Oral Inoculation of Specific-Pathogen-Free Chickens with Chicken Anemia Virus Induces Dose-Dependent Viremia and Transient Anemia
Abstract
1. Introduction
2. Results
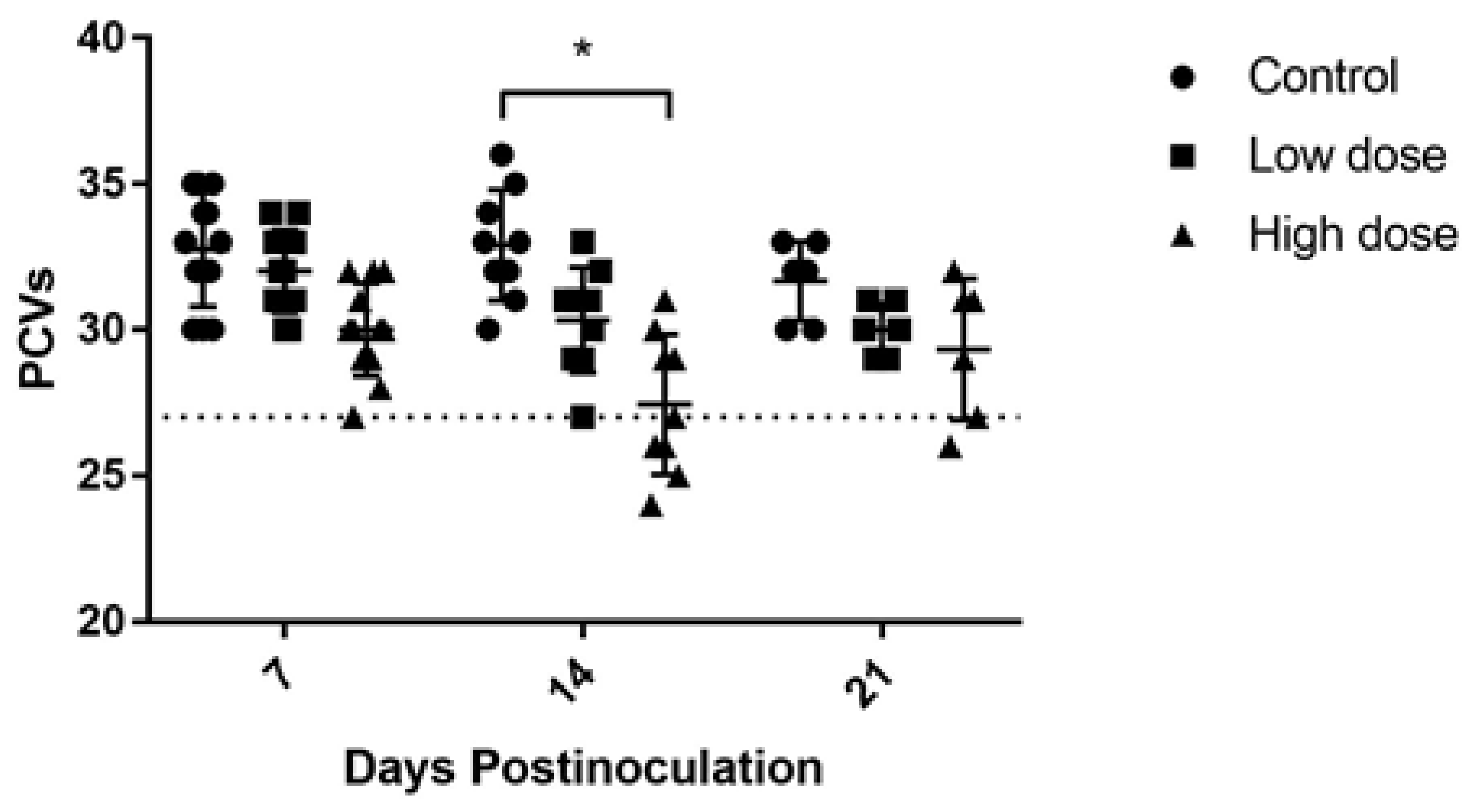
2.1. Hematological Changes
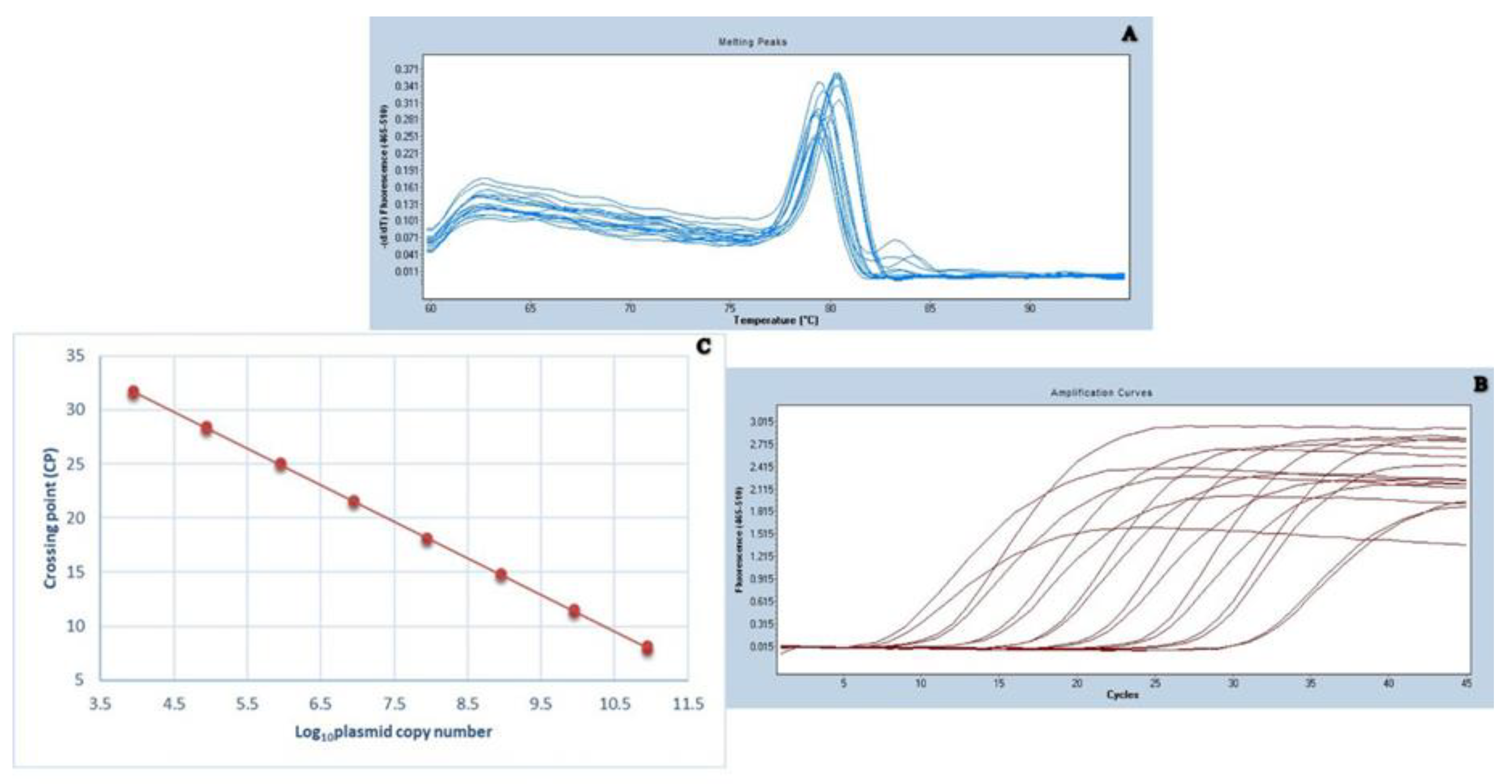
2.2. Standardization of qPCR for Viral Load Detection
2.3. CAV Genome Quantification
2.4. Virus Titration in Blood
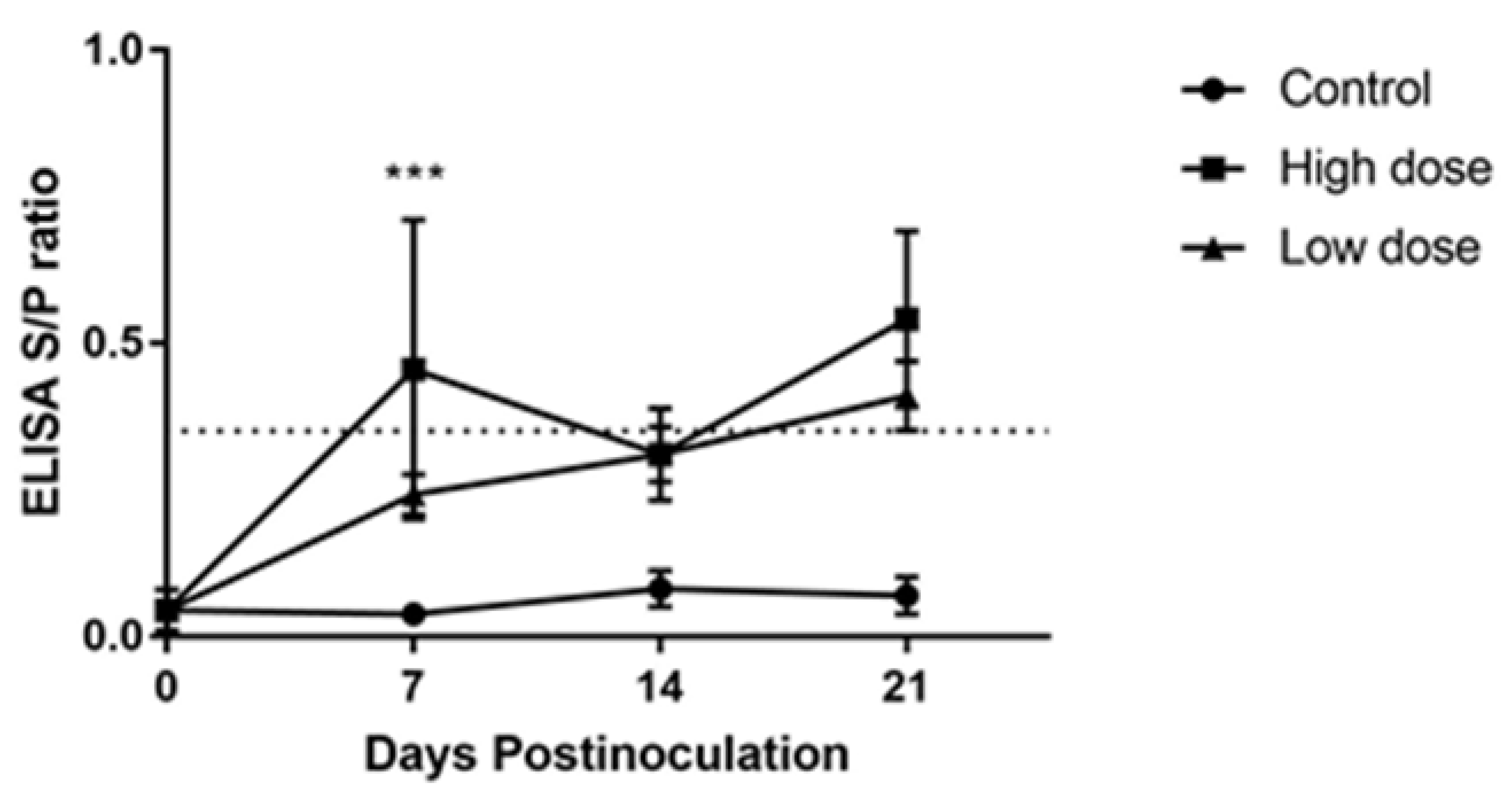
2.5. Humoral Immune Response
3. Discussion
4. Materials and Methods
4.1. Birds and Viral Strain
4.2. Experimental Designs
4.3. Hematology
4.4. Calculation of a Standard Curve
4.5. Tissue DNA Isolation and Viral Load Quantification
4.6. Preparation of Blood Samples and Virus Titration
4.7. Serology
4.8. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rosario, K.; Breitbart, M.; Harrach, B.; Segales, J.; Delwart, E.; Biagini, P.; Varsani, A. Revisiting the taxonomy of the family Circoviridae: Establishment of the genus Cyclovirus and removal of the genus Gyrovirus. Arch. Virol. 2017, 162, 1447–1463. [Google Scholar] [CrossRef] [PubMed]
- Hoerr, F.J. Clinical aspects of immunosuppression in poultry. Avian Dis. 2010, 54, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.M.; Schat, K.A. Chicken infectious anemia virus: An example of the ultimate host-parasite relationship. Avian Dis. 2004, 48, 734–745. [Google Scholar] [CrossRef] [PubMed]
- Wani, M.Y.; Dhama, K.; Tiwari, R.; Barathidasan, R.; Malik, Y.S.; Singh, S.D.; Singh, R.K. Immunosuppressive effects of chicken infectious anaemia virus on T lymphocyte populations using flow cytometry and hematological parameters during experimental subclinical infection in chicks. Adv. Anim. Vet. Sci. 2015, 3, 143–150. [Google Scholar] [CrossRef]
