First Molecular Evidence of Anaplasma bovis and Anaplasma phagocytophilum in Bovine from Central Punjab, Pakistan
Abstract
:1. Introduction
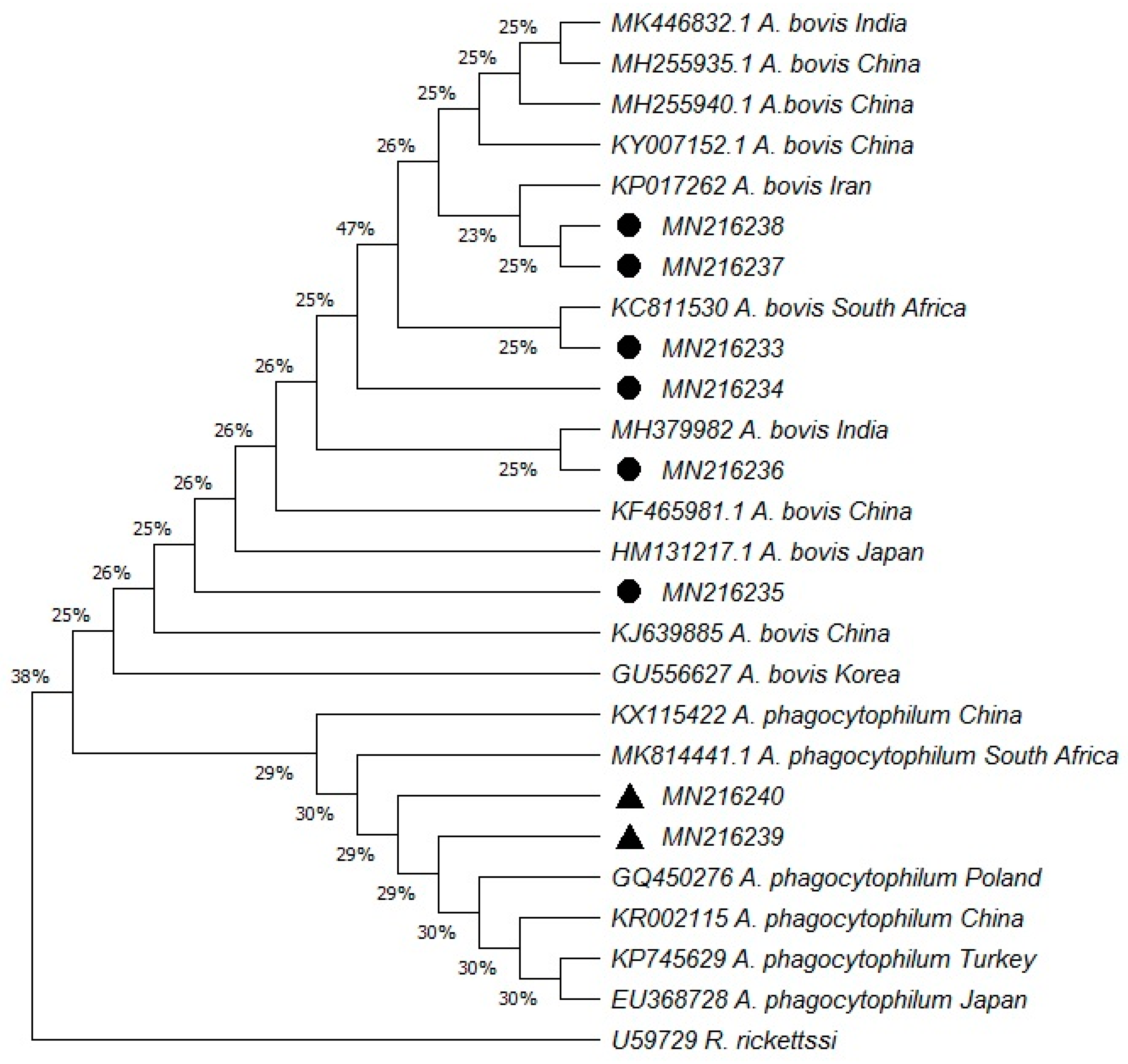
2. Results
3. Discussion
4. Methods
4.1. Sample Collection
4.2. DNA Extraction
4.3. PCR Amplification
DNA Sequencing and Data Analysis
4.4. Phylogenetic Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Ethics Approval and Consent to Participate
Availability of Data and Materials
Abbreviations
| PCR | polymerase chain reaction |
| DNA | deoxyribonucleic acid |
References
- Kocan, K.M.; de la Fuente, J.; Guglielmone, A.A.; Melendez, R.D. Antigens and alternatives for control of Anaplasma marginale infection in cattle. Clin. Microbiol. Rev. 2003, 16, 698–712. [Google Scholar] [CrossRef] [PubMed]
- Sainz, A.; Amusategui, I.; Tesouro, M.A. Ehrlichia platys infection and disease in dogs in Spain. J. Vet. Diagn. Investig. 1999, 11, 382–384. [Google Scholar] [CrossRef] [PubMed]
- Melendez, R.D. Future perspectives on veterinary hemoparasite research in the tropics at the start of this century. Ann. N. Y. Acad. Sci. 2000, 916, 253–258. [Google Scholar] [CrossRef]
- Stuen, S.; Bergstrom, K.; Palmer, E. Reduced weight gain due to subclinical Anaplasma phagocytophilum (formerly Ehrlichia phagocytophila) infection. Exp. Appl. Acarol. 2002, 28, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Stuen, S.; Nevland, S.; Moum, T. Fatal cases of Tick-borne fever (TBF) in sheep caused by several 16S rRNA gene variants of Anaplasma phagocytophilum. Ann. N. Y. Acad. Sci. 2003, 990, 433–434. [Google Scholar] [CrossRef] [PubMed]
- Dumler, J.S.; Barbet, A.F.; Bekker, C.P.; Dasch, G.A.; Palmer, G.H.; Ray, S.C.; Rikihisa, Y.; Rurangirwa, F.R. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: Unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ’HGE agent’ as subjective synonyms of Ehrlichia phagocytophila. Int. J. Syst. Evol. Microbiol. 2001, 51, 2145–2165. [Google Scholar] [CrossRef] [PubMed]
- Rar, V.; Golovljova, I. Anaplasma, Ehrlichia, and “Candidatus Neoehrlichia” bacteria: Pathogenicity, biodiversity, and molecular genetic characteristics, a review. Infect. Genet. Evol. 2011, 11, 1842–1861. [Google Scholar] [CrossRef] [PubMed]
- Ceci, L.; Iarussi, F.; Greco, B.; Lacinio, R.; Fornelli, S.; Carelli, G. Retrospective study of haemoparasites in cattle in Southern Italy by reverse line blot hybridization. J. Vet. Med. Sci. 2014, 76, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Belkahia, H.; Said, M.B.; Alberti, A.; Abdi, K.; Issaoui, Z.; Hattab, D.; Gharbi, M.; Messadi, L. First molecular survey and novel genetic variants’ identification of Anaplasma marginale, A. centrale and A. bovis in cattle from Tunisia. Infect. Genet. Evol. 2015, 34, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, Z.; Liu, Z.; Liu, J.; Yang, J.; Li, Q.; Li, Y.; Luo, J.; Yin, H. Molecular Survey of Anaplasma and Ehrlichia of Red Deer and Sika Deer in Gansu, China in 2013. Transbound Emerg. Dis. 2016, 63, e228–e236. