Babesia microti—Borrelia burgdorferi Coinfection
Abstract
1. Babesiosis is an Important Tick-Borne Disease
2. B. microti is the Most Common Transfusion-Transmitted Pathogen in the U.S.A.
3. B. microti—Borrelia burgdorferi Co-Infection is Common in Vector and Host
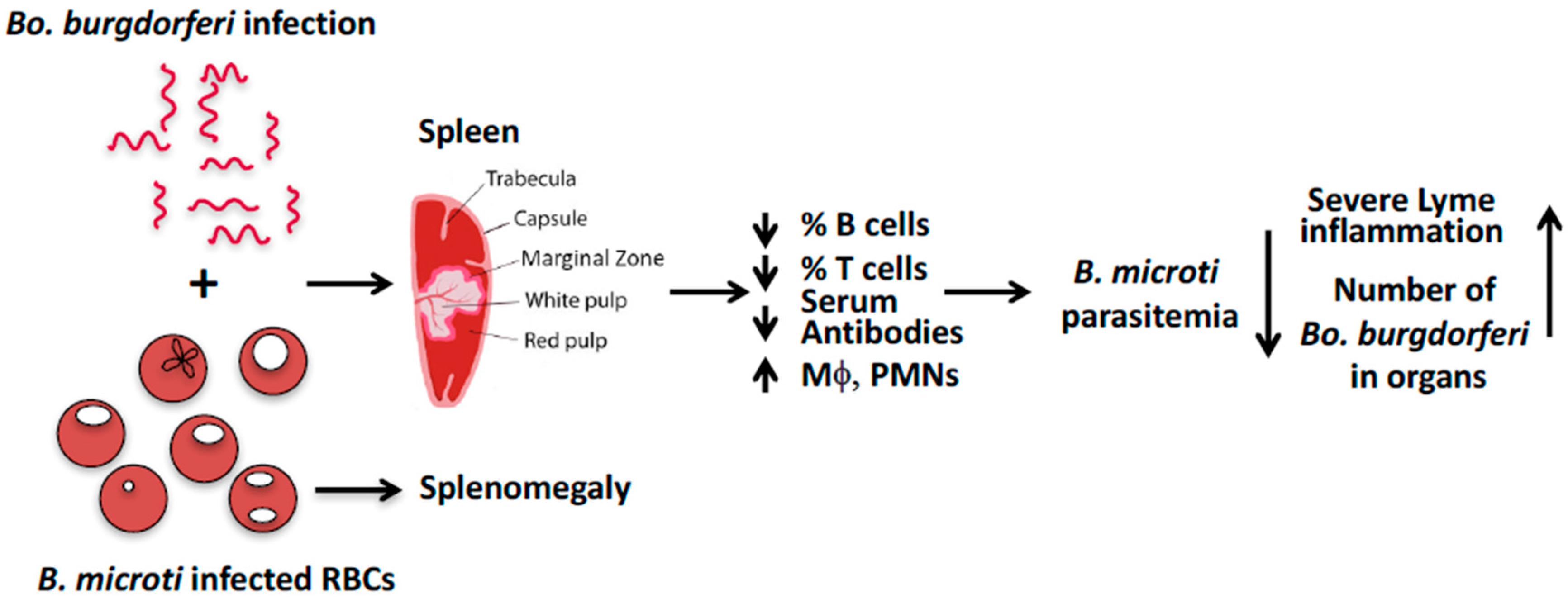
4. Model for B. microti’s Effect on Bo. burgdorferi in C3H Mice
Funding
Conflicts of Interest
References
- Krause, P.J. Human babesiosis. Int. J. Parasitol. 2019, 49, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Vannier, E.; Krause, P.J. Human babesiosis. N. Engl. J. Med. 2012, 366, 2397–2407. [Google Scholar] [CrossRef] [PubMed]
- Persing, D.H.; Herwaldt, B.L.; Glaser, C.; Lane, R.S.; Thomford, J.W.; Mathiesen, D.; Krause, P.J.; Phillip, D.F.; Conrad, P.A. Infection with a babesia-like organism in northern California. N. Engl. J. Med. 1995, 332, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Genda, J.; Negron, E.A.; Lotfipour, M.; Balabhadra, S.; Desai, D.S.; Craft, D.W.; Katzman, M. Severe Babesia microti Infection in an Immunocompetent Host in Pennsylvania. J Investig. Med. High Impact Case Rep. 2016, 4, 2324709616663774. [Google Scholar] [CrossRef] [PubMed]
- Menis, M.; Forshee, R.A.; Kumar, S.; McKean, S.; Warnock, R.; Izurieta, H.S.; Gondalia, R.; Johnson, C.; Mintz, P.D.; Walderhaug, M.O.; et al. Babesiosis Occurrence among the Elderly in the United States, as Recorded in Large Medicare Databases during 2006–2013. PLoS ONE 2015, 10, e0140332. [Google Scholar] [CrossRef] [PubMed]
- Stramer, S.L. The potential threat to blood transfusion safety of emerging infectious disease agents. Clin. Adv. Hematol. Oncol. 2015, 13, 420–422. [Google Scholar]
- Acosta, M.E.; Ender, P.T.; Smith, E.M.; Jahre, J.A. Babesia microti infection, eastern Pennsylvania, USA. Emerg Infect Dis. 2013, 19, 1105–1107. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Babesiosis Surveillance—18 States, 2011. Morb. Mortal. Wkly. Rep. 2012, 61, 505–509. [Google Scholar]
- Herwaldt, B.L.; Linden, J.V.; Bosserman, E.; Young, C.; Olkowska, D.; Wilson, M. Transfusion-associated babesiosis in the United States: A description of cases. Ann. Intern. Med. 2011, 155, 509–519. [Google Scholar] [CrossRef]
- Levin, A.E.; Krause, P.J. Transfusion-transmitted babesiosis: Is it time to screen the blood supply? Curr. Opin. Hematol. 2016, 23, 573–580. [Google Scholar] [CrossRef]
- Saetre, K.; Godhwani, N.; Maria, M.; Patel, D.; Wang, G.; Li, K.; Wormser, G.P.; Nolan, S.M. Congenital Babesiosis After Maternal Infection With Borrelia burgdorferi and Babesia microti. J. Pediatric Infect. Dis. Soc. 2018, 7, e1–e5. [Google Scholar] [CrossRef] [PubMed]
