Ursolic Acid Derivatives as Potential Agents Against Acanthamoeba Spp.
Abstract
1. Introduction
2. Results
2.1. Chemistry
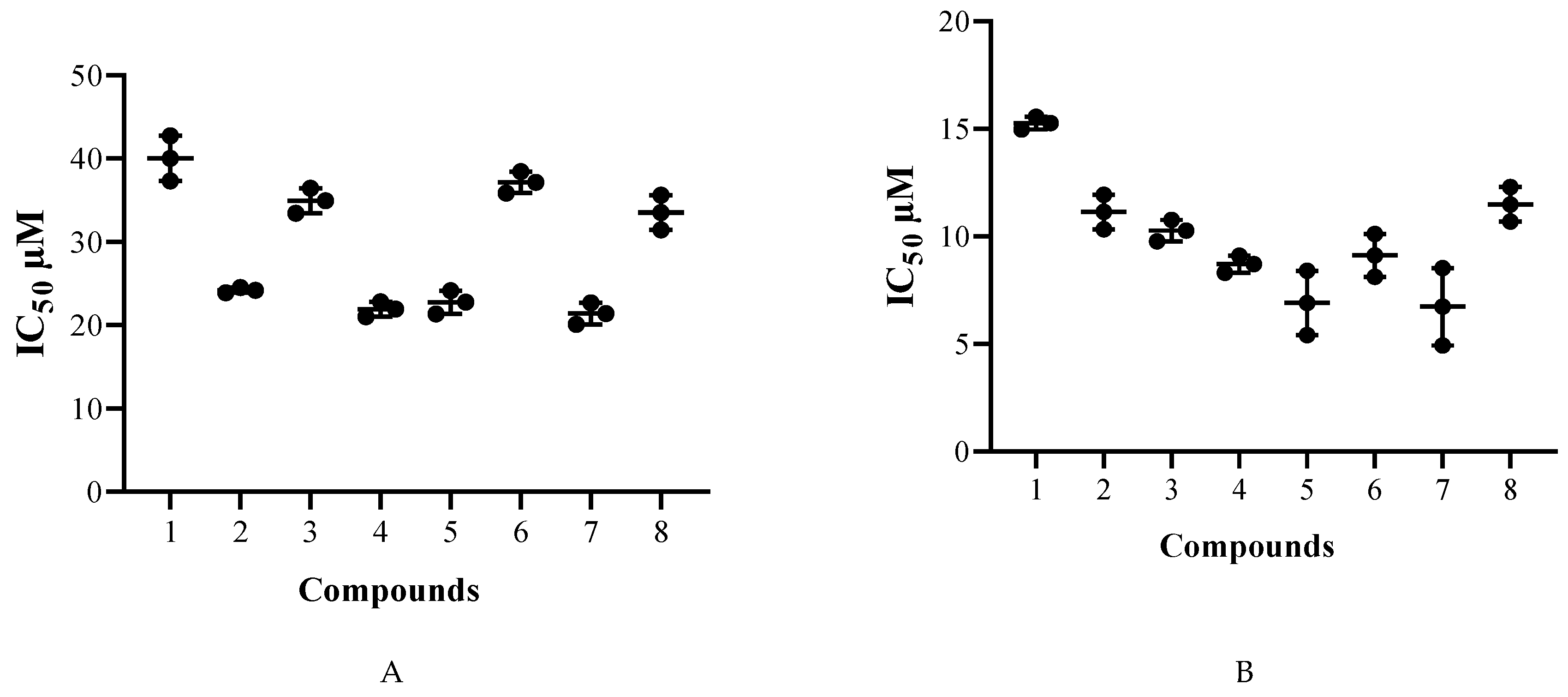
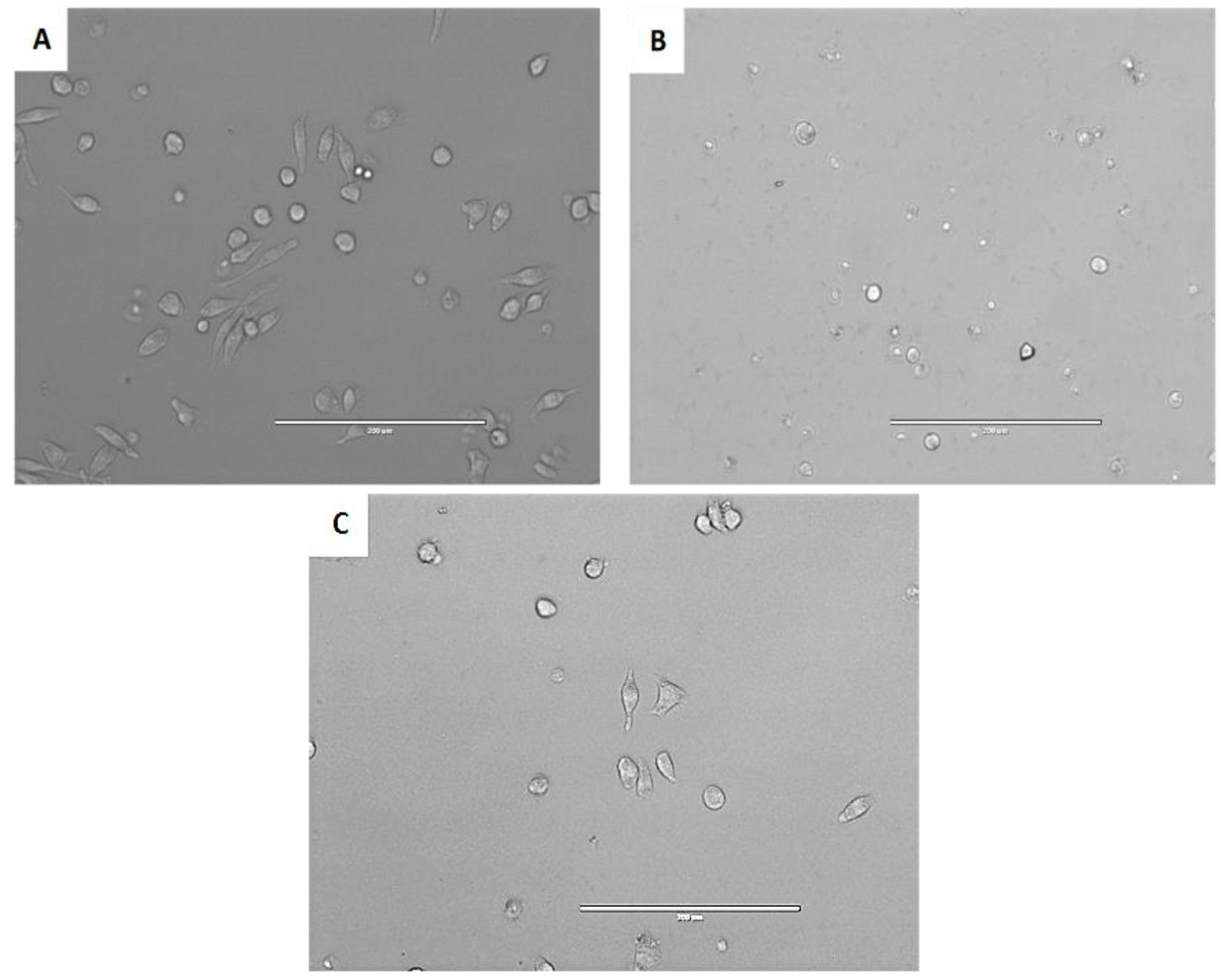
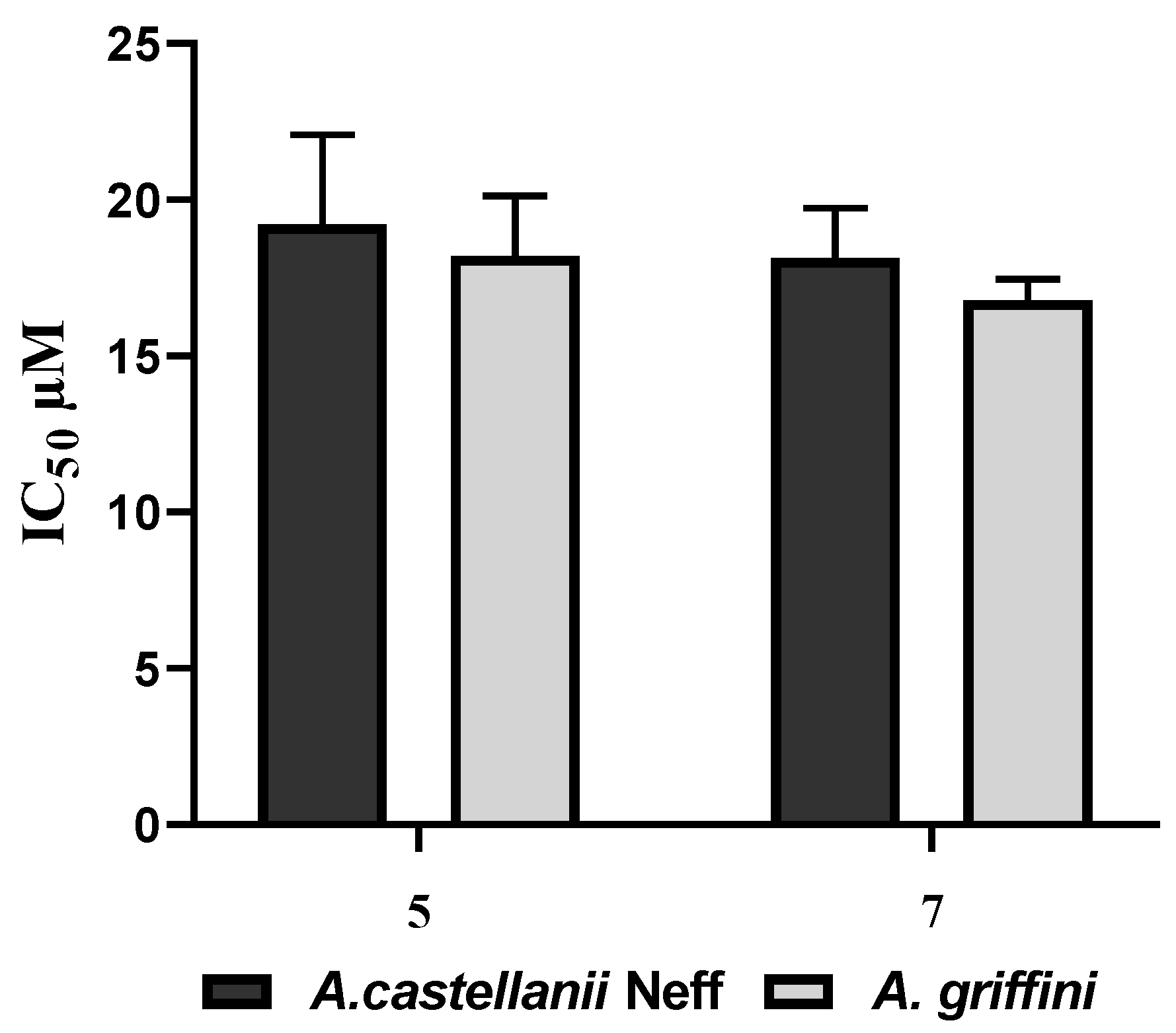
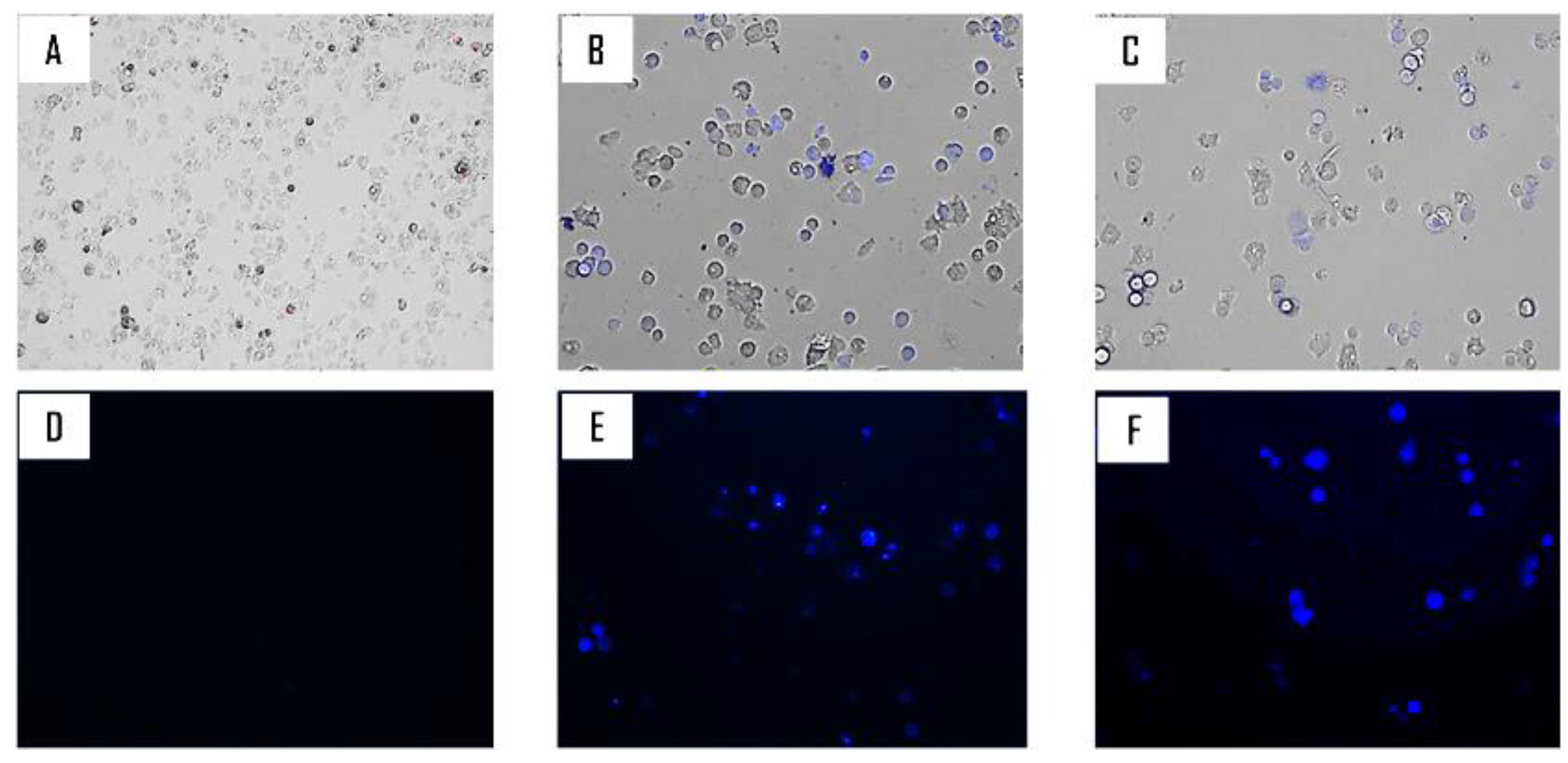
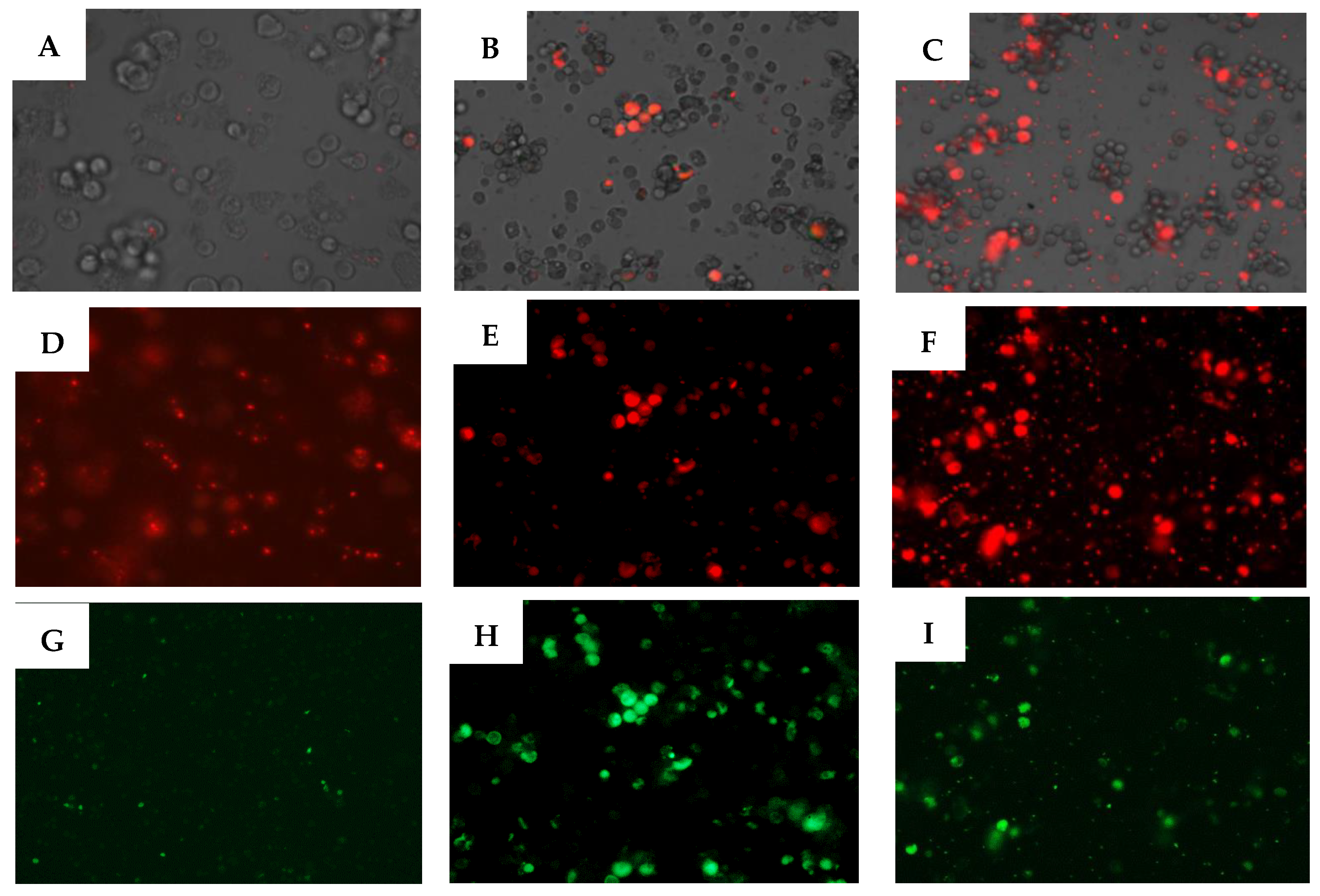
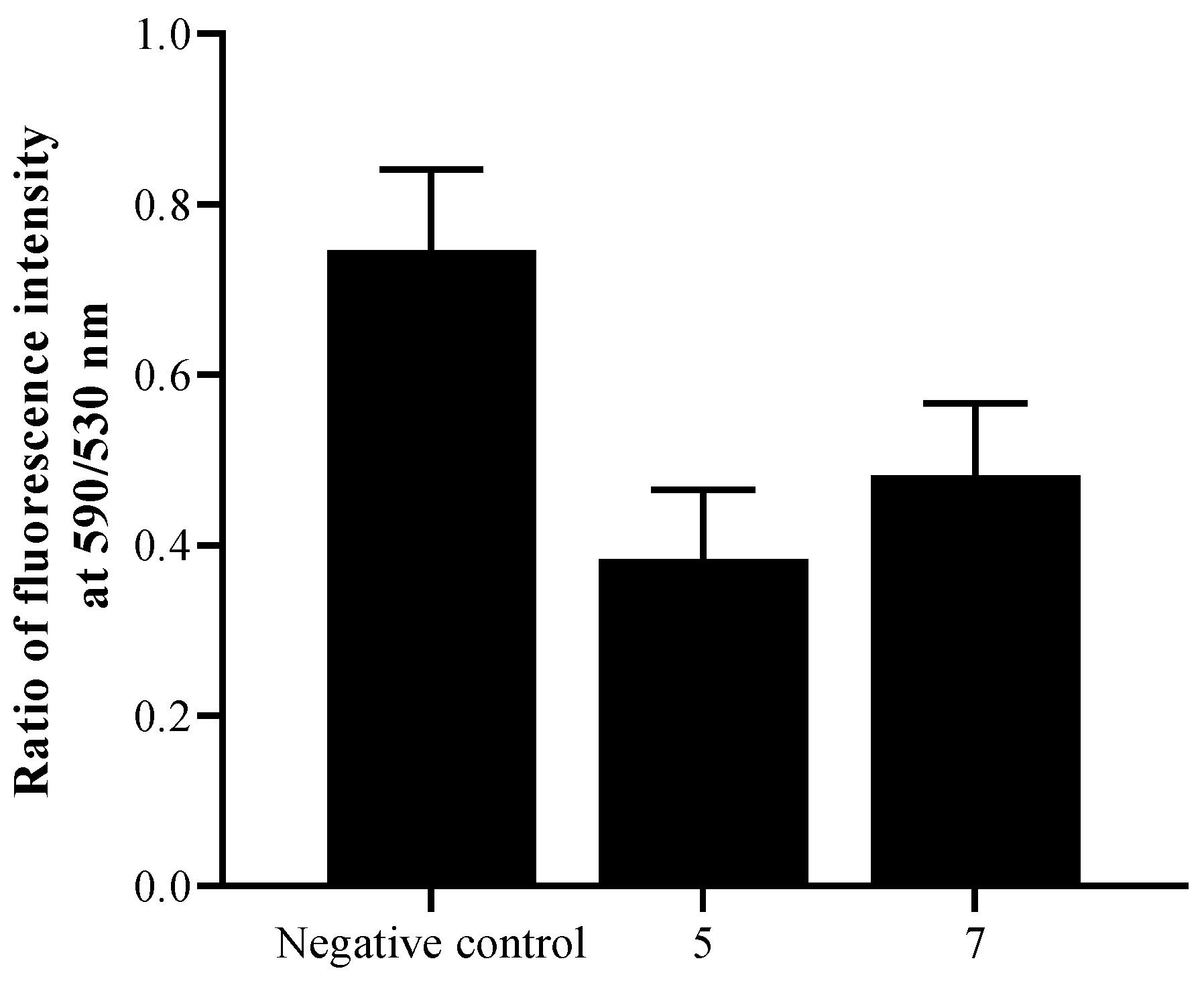
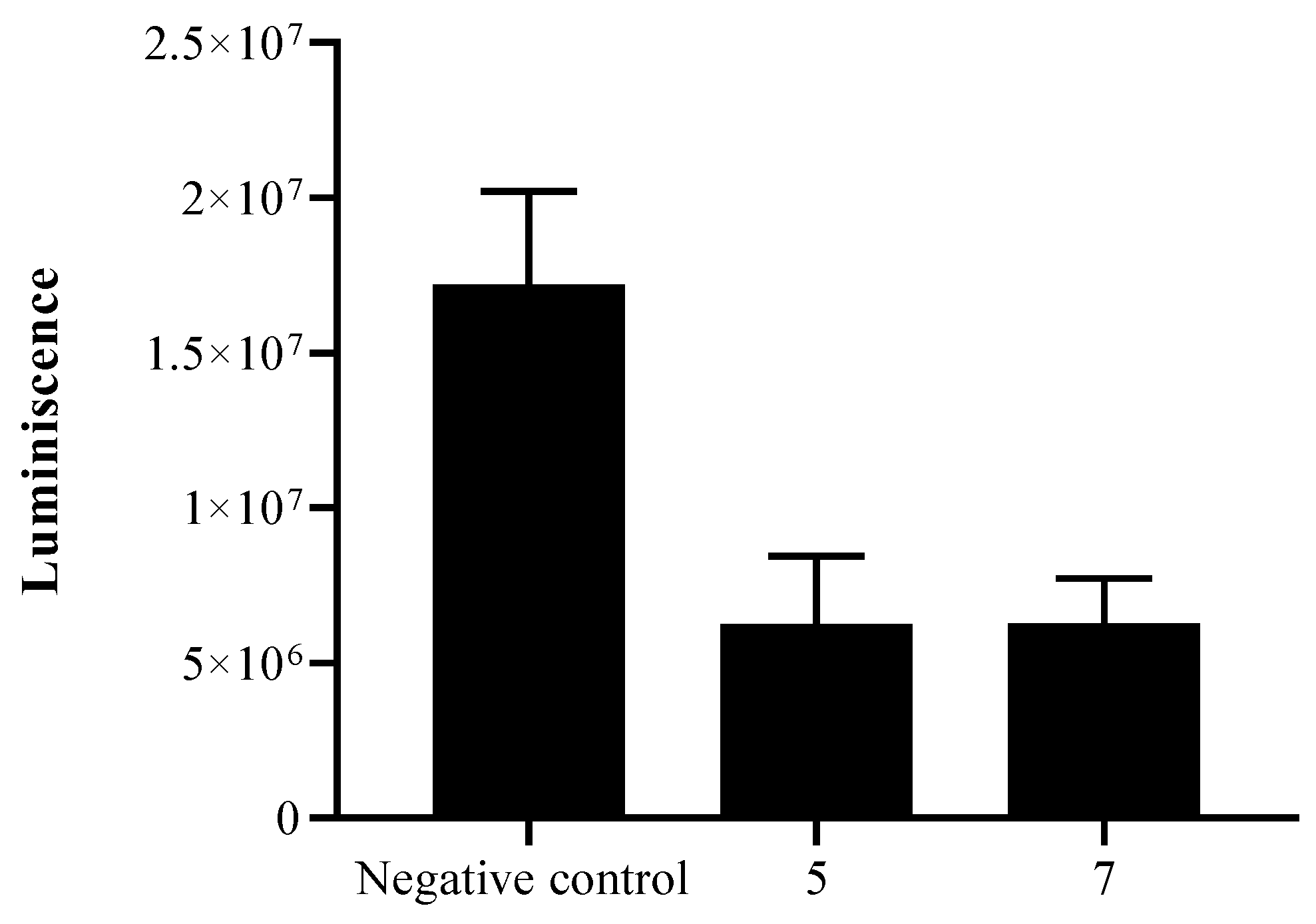
2.2. Biological Study
3. Discussion
4. Materials and Methods
4.1. Molecules/Chemicals
4.2. In Vitro Drug Sensitivity Assay
4.2.1. Acanthamoeba strains
4.2.2. In Vitro Effect Against the Trophozoite Stage of Acanthamoeba spp.
4.2.3. In Vitro Effect Against the Cyst Stage of Acanthamoeba spp.
4.2.4. Cytotoxicity Activity
4.2.5. Double-Stain Assay for Programmed Cell Death Determination
4.2.6. Intracellular ROS Production Using CellROX® Deep Red Staining
4.2.7. Analysis of Mitochondrial Membrane Potential
4.2.8. Measurement of ATP
4.2.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kazakova, O.B.; Giniyatullina, G.V.; Yamansarov, E.Y.; Tolstikov, G.A. Betulin and ursolic acid synthetic derivatives as inhibitors of Papilloma virus. Bioorg. Med. Chem. Lett. 2010, 20, 4088–4090. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.M.; Jhan, Y.L.; Tsai, S.J.; Chou, C.H. The Pleiotropic Antibacterial Mechanisms of Ursolic Acid against Methicillin-Resistant Staphylococcus aureus (MRSA). Molecules 2016, 21, 884. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, D.; Sharma, A.; Tuli, H.S.; Punia, S.; Sharma, A.K. Ursolic Acid and Oleanolic Acid: Pentacyclic Terpenoids with Promising Anti-Inflammatory Activities. Recent Pat. Inflamm. Allergy Drug Discov. 2016, 10, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Gai, W.T.; Yu, D.P.; Wang, X.S.; Wang, P.T. Anti-cancer effect of ursolic acid activates apoptosis through ROCK/PTEN mediated mitochondrial translocation of cofilin-1 in prostate cancer. Oncol. Lett. 2016, 12, 2880–2885. [Google Scholar] [CrossRef] [PubMed]
