZS: median (Q1–Q3) | −1.0 (−2.2– (−0.1)) | −1.9 (−3.1– (−1.0)) | 1.1 (0.9–1.3) | 0.09 | ** | ** |
| Log10pVL: median (Q1–Q3) | 4.2 (3.2–5.0) | 4.3 (4.1–5.0) | 0.8 (0.7–1.1) | 0.13 | 1.1 (0.6–2.3) | 0.69 |
| Variable | Rebound | P Value | HR (95% CI) | aHR (95%CI) | P Value | |
|---|---|---|---|---|---|---|
| Yes | No | |||||
| Age in years: median (Q1–Q3) | 7 (5–13) | 9 (7–12) | 0.12 | 0.9 (0.8–1.0) | 0.9 (0.8–1.1) | 0.45 |
| Sex: N (%) Male Female | 21 (58.3) 15 (41.7) | 36 (62.1) 22 (37.9) | Ref 0.67 | Ref 1.2 (0.6–2.2) | ||
| PDR: N (%) Yes No | 2 (18.2) 9 (81.8) | 1 (3.3) 29 (96.7) | Ref 0.26 | Ref 0.4 (0.1–1.9) | ||
| TB: N (%) Yes No | 32 (88.9) 4 (11.1) | 48 (84.2) 9 (15.8) | Ref 0.93 | Ref 1.0 (0.3–2.7) | ||
| IPT: N (%) Yes No | 9 (25) 27 (75) | 20 (34.5) 38 (65.5) | Ref 0.18 | Ref 1.7 (0.8–3.6) | Ref 2.2 (0.6–7.2) | Ref 0.22 |
| CPT: N (%) Yes No | 22(75.9) 7 (24.1) | 43 (86) 7 (14.0) | Ref 0.04 | Ref 0.4 (0.2–0.9) | Ref 1.1 (0.2–4.2) | Ref 0.94 |
| HCT in %: median (Q1–Q3) | 37.4 (32.9–41.2) | 37.8 (35.9–40.9) | 0.75 | 1.0 (0.9–1.1) | ||
| CD4 cells/mm3 median (Q1–Q3) | 447 (223–744) | 330 (161–741) | 0.85 | 1.0 (1.0–1.0) | ||
| AST in units/dL: median (Q1–Q3) | 37 (33-–54) | 37 (29–45) | 0.50 | 1.0 (1.0–1.0) | ||
| ALT in units/dL: median (Q1–Q3) | 27 (17–38) | 25 (20–34) | 0.37 | 1.0 (1.0–1.0) | ||
| Bil-T in mg/dL: median (Q1–Q3) | 0.8 (0.4–1) | 0.6 (0.4–1.1) | 0.78 | 0.9 (0.5–1.6) | ||
| ALB in mg/dL: median (Q1–Q3) | 3.8 (3–4.3) | 3.8 (3.3–4.2) | 0.83 | 1.0 (0.6–1.4) | ||
| BUN in mg/dL: median (Q1–Q3) | 18 (12–24) | 15.3 (12–25) | 0.51 | 1.0 (0.9–1.0) | ||
| Cr in mg/dL: median (Q1–Q3) | 0.5 (0.4–0.6) | 0.6 (0.5–0.8) | 0.36 | 0.6 (0.2–1.9) | ||
| TChol in mg/dL: median (Q1–Q3) | 108 (89–142) | 128 (99–155) | 0.09 | 1.0 (1.0–1.0) | 1.0 (0.9–1.0) | 0.33 |
| cART Regimen, N (%) ABC AZT TDF | 15 (41.7) 5 (13.9) 16 (44.4) | 19 (32.8) 8 (13.8) 31 (53.5) | Ref 0.25 0.027 | Ref 0.5 (0.2–1.5) 0.4 (0.2–0.9) | Ref 1.0 (0.2–4.2) 1.1 (0.3–4.1) | Ref 0.96 0.88 |
| WHO Stage, N (%) 1 2 3 4 | 18 (51.4) 6 (17.1) 8 (22.7) 3 (8.6) | 20 (34.5) 15 (25.9) 21 (36.2) 2 (3.5) | Ref 0.46 0.37 0.036 | Ref 0.7 (0.3–1.8) 0.7 (0.3–1.6) 4.0 (1.1–14.3) | Ref 0.4 (0.1–1.6) 0.6 (0.2–1.8) 3.6 (0.7–18.8) | Ref 0.22 0.38 0.13 |