- Esernio-Jenssen, D.; Scimeca, P.G.; Benach, J.L.; Tenenbaum, M.J. Transplacental/perinatal babesiosis. J. Pediatric 1987, 110, 570–572. [Google Scholar] [CrossRef]
- Joseph, J.T.; Purtill, K.; Wong, S.J.; Munoz, J.; Teal, A.; Madison-Antenucci, S.; Horowitz, H.W.; Aguero-Rosenfeld, M.E.; Moore, J.M.; Abramowsky, C.; et al. Vertical transmission of Babesia microti, United States. Emerg. Infect. Dis. 2012, 18, 1318–1321. [Google Scholar] [CrossRef] [PubMed]
- New, D.L.; Quinn, J.B.; Qureshi, M.Z.; Sigler, S.J. Vertically transmitted babesiosis. J. Pediatric 1997, 131 Pt 1, 163–164. [Google Scholar] [CrossRef]
- Ather, I.; Pourafshar, N.; Schain, D.; Gupte, A.; Casey, M.J. Babesiosis: An unusual cause of sepsis after kidney transplantation and review of the literature. Transpl. Infect. Dis. 2017, 19. [Google Scholar] [CrossRef]
- Tufts, D.M.; Diuk-Wasser, M.A. Transplacental transmission of tick-borne Babesia microti in its natural host Peromyscus leucopus. Parasites Vectors. 2018, 11, 286. [Google Scholar] [CrossRef]
- Tolkacz, K.; Bednarska, M.; Alsarraf, M.; Dwuznik, D.; Grzybek, M.; Welc-Faleciak, R.; Behnke, J.M.; Bajer, A. Prevalence, genetic identity and vertical transmission of Babesia microti in three naturally infected species of vole, Microtus spp. (Cricetidae). Parasites Vectors 2017, 10, 66. [Google Scholar] [CrossRef]
- Krause, P.J.; Lepore, T.; Sikand, V.K.; Gadbaw, J., Jr.; Burke, G.; Telford, S.R., 3rd; Brassard, P.; Pearl, D.; Azlanzadeh, J.; Christianson, D.; et al. Atovaquone and azithromycin for the treatment of babesiosis. N. Engl. J. Med. 2000, 343, 1454–1458. [Google Scholar] [CrossRef]
- Lawres, L.A.; Garg, A.; Kumar, V.; Bruzual, I.; Forquer, I.P.; Renard, I.; Virji, A.Z.; Boulard, P.; Rodriguez, E.X.; Allen, A.J.; et al. Radical cure of experimental babesiosis in immunodeficient mice using a combination of an endochin-like quinolone and atovaquone. J. Exp. Med. 2016, 213, 1307–1318. [Google Scholar] [CrossRef]
- Nelder, M.P.; Russell, C.B.; Sheehan, N.J.; Sander, B.; Moore, S.; Li, Y.; Johnson, S.; Patel, S.N.; Sider, D. Human pathogens associated with the blacklegged tick Ixodes scapularis: A systematic review. Parasites Vectors 2016, 9, 265. [Google Scholar] [CrossRef]
- Wormser, G.P.; Dattwyler, R.J.; Shapiro, E.D.; Halperin, J.J.; Steere, A.C.; Klempner, M.S.; Krause, P.J.; Bakken, J.S.; Strle, F.; Stanek, G.; et al. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: Clinical practice guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 2006, 43, 1089–1134. [Google Scholar] [CrossRef] [PubMed]