- Yim, E.K.; Lee, K.H.; Namkoong, S.E.; Um, S.J.; Park, J.S. Proteomic analysis of ursolic acid-induced apoptosis in cervical carcinoma cells. Cancer Lett. 2006, 235, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, E.S.; Campos, B.L.; Jesus, J.A.; Laurenti, M.D.; Ribeiro, S.P.; Kallás, E.G.; Rafael-Fernandes, M.; Santos-Gomes, G.; Sessa, D.P.; Lago, J.H.; et al. The Effect of Ursolic Acid on Leishmania (Leishmania) amazonensis Is Related to Programed Cell Death and Presents Therapeutic Potential in Experimental Cutaneous Leishmaniasis. PLoS ONE 2015, 10, e0144946. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A. Acanthamoeba: Biology and increasing importance in human health. FEMS Microbiol. Rev. 2006, 30, 564–595. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-Morales, J.; Khan, N.A.; Walochnik, J. An update on Acanthamoeba keratitis: Diagnosis, pathogenesis and treatment. Parasite 2015, 22. [Google Scholar] [CrossRef]
- Murdoch, D.; Gray, T.B.; Cursons, R.; Parr, D. Acanthamoeba keratitis in New Zealand, including two cases with in vivo resistance to polyhexamethylene biguanide. Aust. N. Z. J. Ophthalmol. 1998, 26, 231–236. [Google Scholar] [CrossRef]
- Huang, F.C.; Shih, M.H.; Chang, K.F.; Huang, J.M.; Shin, J.W.; Lin, W.C. Characterizing clinical isolates of Acanthamoeba castellanii with high resistance to polyhexamethylene biguanide in Taiwan. J. Microbiol. Immunol. Infect. 2017, 50, 570–577. [Google Scholar] [CrossRef]