| LDL in mg/dL: median (Q1–Q3) | 45 (25–65) | 45 (32–63) | 0.90 | 1.0 (0.9–1.0) | ||
| HDL in mg/dL: median (Q1–Q3) | 48 (35–64) | 47 (38–65.2) | 0.66 | 1.0 (1.0–1.0) | ||
| TG in mg/dL: median (Q1–Q3) | 103 (89–163) | 106 (92–152) | 0.24 | 1.0 (1.0–1.0) | ||
| WAZ in ZS: median (Q1–Q3) | −1 (−1.9–(−0.1)) | −1.2 (−2.4–(−0.6)) | 0.36 | 1.1 (1.1–1.2) | ||
| HAZ in ZS: median (Q1–Q3) | −1.3 (−2.1–(−0.5)) | −1.5 (−2.5–(−0.6)) | 0.40 | 1.1 (1.0–1.1) | ||
| BAZ in ZS: median (Q1–Q3) | −0.8 (−1.9–(−0.1)) | −1.2 (−2.2–(−0.1)) | 0.49 | 1.2 (1.0–1.5) | ||
| Log10pVL: median (Q1–Q3) | 4.1 (3.0–5.0) | 4.2 (3.3–4.8) | 0.93 | 0.9 (0.6–1.2) | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tadesse, B.T.; Chala, A.; Mukonzo, J.; Chaka, T.E.; Tadesse, S.; Makonnen, E.; Brumme, Z.L.; Brumme, C.J.; Aklillu, E. Rates and Correlates of Short Term Virologic Response among Treatment-Naïve HIV-Infected Children Initiating Antiretroviral Therapy in Ethiopia: A Multi-Center Prospective Cohort Study. Pathogens 2019, 8, 161. https://doi.org/10.3390/pathogens8040161
Tadesse BT, Chala A, Mukonzo J, Chaka TE, Tadesse S, Makonnen E, Brumme ZL, Brumme CJ, Aklillu E. Rates and Correlates of Short Term Virologic Response among Treatment-Naïve HIV-Infected Children Initiating Antiretroviral Therapy in Ethiopia: A Multi-Center Prospective Cohort Study. Pathogens. 2019; 8(4):161. https://doi.org/10.3390/pathogens8040161
Chicago/Turabian StyleTadesse, Birkneh Tilahun, Adugna Chala, Jackson Mukonzo, Tolosssa Eticha Chaka, Sintayehu Tadesse, Eyasu Makonnen, Zabrina L. Brumme, Chanson J. Brumme, and Eleni Aklillu. 2019. "Rates and Correlates of Short Term Virologic Response among Treatment-Naïve HIV-Infected Children Initiating Antiretroviral Therapy in Ethiopia: A Multi-Center Prospective Cohort Study" Pathogens 8, no. 4: 161. https://doi.org/10.3390/pathogens8040161
APA StyleTadesse, B. T., Chala, A., Mukonzo, J., Chaka, T. E., Tadesse, S., Makonnen, E., Brumme, Z. L., Brumme, C. J., & Aklillu, E. (2019). Rates and Correlates of Short Term Virologic Response among Treatment-Naïve HIV-Infected Children Initiating Antiretroviral Therapy in Ethiopia: A Multi-Center Prospective Cohort Study. Pathogens, 8(4), 161. https://doi.org/10.3390/pathogens8040161