- Diuk-Wasser, M.A.; Vannier, E.; Krause, P.J. Coinfection by Ixodes Tick-Borne Pathogens: Ecological, Epidemiological, and Clinical Consequences. Trends Parasitol. 2016, 32, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.L.; Graham, C.B.; Boegler, K.A.; Cherry, C.C.; Maes, S.E.; Pilgard, M.A.; Hojgaard, A.; Buttke, D.E.; Eisen, R.J. Prevalence and Diversity of Tick-Borne Pathogens in Nymphal Ixodes scapularis (Acari: Ixodidae) in Eastern National Parks. J. Med. Entomol. 2017, 54, 742–751. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rollend, L.; Bent, S.J.; Krause, P.J.; Usmani-Brown, S.; Steeves, T.K.; States, S.L.; Lepore, T.; Ryan, R.; Dias, F.; Ben Mamoun, C.; et al. Quantitative PCR for detection of Babesia microti in Ixodes scapularis ticks and in human blood. Vector Borne Zoonotic Dis. 2013, 13, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Hersh, M.H.; Ostfeld, R.S.; McHenry, D.J.; Tibbetts, M.; Brunner, J.L.; Killilea, M.E.; LoGiudice, K.; Schmidt, K.A.; Keesing, F. Co-infection of blacklegged ticks with Babesia microti and Borrelia burgdorferi is higher than expected and acquired from small mammal hosts. PLoS ONE 2014, 9, e99348. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.F.; Johnson, R.C.; Magnarelli, L.A.; Hyde, F.W.; Myers, J.E. Peromyscus leucopus and Microtus pennsylvanicus simultaneously infected with Borrelia burgdorferi and Babesia microti. J. Clin. Microbiol. 1986, 23, 135–137. [Google Scholar] [PubMed]
- Piesman, J.; Hicks, T.C.; Sinsky, R.J.; Obiri, G. Simultaneous transmission of Borrelia burgdorferi and Babesia microti by individual nymphal Ixodes dammini ticks. J. Clin. Microbiol. 1987, 25, 2012–2013. [Google Scholar] [PubMed]
- Curcio, S.R.; Tria, L.P.; Gucwa, A.L. Seroprevalence of Babesia microti in Individuals with Lyme Disease. Vector Borne Zoonotic Dis. 2016, 16, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, R.I.; Freeman, P.R. Precision medicine: Retrospective chart review and data analysis of 200 patients on dapsone combination therapy for chronic Lyme disease/post-treatment Lyme disease syndrome: Part 1. Int. J. Gen. Med. 2019, 12, 101–119. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.D.; Scott, C.M. Human Babesiosis Caused by Babesia duncani Has Widespread Distribution across Canada. Healthcare (Basel) 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Abraham, A.; Brasov, I.; Thekkiniath, J.; Kilian, N.; Lawres, L.; Gao, R.; DeBus, K.; He, L.; Yu, X.; Zhu, G.; et al. Establishment of a continuous in vitro culture of Babesia duncani in human erythrocytes reveals unusually high tolerance to recommended therapies. J. Biol. Chem. 2018, 293, 19974–19981. [Google Scholar] [CrossRef] [PubMed]
