Chronic Hepatitis E Virus Infection during Lymphoplasmacytic Lymphoma and Ibrutinib Treatment
Abstract
1. Introduction
2. Material and Methods
2.1. Cell Culture
2.2. Compounds and Reagents
2.3. HEV Plasmids and In Vitro Transcription
2.4. HEV Replication Assay
2.5. Luciferase Assay
2.6. Viability Assay
2.7. Antibody-Based Microarray
2.8. Statistical Methods
2.9. Ethics
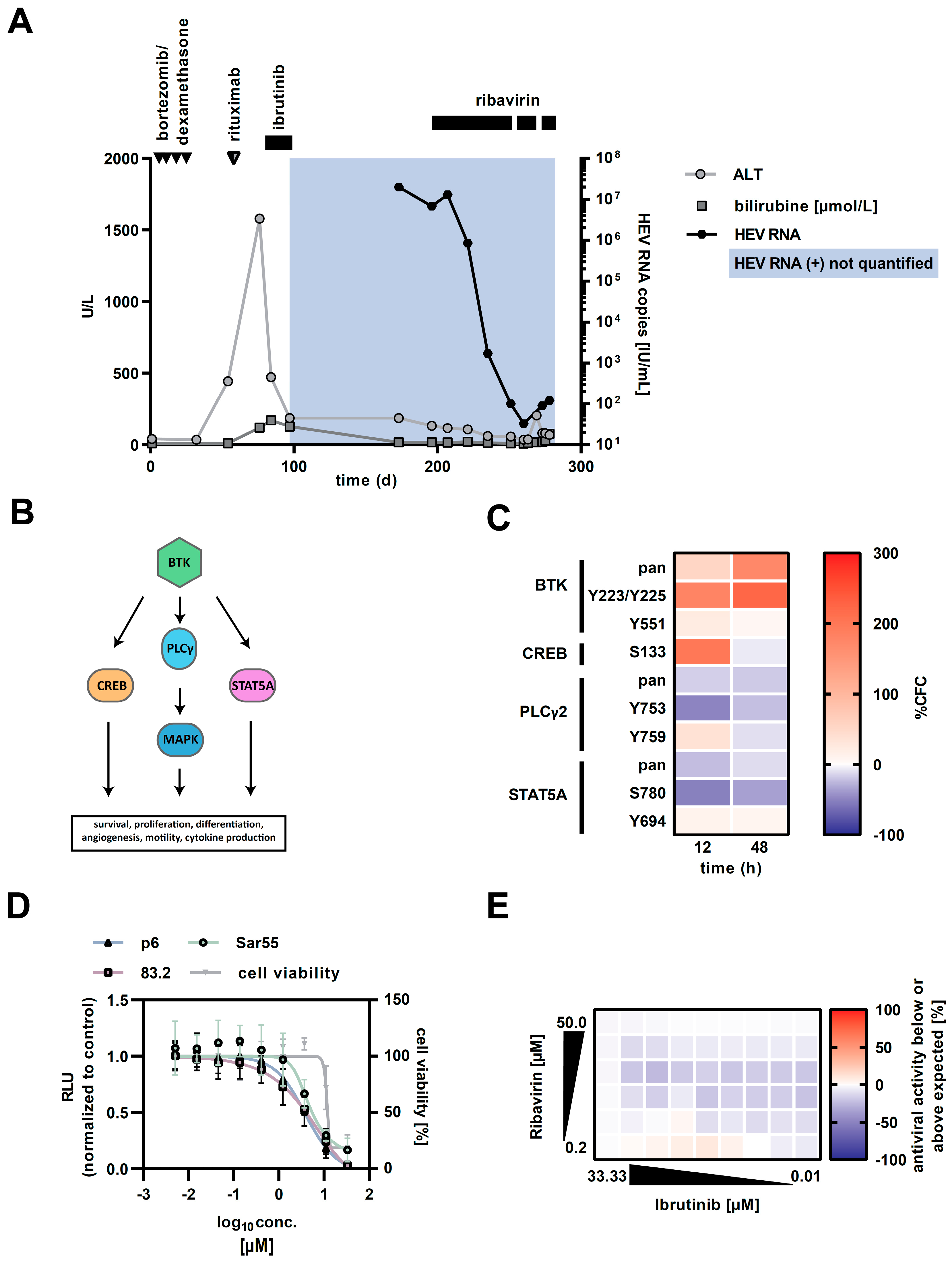
3. Case
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Faber, M.; Willrich, N.; Schemmerer, M.; Rauh, C.; Kuhnert, R.; Stark, K.; Wenzel, J.J. Hepatitis E virus seroprevalence, seroincidence and seroreversion in the German adult population. J. Viral Hepat. 2018, 25, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Kamar, N.; Selves, J.; Mansuy, J.-M.; Ouezzani, L.; Péron, J.-M.; Guitard, J.; Cointault, O.; Esposito, L.; Abravanel, F.; Danjoux, M.; et al. Hepatitis E Virus and Chronic Hepatitis in Organ-Transplant Recipients. N. Engl. J. Med. 2008, 358, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Behrendt, P.; Steinmann, E.; Manns, M.P.; Wedemeyer, H. The impact of hepatitis E in the liver transplant setting. J. Hepatol. 2014, 61, 1418–1429. [Google Scholar] [CrossRef] [PubMed]
- Kamar, N.; Rostaing, L.; Abravanel, F.; Garrouste, C.; Esposito, L.; Cardeau-Desangles, I.; Mansuy, J.M.; Selves, J.; Peron, J.M.; Otal, P.; et al. Pegylated Interferon-α for Treating Chronic Hepatitis E Virus Infection after Liver Transplantation. Clin. Infect. Dis. 2010, 50, e30–e33. [Google Scholar] [CrossRef] [PubMed]
- Kamar, N.; Izopet, J.; Tripon, S.; Bismuth, M.; Hillaire, S.; Dumortier, J.; Radenne, S.; Coilly, A.; Garrigue, V.; D’Alteroche, L.; et al. Ribavirin for Chronic Hepatitis E Virus Infection in Transplant Recipients. N. Engl. J. Med. 2014, 370, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Todt, D.; Meister, T.L.; Steinmann, E. Hepatitis E virus treatment and ribavirin therapy: Viral mechanisms of nonresponse. Curr. Opin. Virol. 2018, 32, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Von Felden, J.; Alric, L.; Pischke, S.; Aitken, C.; Schlabe, S.; Spengler, U.; Giordani, M.T.; Schnitzler, P.; Bettinger, D.; Thimme, R.; et al. The Burden of Hepatitis E among Patients with Hematological Malignancies: A Retrospective European Cohort Study. J. Hepatol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Drave, S.A.; Debing, Y.; Walter, S.; Todt, D.; Engelmann, M.; Friesland, M.; Wedemeyer, H.; Neyts, J.; Behrendt, P.; Steinmann, E. Extra-hepatic replication and infection of hepatitis E virus in neuronal-derived cells. J. Viral Hepat. 