Comparative Pathobiology of the Intestinal Protozoan Parasites Giardia lamblia, Entamoeba histolytica, and Cryptosporidium parvum
Abstract
1. Introduction
2. Etiology and Epidemiology
2.1. Giardia lamblia
2.2. Entamoeba histolytica
2.3. Cryptosporidium sp.
3. Pathogenicity and Virulence
3.1. General Remarks
3.2. Giardia lamblia
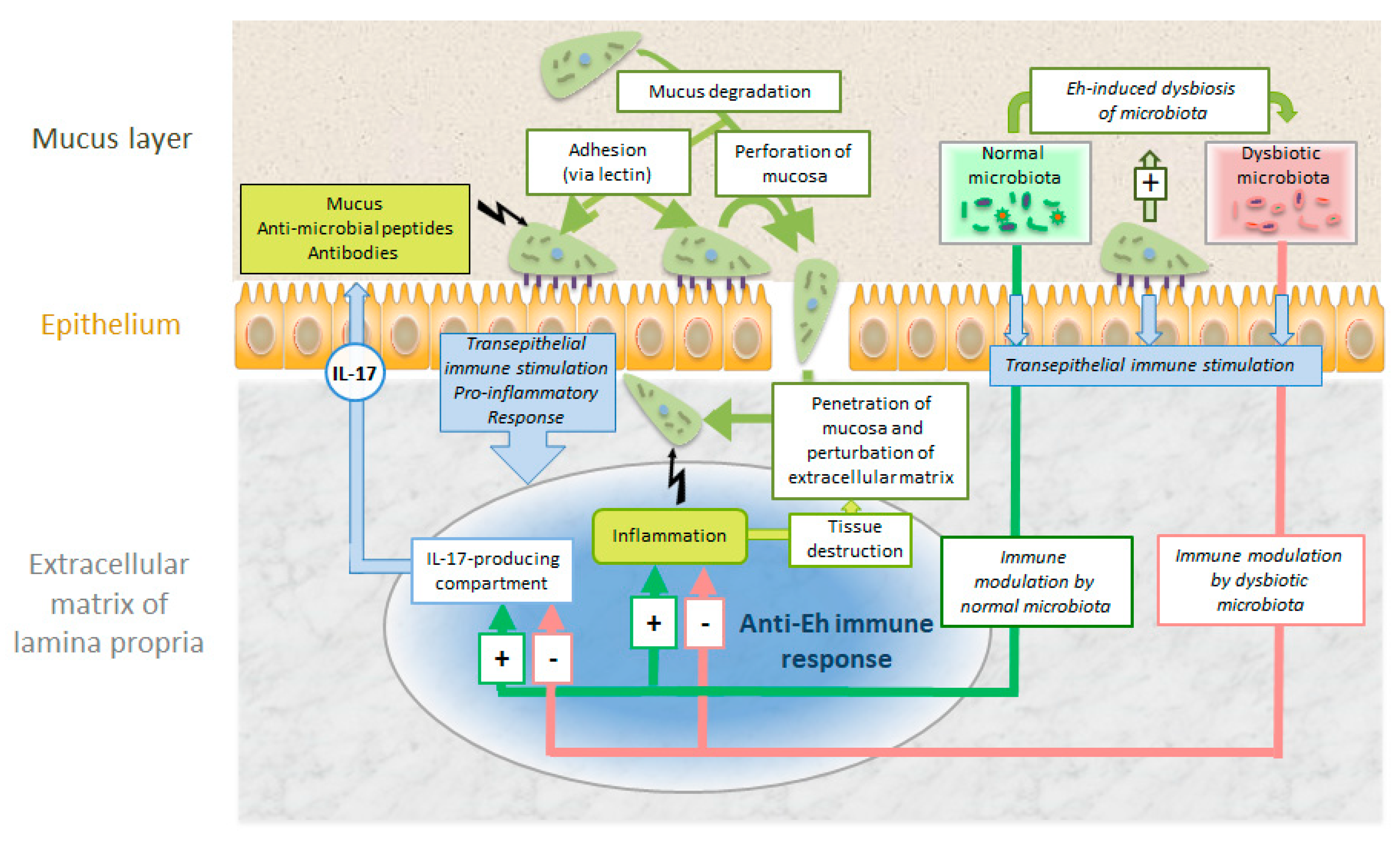
3.3. Entamoeba histolytica
3.4. Cryptosporidium sp.
4. Control and Treatment
4.1. Prevention
4.2. Diagnosis
4.3. Treatment
4.4. Is Vaccination a Suitable Strategy?
5. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van Voorhis, W. Protozoan infections. Sci. Am. Med. 2014. [Google Scholar] [CrossRef]
- Chabe, M.; Lokmer, A.; Segurel, L. Gut protozoa: Friends or foes of the human gut microbiota? Trends Parasitol. 2017, 33, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Stensvold, C.R.; Clark, C.G. Current status of Blastocystis: A personal view. Parasitol. Int. 2016, 65, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.; Stark, D.; Harkness, J.; Ellis, J. Update on the pathogenic potential and treatment options for Blastocystis sp. Gut Pathog. 2014, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Adl, S.M.; Simpson, A.G.; Lane, C.E.; Lukes, J.; Bass, D.; Bowser, S.S.; Brown, M.W.; Burki, F.; Dunthorn, M.; Hampl, V.; et al. The revised classification of eukaryotes. J. Eukaryot. Microbiol. 2012, 59, 429–493. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.B.; White, A.C. An updated review on Cryptosporidium and Giardia. Gastroenterol. Clin. North Am. 2006, 35, 291–314. [Google Scholar] [CrossRef] [PubMed]
- Lujan, H.D. Mechanisms of adaptation in the intestinal parasite Giardia lamblia. Essays Biochem. 2011, 51, 177–191. [Google Scholar] [CrossRef]
- Müller, N.; Müller, J. Giardia. In Molecular Parasitology; Walochnik, J., Duchêne, M., Eds.; Springer: Vienna, Austria, 2016; pp. 93–114. [Google Scholar]
- Upcroft, J.; Upcroft, P. My favorite cell: Giardia. Bioessays 1998, 20, 256–263. [Google Scholar] [CrossRef]
- Schofield, P.J.; Edwards, M.R.; Matthews, J.; Wilson, J.R. The pathway of arginine catabolism in Giardia intestinalis. Mol. Biochem. Parasitol. 1992, 51, 29–36. [Google Scholar] [CrossRef]
- Vermathen, M.; Müller, J.; Furrer, J.; Müller, N.; Vermathen, P. 1H HR-MAS NMR spectroscopy to study the metabolome of the protozoan parasite Giardia lamblia. Talanta 2018, 188, 429–441. [Google Scholar] [CrossRef]
- Efstratiou, A.; Ongerth, J.; Karanis, P. Evolution of monitoring for Giardia and Cryptosporidium in water. Water Res. 2017, 123, 96–112. [Google Scholar] [CrossRef] [PubMed]
- Utaaker, K.S.; Skjerve, E.; Robertson, L.J. Keeping it cool: Survival of Giardia cysts and Cryptosporidium oocysts on lettuce leaves. Int. J. Food Microbiol. 2017, 255, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Waldram, A.; Vivancos, R.; Hartley, C.; Lamden, K. Prevalence of Giardia infection in households of Giardia cases and risk factors for household transmission. BMC Infect. Dis. 2017, 17, 486. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.R.; Daniel, L.A. Occurrence and removal of Giardia spp. cysts and Cryptosporidium spp. oocysts from a municipal wastewater treatment plant in Brazil. Environ. Technol. 2017, 38, 1245–1254. [Google Scholar] [CrossRef] [PubMed]
- Sahagún, J.; Clavel, A.; Goñi, P.; Seral, C.; Llorente, M.T.; Castillo, F.J.; Capilla, S.; Arias, A.; Gómez-Lus, R. Correlation between the presence of symptoms and the Giardia duodenalis genotype. Eur. J. Clin. Microbiol. Infect. Dis. 2008, 27, 81–83. [Google Scholar] [CrossRef]
- Heyworth, M.F. Giardia duodenalis genetic assemblages and hosts. Parasite 2016, 23, 13. [Google Scholar] [CrossRef] [PubMed]
- Allain, T.; Fekete, E.; Buret, A.G. Giardia cysteine proteases: The teeth behind the smile. Trends Parasitol. 2019, 35, 636–648. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, A.; Field, D.; Ryan, U. Molecular typing of Giardia duodenalis in humans in Queensland—First report of Assemblage E. Parasitology 2017, 144, 1154–1161. [Google Scholar] [CrossRef]