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, Y.; Liu, Z.; Liu, J.; Niu, Q.; Ren, Q.; Chen, Z.; Guan, G.; Luo, J.; Yin, H. Molecular detection and characterization of Anaplasma spp. in sheep and cattle from Xinjiang, northwest China. Parasites Vectors 2015, 8, 108. [Google Scholar] [CrossRef] [PubMed]
- Njiiri, N.E.; Bronsvoort, B.M.; Collins, N.E.; Steyn, H.C.; Troskie, M.; Vorster, I.; Thumbi, S.M.; Sibeko, K.P.; Jennings, A.; van Wyk, I.C.; et al. The epidemiology of tick-borne haemoparasites as determined by the reverse line blot hybridization assay in an intensively studied cohort of calves in western Kenya. Vet. Parasitol. 2015, 210, 69–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawahara, M.; Rikihisa, Y.; Lin, Q.; Isogai, E.; Tahara, K.; Itagaki, A.; Hiramitsu, Y.; Tajima, T. Novel genetic variants of Anaplasma phagocytophilum, Anaplasma bovis, Anaplasma centrale, and a novel Ehrlichia sp. in wild deer and ticks on two major islands in Japan. Appl. Environ. Microbiol. 2006, 72, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- M’Ghirbi, Y.; Yaich, H.; Ghorbel, A.; Bouattour, A. Anaplasma phagocytophilum in horses and ticks in Tunisia. Parasites Vectors 2012, 5, 180. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.G.; Kim, H.C.; Choi, C.Y.; Nam, H.Y.; Chae, H.Y.; Chong, S.T.; Klein, T.A.; Ko, S.; Chae, J.S. Molecular detection of Anaplasma, Bartonella, and Borrelia species in ticks collected from migratory birds from Hong-do Island, Republic of Korea. Vectors Borne Zoonotic. Dis. 2013, 13, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Djiba, M.L.; Mediannikov, O.; Mbengue, M.; Thiongane, Y.; Molez, J.F.; Seck, M.T.; Fenollar, F.; Raoult, D.; Ndiaye, M. Survey of Anaplasmataceae bacteria in sheep from Senegal. Trop. Anim. Health. Prod. 2013, 45, 1557–1561. [Google Scholar] [CrossRef] [PubMed]
- Stuen, S.; Pettersen, K.S.; Granquist, E.G.; Bergstrom, K.; Bown, K.J.; Birtles, R.J. Anaplasma phagocytophilum variants in sympatric red deer (Cervus elaphus) and sheep in southern Norway. Ticks Tick Borne Dis. 2013, 4, 197–201. [Google Scholar] [CrossRef]
- Borthakur, S.; Deka, D.; Bhattacharjee, K.; Sarmah, P. Seroprevalence of canine dirofilariosis, granulocytic anaplasmosis and lyme borreliosis of public health importance in dogs from India’s North East. Vet. World 2014, 7, 665–667. [Google Scholar] [CrossRef]