- McNulty, M.S.; Connor, T.J.; McNeilly, F. Influence of virus dose on experimental anaemia due to chicken anaemia agent. Avian Pathol. 1990, 19, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Ou, S.C.; Lin, H.L.; Liu, P.C.; Huang, H.J.; Lee, M.S.; Lien, Y.Y.; Tsai, Y.L. Epidemiology and molecular characterization of chicken anaemia virus from commercial and native chickens in Taiwan. Transbound. Emerg. Dis. 2018, 65, 1493–1501. [Google Scholar] [CrossRef] [PubMed]
- Dren, C.N.; Kant, A.; Roozelaar, D.J.; Hartog, L.; Noteborn, H.M.; Koch, G. Studies on the pathogenesis of chicken infectious anemia virus infection in six-week-old SPF chickens. Acta Vet. Hung. 2000, 48, 455–467. [Google Scholar] [CrossRef]
- Smyth, J.A.; Moffett, D.A.; Connor, T.J.; McNulty, M.S. Chicken anaemia virus inoculated by the oral route causes lymphocyte depletion in the thymus in 3-week-old and 6-week-old chickens. Avian Pathol. 2006, 35, 254–259. [Google Scholar] [CrossRef]
- Tongkamsai, S.; Lee, M.S.; Cheng, M.C.; Chaung, H.C.; Tsai, Y.L.; Lien, Y.Y. Persistent infection with chicken anemia virus in 3-week-old chickens induced by inoculation of the virus by the natural route. Pathogens 2019, 8, 48. [Google Scholar] [CrossRef]
- Wani, M.Y.; Dhama, K.; Latheef, S.K.; Singh, S.D.; Tiwari, R. Correlation between cytokine profile, antibody titre and viral load during sub-clinical chicken anaemia virus infection. Vet. Med. 2014, 59, 33–43. [Google Scholar] [CrossRef]
- Imai, K.; Mase, M.; Tsukamoto, K.; Hihara, H.; Yuasa, N. Persistent infection with chicken anaemia virus and some effects of highly virulent infectious bursal disease virus infection on its persistency. Res. Vet. Sci. 1999, 67, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Smyth, J.A.; Moffett, D.A.; McNulty, M.S.; Todd, D.; Mackie, D.P. A sequential histopathologic and immnunocytochemical study of chicken anemia virus infection at one day of age. Avian Dis. 1993, 37, 324–338. [Google Scholar] [CrossRef] [PubMed]