- Sifaoui, I.; López-Arencibia, A.; Martín-Navarro, C.M.; Reyes-Batlle, M.; Wagner, C.; Chiboub, O.; Mejri, M.; Valladares, B.; Abderrabba, M.; Piñero, J.E.; et al. Programmed cell death in Acanthamoeba castellanii Neff induced by several molecules present in olive leaf extracts. PLoS ONE 2017, 12, e0183795. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.; Green, I.R.; Ali, I.; Khan, I.A.; Ali, Z.; Al-Sadi, A.M.; Ahmed, I. Ursolic acid derivatives for pharmaceutical use: A patent review (2012–2016). Expert Opin. Pat. 2017, 27, 1061–1072. [Google Scholar] [CrossRef] [PubMed]
- Martín-Navarro, C.M.; Lorenzo-Morales, J.; Cabrera-Serra, M.G.; Rancel, F.; Coronado-Alvarez, N.M.; Pinero, J.E.; Valladares, B. The potential pathogenicity of chlorhexidine-sensitive Acanthamoeba strains isolated from contact lens cases from asymptomatic individuals in Tenerife, Canary Islands, Spain. J. Med. Microbiol. 2008, 57, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Davison, E.K.; Brimble, M.A. Natural product derived privileged scaffolds in drug discovery. Curr. Opin. Chem. Biol. 2019, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Campos, K.R.; Coleman, P.J.; Alvarez, J.C.; Dreher, S.D.; Garbaccio, R.M.; Terrett, N.K.; Tillyer, R.D.; Truppo, M.D.; Parmee, E.R. The importance of synthetic chemistry in the pharmaceutical industry. Science 2019, 363, eaat0805. [Google Scholar] [CrossRef] [PubMed]
- Pejin, B.; Iodice, C.; Tommonaro, G.; De Rosa, S. Synthesis and biological activities of thio-avarol derivatives. J. Nat. Prod. 2008, 71, 1850–1853. [Google Scholar] [CrossRef]
- Tommonaro, G.; García-Font, N.; Vitale, R.M.; Pejin, B.; Iodice, C.; Canadas, S.; Marco-Contelles, J.; Oset-Gasque, M.J. Avarol derivatives as competitive AChE inhibitors, non-hepatotoxic and neuroprotective agents for Alzheimer’s disease. Eur. J. Med. Chem. 2016, 122, 326–338. [Google Scholar] [CrossRef]
- Pejin, B.; Iodice, C.; Tommonaro, G.; Bogdanovic, G.; Kojic, V.; De Rosa, S. Further in vitro evaluation of cytotoxicity of the marine natural product derivative 4′-leucine-avarone. Nat. Prod. Res. 2014, 28, 347–350. [Google Scholar] [CrossRef]