- Feng, Y.; Xiao, L. Zoonotic potential and molecular epidemiology of Giardia species and giardiasis. Clin. Microbiol. Rev. 2011, 24, 110–140. [Google Scholar] [CrossRef]
- Rogawski, E.T.; Bartelt, L.A.; Platts-Mills, J.A.; Seidman, J.C.; Samie, A.; Havt, A.; Babji, S.; Trigoso, D.R.; Qureshi, S.; Shakoor, S.; et al. Determinants and Impact of Giardia Infection in the First 2 Years of Life in the MAL-ED Birth Cohort. J. Pediatric Infect. Dis. Soc. 2017, 6, 153–160. [Google Scholar] [CrossRef]
- Gonzalez-Ruiz, A.; Wright, S.G. Disparate amoebae. Lancet 1998, 351, 1672–1673. [Google Scholar] [CrossRef]
- Smith, L.A. Still around and still dangerous: Giardia lamblia and Entamoeba histolytica. Clin. Lab. Sci. J. Am. Soc. Med Technol. 1997, 10, 279–286. [Google Scholar]
- Clode, P.L.; Koh, W.H.; Thompson, R.C.A. Life without a host cell: What is Cryptosporidium? Trends Parasitol. 2015, 31, 614–624. [Google Scholar] [CrossRef]
- Certad, G.; Viscogliosi, E.; Chabe, M.; Caccio, S.M. Pathogenic mechanisms of Cryptosporidium and Giardia. Trends Parasitol. 2017, 33, 561–576. [Google Scholar] [CrossRef]
- Checkley, W.; White, A.C., Jr.; Jaganath, D.; Arrowood, M.J.; Chalmers, R.M.; Chen, X.M.; Fayer, R.; Griffiths, J.K.; Guerrant, R.L.; Hedstrom, L.; et al. A review of the global burden, novel diagnostics, therapeutics, and vaccine targets for Cryptosporidium. Lancet. Infect. Dis. 2015, 15, 85–94. [Google Scholar] [CrossRef]
- Ryan, U.; Zahedi, A.; Paparini, A. Cryptosporidium in humans and animals-a one health approach to prophylaxis. Parasite Immunol. 2016, 38, 535–547. [Google Scholar] [CrossRef]
- Sow, S.O.; Muhsen, K.; Nasrin, D.; Blackwelder, W.C.; Wu, Y.; Farag, T.H.; Panchalingam, S.; Sur, D.; Zaidi, A.K.; Faruque, A.S.; et al. The burden of Cryptosporidium diarrheal disease among children <24 months of age in moderate/high mortality regions of sub-Saharan Africa and South Asia, utilizing data from the Global Enteric Multicenter Study (GEMS). PLoS Negl. Trop. Dis. 2016, 10, e0004729. [Google Scholar] [CrossRef]
- Striepen, B. Parasitic infections: Time to tackle cryptosporidiosis. Nature 2013, 503, 189–191. [Google Scholar] [CrossRef]
- White, A.C.J. Cryptosporidiosis (Cryptosporidium species). In Principles and Practice of Infectious Diseases, 8th ed.; Bennett, J.E., Dolin, R., Blaser, M.K., Eds.; Elsevier Churchill Livingstone: Philadelphia, PA, USA, 2015; pp. 3173–3183. [Google Scholar]
- Buret, A.G.; Motta, J.P.; Allain, T.; Ferraz, J.; Wallace, J.L. Pathobiont release from dysbiotic gut microbiota biofilms in intestinal inflammatory diseases: A role for iron? J. Biomed. Sci. 2019, 26, 1. [Google Scholar] [CrossRef]
- Sorci, G.; Cornet, S.; Faivre, B. Immune evasion, immunopathology and the regulation of the immune system. Pathogens 2013, 2, 71–91. [Google Scholar] [CrossRef]
- Lopez-Romero, G.; Quintero, J.; Astiazaran-Garcia, H.; Velazquez, C. Host defences against Giardia lamblia. Parasite Immunol. 2015, 37, 394–406. [Google Scholar] [CrossRef]
- Koh, W.H.; Geurden, T.; Paget, T.; O’Handley, R.; Steuart, R.F.; Thompson, R.C.; Buret, A.G. Giardia duodenalis assemblage-specific induction of apoptosis and tight junction disruption in human intestinal epithelial cells: Effects of mixed infections. J. Parasitol. 2013, 99, 353–358. [Google Scholar] [CrossRef]
- Cotton, J.A.; Beatty, J.K.; Buret, A.G. Host parasite interactions and pathophysiology in Giardia infections. Int. J. Parasitol. 2011, 41, 925–933. [Google Scholar] [CrossRef]
- Scott, K.G.; Logan, M.R.; Klammer, G.M.; Teoh, D.A.; Buret, A.G. Jejunal brush border microvillous alterations in Giardia muris-infected mice: Role of T lymphocytes and interleukin-6. Infect. Immun. 2000, 68, 3412–3418. [Google Scholar] [CrossRef]
- Kamda, J.D.; Nash, T.E.; Singer, S.M. Giardia duodenalis: Dendritic cell defects in IL-6 deficient mice contribute to susceptibility to intestinal infection. Exp. Parasitol. 2012, 130, 288–291. [Google Scholar] [CrossRef]
- Saghaug, C.S.; Sornes, S.; Peirasmaki, D.; Svard, S.; Langeland, N.; Hanevik, K. Human memory CD4+ T cell immune responses against Giardia lamblia. Clin. Vaccine Immunol. 2015, 23, 11–18. [Google Scholar] [CrossRef]