- Joiner, K.S.; Ewald, S.J.; Hoerr, F.J.; van Santen, V.L.; Toro, H. Oral infection with chicken anemia virus in 4-wk broiler breeders: Lack of effect of major histocompatibility B complex genotype. Avian Dis. 2005, 49, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Wani, M.Y.; Dhama, K.; Malik, Y.S. Impact of virus load on immunocytological and histopathological parameters during clinical chicken anemia virus (CAV) infection in poultry. Microb. Pathog. 2016, 96, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Markowski-Grimsrud, C.J.; Schat, K.A. Infection with chicken anaemia virus impairs the generation of pathogen-specific cytotoxic T lymphocytes. Immunology 2003, 109, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Guedes, P.T.; de Oliveira, B.C.; Manso, P.P.; Caputo, L.F.; Cotta-Pereira, G.; Pelajo-Machado, M. Histological analyses demonstrate the temporary contribution of yolk sac, liver, and bone marrow to hematopoiesis during chicken development. PLoS ONE 2014, 9, e90975. [Google Scholar] [CrossRef] [PubMed]
- Hoop, R.K.; Reece, R.L. The use of immunofluorescence and immunoperoxidase staining in studying the pathogenesis of chicken anaemia agent in experimentally infected chickens. Avian Pathol. 1991, 20, 349–355. [Google Scholar] [CrossRef]
- Kuscu, B.; Gürel, A. Lesions in the thymus and bone marrow in chicks with experimentally induced chicken infectious anemia disease. J. Vet. Sci. 2008, 9, 15–23. [Google Scholar] [CrossRef]
- McNeilly, F.; Adair, B.M.; McNulty, M.S. In vitro infection of mononuclear cells derived from various chicken lymphoid tissues by chicken anaemia virus. Avian Pathol. 1994, 23, 547–556. [Google Scholar] [CrossRef][Green Version]
- Yuasa, N.; Imai, K.; Nakamura, K. Pathogenicity of chicken anaemia agent in bursectomised chickens. Avian Pathol. 1988, 17, 363–369. [Google Scholar] [CrossRef]