- Krause, P.J.; McKay, K.; Thompson, C.A.; Sikand, V.K.; Lentz, R.; Lepore, T.; Closter, L.; Christianson, D.; Telford, S.R.; Persing, D.; et al. Deer-Associated Infection Study G. Disease-specific diagnosis of coinfecting tickborne zoonoses: Babesiosis, human granulocytic ehrlichiosis, and Lyme disease. Clin. Infect. Dis. 2002, 34, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Krause, P.J.; Telford, S.R., 3rd; Spielman, A.; Sikand, V.; Ryan, R.; Christianson, D.; Burke, G.; Brassard, P.; Pollack, R.; Peck, J.; et al. Concurrent Lyme disease and babesiosis. Evid. Increased Sev. Durat. Illn. JAMA 1996, 275, 1657–1660. [Google Scholar] [CrossRef]
- Djokic, V.; Primus, S.; Akoolo, L.; Chakraborti, M.; Parveen, N. Age-Related Differential Stimulation of Immune Response by Babesia microti and Borrelia burgdorferi During Acute Phase of Infection Affects Disease Severity. Front. Immunol. 2018, 9, 2891. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.L.; LeVine, D.; Thill, C.; Kuhlow, C.; Benach, J.L. Babesia microti and Borrelia burgdorferi follow independent courses of infection in mice. J. Infect. Dis. 2005, 192, 1634–1641. [Google Scholar] [CrossRef] [PubMed]
- Moro, M.H.; Zegarra-Moro, O.L.; Bjornsson, J.; Hofmeister, E.K.; Bruinsma, E.; Germer, J.J.; Persing, D.H. Increased arthritis severity in mice coinfected with Borrelia burgdorferi and Babesia microti. J. Infect. Dis. 2002, 186, 428–431. [Google Scholar] [CrossRef]
- Djokic, V.; Akoolo, L.; Primus, S.; Schlachter, S.; Kelly, K.; Bhanot, P.; Parveen, N. Protozoan Parasite Babesia microti Subverts Adaptive Immunity and Enhances Lyme Disease Severity. Front. Microbiol. 2019, 10, 1596. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.M.; Krause, P.J.; Davis, S.; Vannier, E.G.; Fitzpatrick, M.C.; Rollend, L.; Belperron, A.A.; States, S.L.; Stacey, A.; Bockenstedt, L.K.; et al. Borrelia burgdorferi promotes the establishment of Babesia microti in the northeastern United States. PLoS ONE 2014, 9, e115494. [Google Scholar] [CrossRef]
- Hanincova, K.; Ogden, N.H.; Diuk-Wasser, M.; Pappas, C.J.; Iyer, R.; Fish, D.; Schwartz, I.; Kurtenbach, K. Fitness variation of Borrelia burgdorferi sensu stricto strains in mice. Appl. Environ. Microbiol. 2008, 74, 153–157. [Google Scholar] [CrossRef]
- Seinost, G.; Dykhuizen, D.E.; Dattwyler, R.J.; Golde, W.T.; Dunn, J.J.; Wang, I.N.; Wormser, G.P.; Schriefer, M.E.; Luft, B.J. Four clones of Borrelia burgdorferi sensu stricto cause invasive infection in humans. Infect. Immun. 1999, 67, 3518–3524. [Google Scholar]
- Cerar, T.; Strle, F.; Stupica, D.; Ruzic-Sabljic, E.; McHugh, G.; Steere, A.C.; Strle, K. Differences in Genotype, Clinical Features, and Inflammatory Potential of Borrelia burgdorferi sensu stricto Strains from Europe and the United States. Emerg. Infect. Dis. 2016, 22, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Keirans, J.E.; Hutcheson, H.J.; Durden, L.A.; Klompen, J.S. Ixodes (Ixodes) scapularis (Acari:Ixodidae): Redescription of all active stages, distribution, hosts, geographical variation, and medical and veterinary importance. J. Med. Entomol. 1996, 33, 297–318. [Google Scholar] [CrossRef] [PubMed]