- Schneider, C.; O’Leary, C.E.; von Moltke, J.; Liang, H.E.; Ang, Q.Y.; Turnbaugh, P.J.; Radhakrishnan, S.; Pellizzon, M.; Ma, A.; Locksley, R.M. A metabolite-triggered tuft cell-ILC2 circuit drives small intestinal remodeling. Cell 2018, 174, 271–284. [Google Scholar] [CrossRef]
- Stäger, S.; Gottstein, B.; Sager, H.; Jungi, T.W.; Müller, N. Influence of antibodies in mother’s milk on antigenic variation of Giardia lamblia in the murine mother-offspring model of infection. Infect. Immun. 1998, 66, 1287–1292. [Google Scholar]
- Bénéré, E.; Van Assche, T.; Cos, P.; Maes, L. Intrinsic susceptibility of Giardia duodenalis assemblage subtypes A(I), A(II), B and E(III) for nitric oxide under axenic culture conditions. Parasitol. Res. 2012, 110, 1315–1319. [Google Scholar] [CrossRef]
- Fink, M.Y.; Singer, S.M. The Intersection of Immune Responses, Microbiota, and Pathogenesis in Giardiasis. Trends Parasitol. 2017, 33, 901–913. [Google Scholar] [CrossRef]
- Nash, T.E. Surface antigenic variation in Giardia lamblia. Mol. Microbiol. 2002, 45, 585–590. [Google Scholar] [CrossRef]
- Kulakova, L.; Singer, S.M.; Conrad, J.; Nash, T.E. Epigenetic mechanisms are involved in the control of Giardia lamblia antigenic variation. Mol. Microbiol. 2006, 61, 1533–1542. [Google Scholar] [CrossRef]
- Prucca, C.G.; Slavin, I.; Quiroga, R.; Elías, E.V.; Rivero, F.D.; Saura, A.; Carranza, P.G.; Luján, H.D. Antigenic variation in Giardia lamblia is regulated by RNA interference. Nature 2008, 456, 750–754. [Google Scholar] [CrossRef]
- Prucca, C.G.; Lujan, H.D. Antigenic variation in Giardia lamblia. Cell Microbiol 2009, 11, 1706–1715. [Google Scholar] [CrossRef]
- Nash, T.E. Antigenic variation in Giardia lamblia and the host’s immune response. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1997, 352, 1369–1375. [Google Scholar] [CrossRef]
- Müller, J.; Ley, S.; Felger, I.; Hemphill, A.; Müller, N. Identification of differentially expressed genes in a Giardia lamblia WB C6 clone resistant to nitazoxanide and metronidazole. J. Antimicrob. Chemother. 2008, 62, 72–82. [Google Scholar] [CrossRef]
- Emery, S.J.; Baker, L.; Ansell, B.R.E.; Mirzaei, M.; Haynes, P.A.; McConville, M.J.; Svard, S.G.; Jex, A.R. Differential protein expression and post-translational modifications in metronidazole-resistant Giardia duodenalis. Gigascience 2018, 7. [Google Scholar] [CrossRef]
- Müller, J.; Braga, S.; Heller, M.; Müller, N. Resistance formation to nitro drugs in Giardia lamblia: No common markers identified by comparative proteomics. Int. J. Parasitol. Drugs Drug Resist. 2019, 9, 112–119. [Google Scholar] [CrossRef]
- Gargantini, P.R.; Serradell, M.D.C.; Rios, D.N.; Tenaglia, A.H.; Lujan, H.D. Antigenic variation in the intestinal parasite Giardia lamblia. Curr. Opin. Microbiol. 2016, 32, 52–58. [Google Scholar] [CrossRef]
- Cabrera-Licona, A.; Solano-Gonzalez, E.; Fonseca-Linan, R.; Bazan-Tejeda, M.L.; Raul, A.-G.; Bermudez-Cruz, R.M.; Ortega-Pierres, G. Expression and secretion of the Giardia duodenalis variant surface protein 9B10A by transfected trophozoites causes damage to epithelial cell monolayers mediated by protease activity. Exp. Parasitol. 2017, 179, 49–64. [Google Scholar] [CrossRef]
- Deloer, S.; Nakamura, R.; Mi-Ichi, F.; Adachi, K.; Kobayashi, S.; Hamano, S. Mouse models of amoebiasis and culture methods of amoeba. Parasitol. Int. 2016, 65, 520–525. [Google Scholar] [CrossRef]
- Kantor, M.; Abrantes, A.; Estevez, A.; Schiller, A.; Torrent, J.; Gascon, J.; Hernandez, R.; Ochner, C. Entamoeba histolytica: Updates in clinical manifestation, pathogenesis, and vaccine development. Can. J. Gastroenterol. Hepatol. 2018, 2018, 4601420. [Google Scholar] [CrossRef]
- Sanchez, V.; Serrano-Luna, J.; Ramirez-Moreno, E.; Tsutsumi, V.; Shibayama, M. Entamoeba histolytica: Overexpression of the gal/galnac lectin, ehcp2 and ehcp5 genes in an in vivo model of amebiasis. Parasitol. Int. 2016, 65, 665–667. [Google Scholar] [CrossRef]
- Ghosh, S.; Padalia, J.; Moonah, S. Tissue destruction caused by Entamoeba histolytica parasite: Cell death, inflammation, invasion, and the gut microbiome. Curr. Clin. Microbiol. Rep. 2019, 6, 51–57. [Google Scholar] [CrossRef]