- Vaziry, A.; Silim, A.; Bleau, C.; Frenette, D.; Lamontagne, L. Chicken infectious anaemia vaccinal strain persists in the spleen and thymus of young chicks and induces thymic lymphoid cell disorders. Avian Pathol. 2011, 40, 377–385. [Google Scholar] [CrossRef] [PubMed]
- McIlroy, S.G.; McNulty, M.S.; Bruce, D.W.; Smyth, J.A.; Goodall, E.A.; Alcorn, M.J. Economic effects of clinical chicken anemia agent infection on profitable broiler production. Avian Dis. 1992, 36, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Goryo, M.; Suwa, T.; Matsumoto, S.; Umemura, T.; Itakura, C. Serial propagation and purification of chicken anaemia agent in MDCC-MSB1 cell line. Avian Pathol. 1987, 16, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Van Santen, V.L.; Kaltenboeck, B.; Joiner, K.S.; Macklin, K.S.; Norton, R.A. Real-time quantitative PCR-based serum neutralization test for detection and titration of neutralizing antibodies to chicken anemia virus. J. Virol. Methods 2004, 115, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Kaffashi, A.; Noormohammadi, A.H.; Allott, M.L.; Browning, G.F. Viral load in 1-day-old and 6-week-old chickens infected with chicken anaemia virus by the intraocular route. Avian Pathol. 2006, 35, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.R.; Rajeevan, M.S. Evaluation of DNA extraction from granulocytes discarded in the separation medium after isolation of peripheral blood mononuclear cells and plasma from whole blood. BMC Res. Notes 2013, 6, 440. [Google Scholar] [CrossRef] [PubMed]
- Natt, M.P.; Herrick, C.A. A new blood diluent for counting the erythrocytes and leucocytes of chicken. Poult. Sci. 1952, 31, 735–738. [Google Scholar] [CrossRef]
- Ramakrishnan, M.A. Determination of 50% endpoint titer using a simple formula. World J. Virol. 2016, 5, 85–86. [Google Scholar] [CrossRef] [PubMed]