- Razzaq, F.; Khosa, T.; Ahmad, S.; Hussain, M.; Saeed, Z.; Khan, M.; Shaikh, R.; Ali, M.; Iqbal, F. Prevalence of Anaplasma phagocytophilum in horses from Southern Punjab (Pakistan). Trop. Biomed. 2015, 32, 233–239. [Google Scholar]
- Rajput, Z.I.; Hu, S.H.; Arijo, A.G.; Habib, M.; Khalid, M. Comparative study of Anaplasma parasites in tick carrying buffaloes and cattle. J. Zhejiang Univ. Sci. B 2005, 6, 1057–1062. [Google Scholar] [CrossRef]
- Ashraf, Q.U.; Khan, A.U.; Khattak, R.M.; Ali, M.; Shaikh, R.S.; Ali, M.; Iqbal, F. A report on the high prevalence of Anaplasma sp. in buffaloes from two provinces in Pakistan. Ticks Tick Borne Dis. 2013, 4, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, S.H.; Ijaz, M.; Rashid, M.I.; Nabi, H.; Islam, S.; Aqib, A.I.; Hussain, K.; Khan, A.; Rizvi, S.N.B.; Mahmood, S.; et al. Molecular epidemiology of bovine anaplasmosis in Khyber Pakhtunkhwa, Pakistan. Trop. Anim. Health Prod. 2018, 50, 1591–1598. [Google Scholar] [CrossRef] [PubMed]
- De Echaide, S.T.; Bono, M.F.; Lugaresi, C.; Aguirre, N.; Mangold, A.; Moretta, R.; Farber, M.; Mondillo, C. Detection of antibodies against Anaplasma marginale in milk using a recombinant MSP5 indirect ELISA. Vet. Microbiol. 2005, 106, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, S.D.; Garcia Ortiz, M.A.; Jimenez Ocampo, R.; Vega y Murguia, C.A. Molecular epidemiology of bovine anaplasmosis with a particular focus in Mexico. Infect. Genet. Evol. 2009, 9, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Haider, M.; Bilqees, F. Anaplasmosis in certain mammals in Karachi and adjoin areas. Proc. Parasitol. 1988, 6, 85–88. [Google Scholar]
- Molad, T.; Mazuz, M.; Fleiderovitz, L.; Fish, L.; Savitsky, I.; Krigel, Y.; Leibovitz, B.; Molloy, J.; Jongejan, F.; Shkap, V. Molecular and serological detection of A. centrale-and A. marginale-infected cattle grazing within an endemic area. Vet. Microbiol. 2006, 113, 55–62. [Google Scholar] [CrossRef]
- Bekker, C.P.; De Vos, S.; Taoufik, A.; Sparagano, O.A.; Jongejan, F. Simultaneous detection of Anaplasma and Ehrlichia species in ruminants and detection of Ehrlichiaruminantium in Amblyomma variegatum ticks by reverse line blot hybridization. Vet. Microbiol. 2002, 89, 223–238. [Google Scholar] [CrossRef]
- Carelli, G.; Decaro, N.; Lorusso, A.; Elia, G.; Lorusso, E.; Mari, V.; Ceci, L.; Buonavoglia, C. Detection and quantification of Anaplasma marginale DNA in blood samples of cattle by real-time PCR. Vet. Microbiol. 2007, 124, 107–114. [Google Scholar] [CrossRef]
- Noaman, V.; Shayan, P.; Amininia, N. Molecular diagnostic of Anaplasma marginale in carrier cattle. Iran. J. Parasitol. 2009, 4, 26–33. [Google Scholar]