- Ayala-Sumuano, J.T.; Tellez-Lopez, V.M.; Dominguez-Robles Mdel, C.; Shibayama-Salas, M.; Meza, I. Toll-like receptor signaling activation by Entamoeba histolytica induces beta defensin 2 in human colonic epithelial cells: Its possible role as an element of the innate immune response. PLoS Negl. Trop. Dis. 2013, 7, e2083. [Google Scholar] [CrossRef]
- Mortimer, L.; Chadee, K. The immunopathogenesis of Entamoeba histolytica. Exp. Parasitol. 2010, 126, 366–380. [Google Scholar] [CrossRef]
- Deloer, S.; Nakamura, R.; Kikuchi, M.; Moriyasu, T.; Kalenda, Y.D.J.; Mohammed, E.S.; Senba, M.; Iwakura, Y.; Yoshida, H.; Hamano, S. IL-17A contributes to reducing IFN-gamma/IL-4 ratio and persistence of Entamoeba histolytica during intestinal amebiasis. Parasitol. Int. 2017, 66, 817–823. [Google Scholar] [CrossRef]
- Cornick, S.; Chadee, K. Entamoeba histolytica: Host parasite interactions at the colonic epithelium. Tissue Barriers 2017, 5, e1283386. [Google Scholar] [CrossRef]
- Salles, J.M.; Moraes, L.A.; Salles, M.C. Hepatic amebiasis. Braz. J. Infect. Dis. Off. Publ. Braz. Soc. Infect. Dis. 2003, 7, 96–110. [Google Scholar] [CrossRef]
- Perez-Tamayo, R.; Montfort, I.; Garcia, A.O.; Ramos, E.; Ostria, C.B. Pathogenesis of acute experimental liver amebiasis. Arch. Med. Res. 2006, 37, 203–209. [Google Scholar] [CrossRef]
- Varet, H.; Shaulov, Y.; Sismeiro, O.; Trebicz-Geffen, M.; Legendre, R.; Coppee, J.Y.; Ankri, S.; Guillen, N. Enteric bacteria boost defences against oxidative stress in Entamoeba histolytica. Sci. Rep. 2018, 8, 9042. [Google Scholar] [CrossRef]
- Watanabe, K.; Gilchrist, C.A.; Uddin, M.J.; Burgess, S.L.; Abhyankar, M.M.; Moonah, S.N.; Noor, Z.; Donowitz, J.R.; Schneider, B.N.; Arju, T.; et al. Microbiome-mediated neutrophil recruitment via CXCR2 and protection from amebic colitis. PLoS Pathog. 2017, 13, e1006513. [Google Scholar] [CrossRef]
- Nakada-Tsukui, K.; Nozaki, T. Immune response of amebiasis and immune evasion by Entamoeba histolytica. Front. Immunol. 2016, 7, 175. [Google Scholar] [CrossRef]
- Müller, J.; Hemphill, A. In vitro culture systems for the study of apicomplexan parasites in farm animals. Int. J. Parasitol. 2013, 43, 115–124. [Google Scholar] [CrossRef]
- Pawlowic, M.C.; Vinayak, S.; Sateriale, A.; Brooks, C.F.; Striepen, B. Generating and maintaining transgenic Cryptosporidium parvum parasites. Curr. Protoc. Microbiol. 2017, 46, 20B.2.1–20B.2.32. [Google Scholar] [CrossRef]
- Vinayak, S.; Pawlowic, M.C.; Sateriale, A.; Brooks, C.F.; Studstill, C.J.; Bar-Peled, Y.; Cipriano, M.J.; Striepen, B. Genetic modification of the diarrhoeal pathogen Cryptosporidium parvum. Nature 2015, 523, 477–480. [Google Scholar] [CrossRef]
- King, B.J.; Keegan, A.R.; Robinson, B.S.; Monis, P.T. Cryptosporidium cell culture infectivity assay design. Parasitology 2011, 138, 671–681. [Google Scholar] [CrossRef]
- Karanis, P.; Aldeyarbi, H.M. Evolution of Cryptosporidium in vitro culture. Int. J. Parasitol. 2011, 41, 1231–1242. [Google Scholar] [CrossRef]
- Castellanos-Gonzalez, A.; Cabada, M.M.; Nichols, J.; Gomez, G.; White, A.C., Jr. Human primary intestinal epithelial cells as an improved in vitro model for Cryptosporidium parvum infection. Infect. Immun. 2013, 81, 1996–2001. [Google Scholar] [CrossRef]
- Huang, B.Q.; Chen, X.M.; LaRusso, N.F. Cryptosporidium parvum attachment to and internalization by human biliary epithelia in vitro: A morphologic study. J. Parasitol. 2004, 90, 212–221. [Google Scholar] [CrossRef]
- Zhang, S.; Jian, F.; Zhao, G.; Huang, L.; Zhang, L.; Ning, C.; Wang, R.; Qi, M.; Xiao, L. Chick embryo tracheal organ: A new and effective in vitro culture model for Cryptosporidium baileyi. Vet. Parasitol. 2012, 188, 376–381. [Google Scholar] [CrossRef]
- Josse, L.; Bones, A.J.; Purton, T.; Michaelis, M.; Tsaousis, A.D. A Cell Culture Platform for the Cultivation of Cryptosporidium parvum. Curr. Protoc. Microbiol. 2019, 53, e80. [Google Scholar] [CrossRef]