- Chen, H.; Gao, Y.; Wang, A.; Zhou, X.; Zheng, Y.; Zhou, J. Evolution in medicinal chemistry of ursolic acid derivatives as anticancer agents. Eur. J. Med. Chem. 2015, 92, 648–655. [Google Scholar] [CrossRef]
- Kashiwada, Y.; Nagao, T.; Hashimoto, A.; Ikeshiro, Y.; Okabe, H.; Cosentino, L.M.; Lee, K.H. Anti-AIDS agents 38. Anti-HIV activity of 3-O-acyl ursolic acid derivatives. J. Nat. Prod. 2000, 63, 1619–1622. [Google Scholar] [CrossRef]
- Innocente, A.; Silva, G.; Cruz, L.; Moraes, M.; Nakabashi, M.; Sonnet, P.; Gosmann, G.; Garcia, C.R.; Gnoatto, S. Synthesis and antiplasmodial activity of betulinic acid and ursolic acid analogues. Molecules 2012, 17, 12003–12014. [Google Scholar] [CrossRef] [PubMed]
- Jesus, J.A.; Lago, J.H.G.; Laurenti, M.D.; Yamamoto, E.S.; Passero, L.F.D. Antimicrobial activity of oleanolic and ursolic acids: An update. Evid. Based Complement. Altern. Med. 2015, 2015, 14. [Google Scholar] [CrossRef] [PubMed]
- Suffness, M. Assays related to cancer drug discovery. Methods Plant Biochem. Assays Bioact. 1990, 6, 71–133. [Google Scholar]
- Meng, Y.Q.; Liu, D.; Cai, L.L.; Chen, H.; Cao, B.; Wang, Y.Z. The synthesis of ursolic acid derivatives with cytotoxic activity and the investigation of their preliminary mechanism of action. Bioorg. Med. Chem. 2009, 17, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Kahnt, M.; Hoenke, S.; Fischer, L.; Al-Harrasi, A.; Csuk, R. Synthesis and Cytotoxicity Evaluation of DOTA-Conjugates of Ursolic Acid. Molecules 2019, 24, 2254. [Google Scholar] [CrossRef] [PubMed]
- Kaczanowski, S.; Sajid, M.; Reece, S.E. Evolution of apoptosis-like programmed cell death in unicellular protozoan parasites. Parasit. Vectors 2011, 25, 44. [Google Scholar] [CrossRef] [PubMed]