2016, 23, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Protin, C.; Abravanel, F.; Laurent, G.; Tavitian, S.; Oberic, L.; Izopet, J.; Martin-Blondel, G.; Ysebaert, L.; Alric, L. Hepatitis E virus infection and ribavirin treatment in patients with chronic lymphocytic leukemia receiving ibrutinib. J. Hepatol. 2017, 66, S250. [Google Scholar] [CrossRef]
- Boudin, L.; Patient, M.; Tsogou, P.T.N.; Roméo, E.; Bladé, J.-S.; De Jauréguiberry, J.-P. Successful treatment with ribavirine for chronic hepatitis E in chronic lymphocytic leukemia treated with Ibrutinib. Bull. Cancer 2019, 106, 84–85. [Google Scholar] [CrossRef] [PubMed]
- Ngoma, P.D.J.; Kabamba, B.; Dahlqvist, G.; Sempoux, C.; Lanthier, N.; Shindano, T.; Neste, E.V.D.; Horsmans, Y. Occult HBV reactivation induced by ibrutinib treatment: A case report. Acta Gastro-Enterol. Belg. 2015, 78, 424–426. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schlevogt, B.; Kinast, V.; Reusch, J.; Kerkhoff, A.; Praditya, D.; Todt, D.; Schmidt, H.H.; Steinmann, E.; Behrendt, P. Chronic Hepatitis E Virus Infection during Lymphoplasmacytic Lymphoma and Ibrutinib Treatment. Pathogens 2019, 8, 129. https://doi.org/10.3390/pathogens8030129
Schlevogt B, Kinast V, Reusch J, Kerkhoff A, Praditya D, Todt D, Schmidt HH, Steinmann E, Behrendt P. Chronic Hepatitis E Virus Infection during Lymphoplasmacytic Lymphoma and Ibrutinib Treatment. Pathogens. 2019; 8(3):129. https://doi.org/10.3390/pathogens8030129
Chicago/Turabian StyleSchlevogt, Bernhard, Volker Kinast, Julia Reusch, Andrea Kerkhoff, Dimas Praditya, Daniel Todt, Hartmut H. Schmidt, Eike Steinmann, and Patrick Behrendt. 2019. "Chronic Hepatitis E Virus Infection during Lymphoplasmacytic Lymphoma and Ibrutinib Treatment" Pathogens 8, no. 3: 129. https://doi.org/10.3390/pathogens8030129
APA StyleSchlevogt, B., Kinast, V., Reusch, J., Kerkhoff, A., Praditya, D., Todt, D., Schmidt, H. H., Steinmann, E., & Behrendt, P. (2019). Chronic Hepatitis E Virus Infection during Lymphoplasmacytic Lymphoma and Ibrutinib Treatment. Pathogens, 8(3), 129. https://doi.org/10.3390/pathogens8030129