- Shahnawaz, S.; Ali, M.; Aslam, M.A.; Fatima, R.; Chaudhry, Z.I.; Hassan, M.U.; Ali, M.; Iqbal, F. A study on the prevalence of a tick-transmitted pathogen, Theileria annulata, and hematological profile of cattle from Southern Punjab (Pakistan). Parasitol. Res. 2011, 109, 1155–1160. [Google Scholar] [CrossRef]
- Khan, M.; Zahoor, A.; Jahangir, M.; Mirza, M.A. Prevalence of blood parasites in cattle and buffaloes. Pak. Vet. J. 2004, 24, 193–194. [Google Scholar]
- Hosseini-Vasoukolaei, N.; Oshaghi, M.A.; Shayan, P.; Vatandoost, H.; Babamahmoudi, F.; Yaghoobi-Ershadi, M.R.; Telmadarraiy, Z.; Mohtarami, F. Anaplasma Infection in Ticks, Livestock and Human in Ghaemshahr, Mazandaran Province, Iran. J. Arthropod. Borne Dis. 2014, 8, 204–211. [Google Scholar]
- Sharma, A.; Singla, L.D.; Kaur, P.; Bal, M.S. PCR and ELISA vis-a-vis microscopy for detection of bovine anaplasmosis: A study on associated risk of an upcoming problem in North India. Sci. World J. 2015, 2015, 352519. [Google Scholar] [CrossRef]
- Atif, F.A.; Khan, M.S.; Iqbal, H.J.; Arshad, G.M.; Ashraf, E.; Ullah, S. Prevalence of Anaplasma marginale, Babesia bigemina and Theileria annulata infections among cattle in Sargodha District, Pakistan. Afr. J. Agric. Res. 2012, 7, 302–3307. [Google Scholar]
- Sajid, M.; Siddique, R.; Khan, S.; Zafar, I.; Khan, M. Prevalence and risk factors of anaplasmosis in cattle and buffalo populations of district Khanewal, Punjab, Pakistan. Glob. Vet. 2014, 12, 146–153. [Google Scholar]
- Barlough, J.E.; Madigan, J.E.; DeRock, E.; Bigornia, L. Nested polymerase chain reaction for detection of Ehrlichia equi genomic DNA in horses and ticks (Ixodes pacificus). Vet. Parasitol. 1996, 63, 319–329. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, N.; Mukhtar, M.U.; Yang, J.; Sajid, M.S.; Niu, Q.; Guan, G.; Liu, Z.; Yin, H. First Molecular Evidence of Anaplasma bovis and Anaplasma phagocytophilum in Bovine from Central Punjab, Pakistan. Pathogens 2019, 8, 155. https://doi.org/10.3390/pathogens8030155
Iqbal N, Mukhtar MU, Yang J, Sajid MS, Niu Q, Guan G, Liu Z, Yin H. First Molecular Evidence of Anaplasma bovis and Anaplasma phagocytophilum in Bovine from Central Punjab, Pakistan. Pathogens. 2019; 8(3):155. https://doi.org/10.3390/pathogens8030155
Chicago/Turabian StyleIqbal, Naveed, Muhammad Uzair Mukhtar, Jifei Yang, Muhammad Sohail Sajid, Qingli Niu, Guiquan Guan, Zhijie Liu, and Hong Yin. 2019. "First Molecular Evidence of Anaplasma bovis and Anaplasma phagocytophilum in Bovine from Central Punjab, Pakistan" Pathogens 8, no. 3: 155. https://doi.org/10.3390/pathogens8030155
APA StyleIqbal, N., Mukhtar, M. U., Yang, J., Sajid, M. S., Niu, Q., Guan, G., Liu, Z., & Yin, H. (2019). First Molecular Evidence of Anaplasma bovis and Anaplasma phagocytophilum in Bovine from Central Punjab, Pakistan. Pathogens, 8(3), 155. https://doi.org/10.3390/pathogens8030155