- Heo, I.; Dutta, D.; Schaefer, D.A.; Iakobachvili, N.; Artegiani, B.; Sachs, N.; Boonekamp, K.E.; Bowden, G.; Hendrickx, A.P.A.; Willems, R.J.L.; et al. Modelling Cryptosporidium infection in human small intestinal and lung organoids. Nat. Microbiol. 2018, 3, 814–823. [Google Scholar] [CrossRef]
- Morada, M.; Lee, S.; Gunther-Cummins, L.; Weiss, L.M.; Widmer, G.; Tzipori, S.; Yarlett, N. Continuous culture of Cryptosporidium parvum using hollow fiber technology. Int. J. Parasitol. 2016, 46, 21–29. [Google Scholar] [CrossRef]
- Bouzid, M.; Hunter, P.R.; Chalmers, R.M.; Tyler, K.M. Cryptosporidium pathogenicity and virulence. Clin. Microbiol. Rev. 2013, 26, 115–134. [Google Scholar] [CrossRef]
- O’Hara, S.P.; Chen, X.M. The cell biology of Cryptosporidium infection. Microbes Infect. 2011, 13, 721–730. [Google Scholar] [CrossRef]
- Widmer, G.; Yang, Y.L.; Bonilla, R.; Tanriverdi, S.; Ciociola, K.M. Preferential infection of dividing cells by Cryptosporidium parvum. Parasitology 2006, 133, 131–138. [Google Scholar] [CrossRef]
- Mirhashemi, M.E.; Noubary, F.; Chapman-Bonofiglio, S.; Tzipori, S.; Huggins, G.S.; Widmer, G. Transcriptome analysis of pig intestinal cell monolayers infected with Cryptosporidium parvum asexual stages. Parasites Vectors 2018, 11, 176. [Google Scholar] [CrossRef]
- Liu, J.; Deng, M.; Lancto, C.A.; Abrahamsen, M.S.; Rutherford, M.S.; Enomoto, S. Biphasic modulation of apoptotic pathways in Cryptosporidium parvum-infected human intestinal epithelial cells. Infect. Immun. 2009, 77, 837–849. [Google Scholar] [CrossRef]
- Mele, R.; Gomez Morales, M.A.; Tosini, F.; Pozio, E. Cryptosporidium parvum at different developmental stages modulates host cell apoptosis in vitro. Infect. Immun. 2004, 72, 6061–6067. [Google Scholar] [CrossRef]
- Ojcius, D.M.; Perfettini, J.L.; Bonnin, A.; Laurent, F. Caspase-dependent apoptosis during infection with Cryptosporidium parvum. Microbes Infect. 1999, 1, 1163–1168. [Google Scholar] [CrossRef]
- Luder, C.G.; Gross, U. Apoptosis and its modulation during infection with Toxoplasma gondii: Molecular mechanisms and role in pathogenesis. Curr. Top. Microbiol. Immunol. 2005, 289, 219–237. [Google Scholar]
- Heussler, V.T.; Rottenberg, S.; Schwab, R.; Küenzi, P.; Fernandez, P.C.; McKellar, S.; Shiels, B.; Chen, Z.J.; Orth, K.; Wallach, D.; et al. Hijacking of host cell IKK signalosomes by the transforming parasite Theileria. Science 2002, 298, 1033–1036. [Google Scholar] [CrossRef]
- Heussler, V.T.; Machado, J.; Fernandez, P.C.; Botteron, C.; Chen, C.G.; Pearse, M.J.; Dobbelaere, D.A. The intracellular parasite Theileria parva protects infected T cells from apoptosis. Proc. Natl. Acad. Sci. USA 1999, 96, 7312–7317. [Google Scholar] [CrossRef]
- Bhat, N.; Joe, A.; PereiraPerrin, M.; Ward, H.D. Cryptosporidium p30, a galactose/N-acetylgalactosamine-specific lectin, mediates infection in vitro. J. Biol. Chem. 2007, 282, 34877–34887. [Google Scholar] [CrossRef]
- Howe, D.K.; Gaji, R.Y.; Mroz-Barrett, M.; Gubbels, M.J.; Striepen, B.; Stamper, S. Sarcocystis neurona merozoites express a family of immunogenic surface antigens that are orthologues of the Toxoplasma gondii surface antigens (SAGs) and SAG-related sequences. Infect. Immun. 2005, 73, 1023–1033. [Google Scholar] [CrossRef]
- Cardoso, R.; Soares, H.; Hemphill, A.; Leitao, A. Apicomplexans pulling the strings: Manipulation of the host cell cytoskeleton dynamics. Parasitology 2016, 143, 957–970. [Google Scholar] [CrossRef]
- Pollok, R.C.; McDonald, V.; Kelly, P.; Farthing, M.J. The role of Cryptosporidium parvum-derived phospholipase in intestinal epithelial cell invasion. Parasitol. Res. 2003, 90, 181–186. [Google Scholar] [CrossRef]
- Padda, R.S.; Tsai, A.; Chappell, C.L.; Okhuysen, P.C. Molecular cloning and analysis of the Cryptosporidium parvum aminopeptidase N gene. Int. J. Parasitol. 2002, 32, 187–197. [Google Scholar] [CrossRef]
- Kniel, K.E.; Sumner, S.S.; Pierson, M.D.; Zajac, A.M.; Hackney, C.R.; Fayer, R.; Lindsay, D.S. Effect of hydrogen peroxide and other protease inhibitors on Cryptosporidium parvum excystation and in vitro development. J. Parasitol. 2004, 90, 885–888. [Google Scholar] [CrossRef]