| Group | Blood Component | Days Post-Inoculation | ||
|---|---|---|---|---|
| 7 | 14 | 21 | ||
| High-dose inoculated | Plasma * | + | − | − |
| Mononuclear cells | 2.83 ± 1.52 a A | 4.50 ± 0.00 a B | 3.16 ± 1.52 a D | |
| Granulocytes | + | 2.00 ± 0.50 b C | 1.66 ± 0.28 b D | |
| Erythrocytes | 1.16 ± 0.28 c A | 3.50 ± 1.00 d B C | 2.16 ± 0.57 c d D | |
| Low-dose inoculated | Plasma * | − | - | - |
| Mononuclear cells | + | 2.00 ± 0.50 f C | 1.33 ± 0.28 f D | |
| Granulocytes | + | 1.50 ± 0.00 C | − | |
| Erythrocytes | − | 2.16 ± 0.76 C | − | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tongkamsai, S.; Lee, M.-S.; Tsai, Y.-L.; Chung, H.-H.; Lai, G.-H.; Cheng, J.-H.; Cheng, M.-C.; Lien, Y.-Y. Oral Inoculation of Specific-Pathogen-Free Chickens with Chicken Anemia Virus Induces Dose-Dependent Viremia and Transient Anemia. Pathogens 2019, 8, 141. https://doi.org/10.3390/pathogens8030141
Tongkamsai S, Lee M-S, Tsai Y-L, Chung H-H, Lai G-H, Cheng J-H, Cheng M-C, Lien Y-Y. Oral Inoculation of Specific-Pathogen-Free Chickens with Chicken Anemia Virus Induces Dose-Dependent Viremia and Transient Anemia. Pathogens. 2019; 8(3):141. https://doi.org/10.3390/pathogens8030141
Chicago/Turabian StyleTongkamsai, Suttitas, Meng-Shiou Lee, Yi-Lun Tsai, Hsyang-Hsun Chung, Guan-Hua Lai, Jai-Hong Cheng, Ming-Chu Cheng, and Yi-Yang Lien. 2019. "Oral Inoculation of Specific-Pathogen-Free Chickens with Chicken Anemia Virus Induces Dose-Dependent Viremia and Transient Anemia" Pathogens 8, no. 3: 141. https://doi.org/10.3390/pathogens8030141
APA StyleTongkamsai, S., Lee, M.-S., Tsai, Y.-L., Chung, H.-H., Lai, G.-H., Cheng, J.-H., Cheng, M.-C., & Lien, Y.-Y. (2019). Oral Inoculation of Specific-Pathogen-Free Chickens with Chicken Anemia Virus Induces Dose-Dependent Viremia and Transient Anemia. Pathogens, 8(3), 141. https://doi.org/10.3390/pathogens8030141