- Bidle, K.D. Programmed cell death in unicellular phytoplankton. Curr. Biol. 2016, 26, R594–R607. [Google Scholar] [CrossRef]
- Gregoire, M.; Hernandez-Verdun, D.; Bouteille, M. Visualization of chromatin distribution in living PTO cells by Hoechst 33342 fluorescent staining. Exp. Cell Res. 1984, 152, 38–46. [Google Scholar] [CrossRef]
- Chen, C.J.; Shih, Y.L.; Yeh, M.Y.; Liao, N.C.; Chung, H.Y.; Liu, K.L.; Lee, M.H.; Chou, P.Y.; Hou, H.Y.; Chung, J.G. Ursolic Acid Induces Apoptotic Cell Death Through AIF and Endo G Release through a Mitochondria-dependent Pathway in NCI-H292 Human Lung Cancer Cells In Vitro. In Vivo 2019, 33, 383–391. [Google Scholar] [CrossRef]
- Hsieh, Y.J.; Wu, C.C.; Chang, C.J.; Yu, J.S. Subcellular localization of Photofrin determines the death phenotype of human epidermoid carcinoma A431 cells triggered by photodynamic therapy: When plasma membranes are the main targets. J. Cell Physiol. 2003, 194, 363–375. [Google Scholar] [CrossRef]
- Harmand, P.O.; Duval, R.; Delage, C.; Simon, A. Ursolic acid induces apoptosis through mitochondrial intrinsic pathway and caspase-3 activation in M4Beu melanoma cells. Int. J. Cancer 2005, 114, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, X.; Qi, H.; Li, X.; Xiao, X.; Gao, J. Ursolic acid induces apoptosis through mitochondrial intrinsic pathway and suppression of ERK1/2 MAPK in HeLa cells. J. Pharm. Sci. 2014, 125, 202–210. [Google Scholar] [CrossRef]
- Kim, K.H.; Seo, H.S.; Choi, H.S.; Choi, I.; Shin, Y.C.; Ko, S.G. Induction of apoptotic cell death by ursolic acid through mitochondrial death pathway and extrinsic death receptor pathway in MDA-MB-231 cells. Arch. Pharmacal. Res. 2011, 34, 1363. [Google Scholar] [CrossRef] [PubMed]