- Perez-Cordon, G.; Nie, W.; Schmidt, D.; Tzipori, S.; Feng, H. Involvement of host calpain in the invasion of Cryptosporidium parvum. Microbes Infect. 2011, 13, 103–107. [Google Scholar] [CrossRef]
- Wanyiri, J.W.; Techasintana, P.; O’Connor, R.M.; Blackman, M.J.; Kim, K.; Ward, H.D. Role of CpSUB1, a subtilisin-like protease, in Cryptosporidium parvum infection in vitro. Eukaryot. Cell 2009, 8, 470–477. [Google Scholar] [CrossRef]
- McDonald, V.; Korbel, D.S.; Barakat, F.M.; Choudhry, N.; Petry, F. Innate immune responses against Cryptosporidium parvum infection. Parasite Immunol. 2013, 35, 55–64. [Google Scholar] [CrossRef]
- Perez-Cordon, G.; Yang, G.; Zhou, B.; Nie, W.; Li, S.; Shi, L.; Tzipori, S.; Feng, H. Interaction of Cryptosporidium parvum with mouse dendritic cells leads to their activation and parasite transportation to mesenteric lymph nodes. Pathog. Dis. 2014, 70, 17–27. [Google Scholar] [CrossRef]
- Drinkall, E.; Wass, M.J.; Coffey, T.J.; Flynn, R.J. A rapid IL-17 response to Cryptosporidium parvum in the bovine intestine. Vet. Immunol. Immunopathol. 2017, 191, 1–4. [Google Scholar] [CrossRef]
- Ludington, J.G.; Ward, H.D. Systemic and mucosal immune responses to Cryptosporidium-vaccine development. Curr. Trop. Med. Rep. 2015, 2, 171–180. [Google Scholar] [CrossRef]
- Carryn, S.; Schaefer, D.A.; Imboden, M.; Homan, E.J.; Bremel, R.D.; Riggs, M.W. Phospholipases and cationic peptides inhibit Cryptosporidium parvum sporozoite infectivity by parasiticidal and non-parasiticidal mechanisms. J. Parasitol. 2012, 98, 199–204. [Google Scholar] [CrossRef]
- Schaap, P.; Schilde, C. Encystation: The most prevalent and underinvestigated differentiation pathway of eukaryotes. Microbiology 2018, 164, 727–739. [Google Scholar] [CrossRef]
- Al-Sabi, M.N.; Gad, J.A.; Riber, U.; Kurtzhals, J.A.; Enemark, H.L. New filtration system for efficient recovery of waterborne Cryptosporidium oocysts and Giardia cysts. J. Appl. Microbiol. 2015, 119, 894–903. [Google Scholar] [CrossRef]
- Soliman, A.; El-Adawy, A.; Abd El-Aal, A.A.; Elmallawany, M.A.; Nahnoush, R.K.; Eiaghni, A.R.A.; Negm, M.S.; Mohsen, A. Usefulness of sunlight and artificial UV radiation versus chlorine for the inactivation of Cryptosporidium oocysts: An in vivo animal study. Open Access Maced. J. Med Sci. 2018, 6, 975–981. [Google Scholar] [CrossRef]
- La Hoz, R.M.; Morris, M.I.; AST Infectious Diseases Community of Practice. Intestinal parasites including Cryptosporidium, Cyclospora, Giardia, and Microsporidia, Entamoeba histolytica, Strongyloides, schistosomiasis, and Echinococcus: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transpl. 2019, e13618. [Google Scholar] [CrossRef]
- Freitas, M.A.; Vianna, E.N.; Martins, A.S.; Silva, E.F.; Pesquero, J.L.; Gomes, M.A. A single step duplex PCR to distinguish Entamoeba histolytica from Entamoeba dispar. Parasitology 2004, 128, 625–628. [Google Scholar] [CrossRef]
- Roy, S.; Kabir, M.; Mondal, D.; Ali, I.K.; Petri, W.A., Jr.; Haque, R. Real-time-PCR assay for diagnosis of Entamoeba histolytica infection. J. Clin. Microbiol. 2005, 43, 2168–2172. [Google Scholar] [CrossRef]
- Parcina, M.; Reiter-Owona, I.; Mockenhaupt, F.P.; Vojvoda, V.; Gahutu, J.B.; Hoerauf, A.; Ignatius, R. Highly sensitive and specific detection of Giardia duodenalis, Entamoeba histolytica, and Cryptosporidium spp. in human stool samples by the BD MAX Enteric Parasite Panel. Parasitol. Res. 2018, 117, 447–451. [Google Scholar] [CrossRef]
- Leitsch, D. A review on metronidazole: An old warhorse in antimicrobial chemotherapy. Parasitology 2017, 1–12. [Google Scholar] [CrossRef]
- Adagu, I.S.; Nolder, D.; Warhurst, D.C.; Rossignol, J.F. In vitro activity of nitazoxanide and related compounds against isolates of Giardia intestinalis, Entamoeba histolytica and Trichomonas vaginalis. J. Antimicrob. Chemother. 2002, 49, 103–111. [Google Scholar] [CrossRef]
- Hemphill, A.; Müller, N.; Müller, J. Thiazolides, a novel class of anti-infective drugs, effective against viruses, bacteria, intracellular and extracellular protozoan parasites and proliferating mammalian cells. Antiinfect. Agents 2013, 11, 22–30. [Google Scholar] [CrossRef]