- Piesman, J.; Spielman, A. Human babesiosis on Nantucket Island: Prevalence of Babesia microti in ticks. Am. J. Trop. Med. Hyg. 1980, 29, 742–746. [Google Scholar] [CrossRef] [PubMed]
- Medica, D.L.; Sinnis, P. Quantitative dynamics of Plasmodium yoelii sporozoite transmission by infected anopheline mosquitoes. Infect. Immun. 2005, 73, 4363–4369. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Kebaier, C.; Vanderberg, J. Direct microscopic quantification of dynamics of Plasmodium berghei sporozoite transmission from mosquitoes to mice. Infect. Immun. 2007, 75, 5532–5539. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sinnis, P.; Zavala, F. The skin: Where malaria infection and the host immune response begin. Semin. Immunopathol. 2012, 34, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Simo, L.; Kazimirova, M.; Richardson, J.; Bonnet, S.I. The Essential Role of Tick Salivary Glands and Saliva in Tick Feeding and Pathogen Transmission. Front. Cell. Infect. Microbiol. 2017, 7, 281. [Google Scholar] [CrossRef] [PubMed]
- Mather, T.N.; Telford, S.R., 3rd; Moore, S.I.; Spielman, A. Borrelia burgdorferi and Babesia microti: Efficiency of transmission from reservoirs to vector ticks (Ixodes dammini). Exp. Parasitol. 1990, 70, 55–61. [Google Scholar] [CrossRef]
- Vannier, E.; Borggraefe, I.; Telford, S.R., 3rd; Menon, S.; Brauns, T.; Spielman, A.; Gelfand, J.A.; Wortis, H.H. Age-associated decline in resistance to Babesia microti is genetically determined. J. Infect. Dis. 2004, 189, 1721–1728. [Google Scholar] [CrossRef]
- Sasaki, M.; Fujii, Y.; Iwamoto, M.; Ikadai, H. Effect of sex steroids on Babesia microti infection in mice. Am. J. Trop. Med. Hyg. 2013, 88, 367–375. [Google Scholar] [CrossRef]
- Hughes, V.L.; Randolph, S.E. Testosterone increases the transmission potential of tick-borne parasites. Parasitology 2001, 123 Pt 4, 365–371. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parveen, N.; Bhanot, P. Babesia microti—Borrelia burgdorferi Coinfection. Pathogens 2019, 8, 117. https://doi.org/10.3390/pathogens8030117
Parveen N, Bhanot P. Babesia microti—Borrelia burgdorferi Coinfection. Pathogens. 2019; 8(3):117. https://doi.org/10.3390/pathogens8030117
Chicago/Turabian StyleParveen, Nikhat, and Purnima Bhanot. 2019. "Babesia microti—Borrelia burgdorferi Coinfection" Pathogens 8, no. 3: 117. https://doi.org/10.3390/pathogens8030117
APA StyleParveen, N., & Bhanot, P. (2019). Babesia microti—Borrelia burgdorferi Coinfection. Pathogens, 8(3), 117. https://doi.org/10.3390/pathogens8030117