- Joshi, D.C.; Bakowska, J.C. Determination of mitochondrial membrane potential and reactive oxygen species in live rat cortical neurons. J. Vis. Exp. 2011, 51, 2704. [Google Scholar] [CrossRef] [PubMed]
- Jara, J.A.; Castro-Castillo, V.; Saavedra-Olavarría, J.; Peredo, L.; Pavanni, M.; Jana, F.; Letelier, M.E.; Parra, E.; Becker, M.I.; Morello, A.; et al. Antiproliferative and uncoupling effects of delocalized, lipophilic, cationic gallic acid derivatives on cancer cell lines. Validation in vivo in singenic mice. J. Med. Chem. 2014, 57, 2440–2454. [Google Scholar] [CrossRef] [PubMed]
- Orrenius, S.; Gogvadze, V.; Zhivotovsky, B. Mitochondrial oxidative stress: Implications for cell death. Ann. Rev. Pharmacol. Toxicol. 2007, 47, 143–183. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.W.; Huang, A.M.; Lin, C.C.; Chang, C.C.; Hsu, W.C.; Hour, T.C.; Pu, Y.S.; Lin, C.N. Anti-cancer effects of ursane triterpenoid as a single agent and in combination with cisplatin in bladder cancer. Eur. J. Pharm. 2014, 740, 742–751. [Google Scholar] [CrossRef]
- Leal, A.S.; Wang, R.; Salvador, J.A.; Jing, Y. Semisynthetic Ursolic Acid Fluorolactone Derivatives Inhibit Growth with Induction of p21waf1 and Induce Apoptosis with Upregulation of NOXA and Downregulation of c-FLIP in Cancer Cells. Chem. Med. Chem. 2012, 7, 1635–1646. [Google Scholar] [CrossRef]
- Kwon, T.H.; Lee, B.M.; Chung, S.H.; Kim, D.H.; Lee, Y.S. Synthesis and NO production inhibitory activities of ursolic acid and oleanolic acid derivatives. Bull Korean Chem. Soc. 2009, 30, 119–123. [Google Scholar] [CrossRef]
- Loesche, A.; Köwitsch, A.; Lucas, S.D.; Al-Halabi, Z.; Sippl, W.; Al-Harrasi, A.; Csuk, R. Ursolic and oleanolic acid derivatives with cholinesterase inhibiting potential. Bioorg. Chem. 2019, 85, 23–32. [Google Scholar] [CrossRef]
- Siewert, B.; Wiemann, J.; Köwitsch, A.; Csuk, R. The chemical and biological potential of C ring modified triterpenoids. Eur. J. Med. Chem. 2014, 72, 84–101. [Google Scholar] [CrossRef] [PubMed]