- Leitsch, D. Drug resistance in the microaerophilic parasite Giardia lamblia. Curr. Trop. Med. Rep. 2015, 2, 128–135. [Google Scholar] [CrossRef]
- Pungpak, S.; Singhasivanon, V.; Bunnag, D.; Radomyos, B.; Nibaddhasopon, P.; Harinasuta, K.T. Albendazole as a treatment for Giardia infection. Ann. Trop Med. Parasitol 1996, 90, 563–565. [Google Scholar] [CrossRef]
- Müller, J.; Hemphill, A.; Müller, N. Treatment of giardiasis and drug resistance. In Giardia: A Model Organism; Luján, H., Svärd, S., Eds.; Springer: Wien, Austria; New York, NY, USA, 2011; Available online: https://pdfs.semanticscholar.org/bca9/a2549ee99e86164d17471aa42d1de5e83adf.pdf (accessed on 25 July 2019).
- Bansal, D.; Malla, N.; Mahajan, R.C. Drug resistance in amoebiasis. Indian J. Med. Res. 2006, 123, 115–118. [Google Scholar]
- Müller, J.; Hemphill, A. New approaches for the identification of drug targets in protozoan parasites. Int. Rev. Cell Mol. Biol. 2013, 301, 359–401. [Google Scholar] [CrossRef]
- Ali, V.; Nozaki, T. Current therapeutics, their problems, and sulfur-containing-amino-acid metabolism as a novel target against infections by “amitochondriate” protozoan parasites. Clin. Microbiol. Rev. 2007, 20, 164–187. [Google Scholar] [CrossRef]
- Ramos-Martinez, E.; Olivos-Garcia, A.; Saavedra, E.; Nequiz, M.; Sanchez, E.C.; Tello, E.; El-Hafidi, M.; Saralegui, A.; Pineda, E.; Delgado, J.; et al. Entamoeba histolytica: Oxygen resistance and virulence. Int. J. Parasitol. 2009, 39, 693–702. [Google Scholar] [CrossRef]
- Arias, D.G.; Regner, E.L.; Iglesias, A.A.; Guerrero, S.A. Entamoeba histolytica thioredoxin reductase: Molecular and functional characterization of its atypical properties. Biochim. Biophys. Acta 2012, 1820, 1859–1866. [Google Scholar] [CrossRef]
- Debnath, A.; Parsonage, D.; Andrade, R.M.; He, C.; Cobo, E.R.; Hirata, K.; Chen, S.; Garcia-Rivera, G.; Orozco, E.; Martinez, M.B.; et al. A high-throughput drug screen for Entamoeba histolytica identifies a new lead and target. Nat. Med. 2012, 18, 956–960. [Google Scholar] [CrossRef]
- Andrade, R.M.; Reed, S.L. New drug target in protozoan parasites: The role of thioredoxin reductase. Front. Microbiol. 2015, 6, 975. [Google Scholar] [CrossRef]
- Gargala, G.; Francois, A.; Favennec, L.; Rossignol, J.F. Activity of halogeno-thiazolides against Cryptosporidium parvum in experimentally infected immunosuppressed gerbils (Meriones unguiculatus). Antimicrob. Agents Chemother. 2013, 57, 2821–2823. [Google Scholar] [CrossRef]
- Masur, H.; Brooks, J.T.; Benson, C.A.; Holmes, K.K.; Pau, A.K.; Kaplan, J.E.; National Institutes of Health; Centers for Disease Control and Prevention; HIV Medicine Association of the Infectious Diseases Society of America. Prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: Updated guidelines from the Centers for Disease Control and Prevention, National Institutes of Health, and HIV Medicine Association of the Infectious Diseases Society of America. Clin. Infect. Dis. 2014, 58, 1308–1311. [Google Scholar] [CrossRef]
- Rossignol, J.F.; Ayoub, A.; Ayers, M.S. Treatment of diarrhea caused by Cryptosporidium parvum: A prospective randomized, double-blind, placebo-controlled study of Nitazoxanide. J. Infect. Dis. 2001, 184, 103–106. [Google Scholar] [CrossRef]
- Rossignol, J.F.; Kabil, S.M.; El-Gohary, Y.; Younis, A.M. Effect of nitazoxanide in diarrhea and enteritis caused by Cryptosporidium species. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2006, 4, 320–324. [Google Scholar] [CrossRef]
- Abubakar, I.; Aliyu, S.H.; Arumugam, C.; Hunter, P.R.; Usman, N.K. Prevention and treatment of cryptosporidiosis in immunocompromised patients. Cochrane Database Syst. Rev. 2007, CD004932. [Google Scholar] [CrossRef]
- Amadi, B.; Mwiya, M.; Sianongo, S.; Payne, L.; Watuka, A.; Katubulushi, M.; Kelly, P. High dose prolonged treatment with nitazoxanide is not effective for cryptosporidiosis in HIV positive Zambian children: A randomised controlled trial. BMC Infect. Dis. 2009, 9, 195. [Google Scholar] [CrossRef]