- Mclean, J.; Silverstone, G.A.; Spring, F.S. 210. Triterpene resinols and related acids. Part XX. Conversion of α-amyrin into iso-α-amyranone (12-ketoursane). J. Chem. Soc. (Resumed) 1951, 935–938. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, H.; Lü, G.; Liu, C.; Tang, Y.; Liu, L. Synthesis of Novel 8, 14-Secoursane Derivatives: Key Intermediates for the Preparation of Chiral Decalin Synthons from Ursolic Acid. Helv. Chim. Acta 2012, 95, 1026–1032. [Google Scholar] [CrossRef]
- González-Robles, A.; Salazar-Villatoro, L.; Omaña-Molina, M.; Reyes-Batlle, M.; Martín-Navarro, C.M.; Lorenzo-Morales, J. Morphological features and in vitro cytopathic effect of Acanthamoeba griffini trophozoites isolated from a clinical case. J. Parasitol. Res. 2014, 2014, 256310. [Google Scholar] [CrossRef] [PubMed]
- McBride, J.; Ingram, P.R.; Henríquez, F.L. Development of colorimetric microtiter plate assay for assessment of antimicrobials against Acanthamoeba. J. Clin. Microbiol. 2005, 43, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-Morales, J.; Kliescikova, J.; Martinez-Carretero, E.; De Pablos, L.M.; Profotova, B.; Nohynkova, E.; Osuna, A.; Valladares, B. Glycogen phosphorylase in Acanthamoeba spp.: Determining the role of the enzyme during the encystment process using RNA interference. Eukaryot. Cell 2008, 7, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Sifaoui, I.; López-Arencibia, A.; Martín-Navarro, C.M.; Reyes-Batlle, M.; Mejri, M.; Valladares, B.; Lorenzo-Morales, J.; Manef Abderabba, M.; Piñero, J.E. Selective activity of Oleanolic and Maslinic Acids on the Amastigote form of Leishmania spp. Iran. J. Pharm. Res. 2017, 16, 1190. [Google Scholar] [PubMed]


| Compounds | A. Castellanii Neff IC50 | A. Griffini IC50 | Murine Macrophages CC50 | SI for A. Neff | SI for A. Griffini |
|---|---|---|---|---|---|
| 1 | 40.0 ± 2.7 | 15.2 ± 0.3 | 17.7 ± 3.3 | 0.4 | 1.2 |
| 2 | 24.2 ± 0.3 | 11.1 ± 0.8 | 93.2 ± 7.2 | 3.9 | 8.4 |
| 3 | 34.9 ± 1.5 | 10.2 ± 0.5 | >100 | >2.9 | >9.7 |
| 4 | 21.9 ± 0.9 | 8.7 ± 0.4 | 26.3 ± 1.7 | 1.2 | 3.0 |
| 5 | 22.7 ± 1.4 | 6.9 ± 1.5 | >100 | >4.4 | >14.5 |
| 6 | 37.1 ± 1.3 | 9.1 ± 1.0 | >100 | >2.7 | >11.0 |
| 7 | 21.4 ± 1.3 | 6.7 ± 1.8 | >100 | >4.7 | >14.9 |
| 8 | 33.5 ± 2.1 | 11.5 ± 0.8 | >100 | >3.0 | >8.7 |
| Chlorhexidine* | 3.02 ± 0.89 | 3.73 ± 0.98 | 7.40 ± 0.39 | 2.5 | 2.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sifaoui, I.; Rodríguez-Expósito, R.L.; Reyes-Batlle, M.; Rizo-Liendo, A.; Piñero, J.E.; Bazzocchi, I.L.; Lorenzo-Morales, J.; Jiménez, I.A. Ursolic Acid Derivatives as Potential Agents Against Acanthamoeba Spp. Pathogens 2019, 8, 130. https://doi.org/10.3390/pathogens8030130
Sifaoui I, Rodríguez-Expósito RL, Reyes-Batlle M, Rizo-Liendo A, Piñero JE, Bazzocchi IL, Lorenzo-Morales J, Jiménez IA. Ursolic Acid Derivatives as Potential Agents Against Acanthamoeba Spp. Pathogens. 2019; 8(3):130. https://doi.org/10.3390/pathogens8030130
Chicago/Turabian StyleSifaoui, Ines, Rubén L. Rodríguez-Expósito, María Reyes-Batlle, Aitor Rizo-Liendo, José E. Piñero, Isabel L. Bazzocchi, Jacob Lorenzo-Morales, and Ignacio A. Jiménez. 2019. "Ursolic Acid Derivatives as Potential Agents Against Acanthamoeba Spp." Pathogens 8, no. 3: 130. https://doi.org/10.3390/pathogens8030130
APA StyleSifaoui, I., Rodríguez-Expósito, R. L., Reyes-Batlle, M., Rizo-Liendo, A., Piñero, J. E., Bazzocchi, I. L., Lorenzo-Morales, J., & Jiménez, I. A. (2019). Ursolic Acid Derivatives as Potential Agents Against Acanthamoeba Spp. Pathogens, 8(3), 130. https://doi.org/10.3390/pathogens8030130