- Chavez, M.A.; White, A.C., Jr. Novel treatment strategies and drugs in development for cryptosporidiosis. Expert Rev. Anti Infect. Ther. 2018, 16, 1–7. [Google Scholar] [CrossRef]
- Johnson, C.R.; Gorla, S.K.; Kavitha, M.; Zhang, M.; Liu, X.; Striepen, B.; Mead, J.R.; Cuny, G.D.; Hedstrom, L. Phthalazinone inhibitors of inosine-5′-monophosphate dehydrogenase from Cryptosporidium parvum. Bioorg. Med. Chem. Lett. 2013, 23, 1004–1007. [Google Scholar] [CrossRef]
- Hulverson, M.A.; Vinayak, S.; Choi, R.; Schaefer, D.A.; Castellanos-Gonzalez, A.; Vidadala, R.S.R.; Brooks, C.F.; Herbert, G.T.; Betzer, D.P.; Whitman, G.R.; et al. Bumped-kinase inhibitors for cryptosporidiosis therapy. J. Infect. Dis. 2017, 215, 1275–1284. [Google Scholar] [CrossRef]
- Manjunatha, U.H.; Vinayak, S.; Zambriski, J.A.; Chao, A.T.; Sy, T.; Noble, C.G.; Bonamy, G.M.C.; Kondreddi, R.R.; Zou, B.; Gedeck, P.; et al. A Cryptosporidium PI(4)K inhibitor is a drug candidate for cryptosporidiosis. Nature 2017, 546, 376–380. [Google Scholar] [CrossRef]
- Rivero, F.D.; Saura, A.; Prucca, C.G.; Carranza, P.G.; Torri, A.; Lujan, H.D. Disruption of antigenic variation is crucial for effective parasite vaccine. Nat. Med. 2010, 16, 551–557. [Google Scholar] [CrossRef]
- Harp, J.A.; Goff, J.P. Protection of calves with a vaccine against Cryptosporidium parvum. J. Parasitol. 1995, 81, 54–57. [Google Scholar] [CrossRef]
- Lillehoj, E.P.; Yun, C.H.; Lillehoj, H.S. Vaccines against the avian enteropathogens Eimeria, Cryptosporidium and Salmonella. Anim. Health Res. Rev. 2000, 1, 47–65. [Google Scholar] [CrossRef]
- Askari, N.; Shayan, P.; Mokhber-Dezfouli, M.R.; Ebrahimzadeh, E.; Lotfollahzadeh, S.; Rostami, A.; Amininia, N.; Ragh, M.J. Evaluation of recombinant P23 protein as a vaccine for passive immunization of newborn calves against Cryptosporidium parvum. Parasite Immunol. 2016, 38, 282–289. [Google Scholar] [CrossRef]
- Yang, Y.; Xue, X.; Yang, Y.; Chen, X.; Du, A. Efficacy of a potential DNA vaccine encoding Cryptosporidium baileyi rhomboid protein against homologous challenge in chickens. Vet. Parasitol. 2016, 225, 5–11. [Google Scholar] [CrossRef]
- Haserick, J.R.; Klein, J.A.; Costello, C.E.; Samuelson, J. Cryptosporidium parvum vaccine candidates are incompletely modified with O-linked-N-acetylgalactosamine or contain N-terminal N-myristate and S-palmitate. PLoS ONE 2017, 12, e0182395. [Google Scholar] [CrossRef]
- Mead, J.R. Challenges and prospects for a Cryptosporidium vaccine. Future Microbiol. 2010, 5, 335–337. [Google Scholar] [CrossRef]
- Quach, J.; St-Pierre, J.; Chadee, K. The future for vaccine development against Entamoeba histolytica. Hum. Vaccines Immunother. 2014, 10, 1514–1521. [Google Scholar] [CrossRef]
- Lotter, H.; Tannich, E. The galactose-inhibitable surface lectin of Entamoeba histolytica, a possible candidate for a subunit vaccine to prevent amoebiasis. Behring Inst. Mitt. 1997, 99, 112–116. [Google Scholar]
- Mann, B.J.; Burkholder, B.V.; Lockhart, L.A. Protection in a gerbil model of amebiasis by oral immunization with Salmonella expressing the galactose/N-acetyl D-galactosamine inhibitable lectin of Entamoeba histolytica. Vaccine 1997, 15, 659–663. [Google Scholar] [CrossRef]
- Ivory, C.P.; Chadee, K. Intranasal immunization with Gal-inhibitable lectin plus an adjuvant of CpG oligodeoxynucleotides protects against Entamoeba histolytica challenge. Infect. Immun. 2007, 75, 4917–4922. [Google Scholar] [CrossRef]
- Abd Alla, M.D.; Wolf, R.; White, G.L.; Kosanke, S.D.; Cary, D.; Verweij, J.J.; Zhang, M.J.; Ravdin, J.I. Efficacy of a Gal-lectin subunit vaccine against experimental Entamoeba histolytica infection and colitis in baboons (Papio sp.). Vaccine 2012, 30, 3068–3075. [Google Scholar] [CrossRef]
- Barroso, L.; Abhyankar, M.; Noor, Z.; Read, K.; Pedersen, K.; White, R.; Fox, C.; Petri, W.A., Jr.; Lyerly, D. Expression, purification, and evaluation of recombinant LecA as a candidate for an amebic colitis vaccine. Vaccine 2014, 32, 1218–1224. [Google Scholar] [CrossRef][Green Version]
- Chaudhry, O.A.; Petri, W.A., Jr. Vaccine prospects for amebiasis. Expert Rev. Vaccines 2005, 4, 657–668. [Google Scholar] [CrossRef]
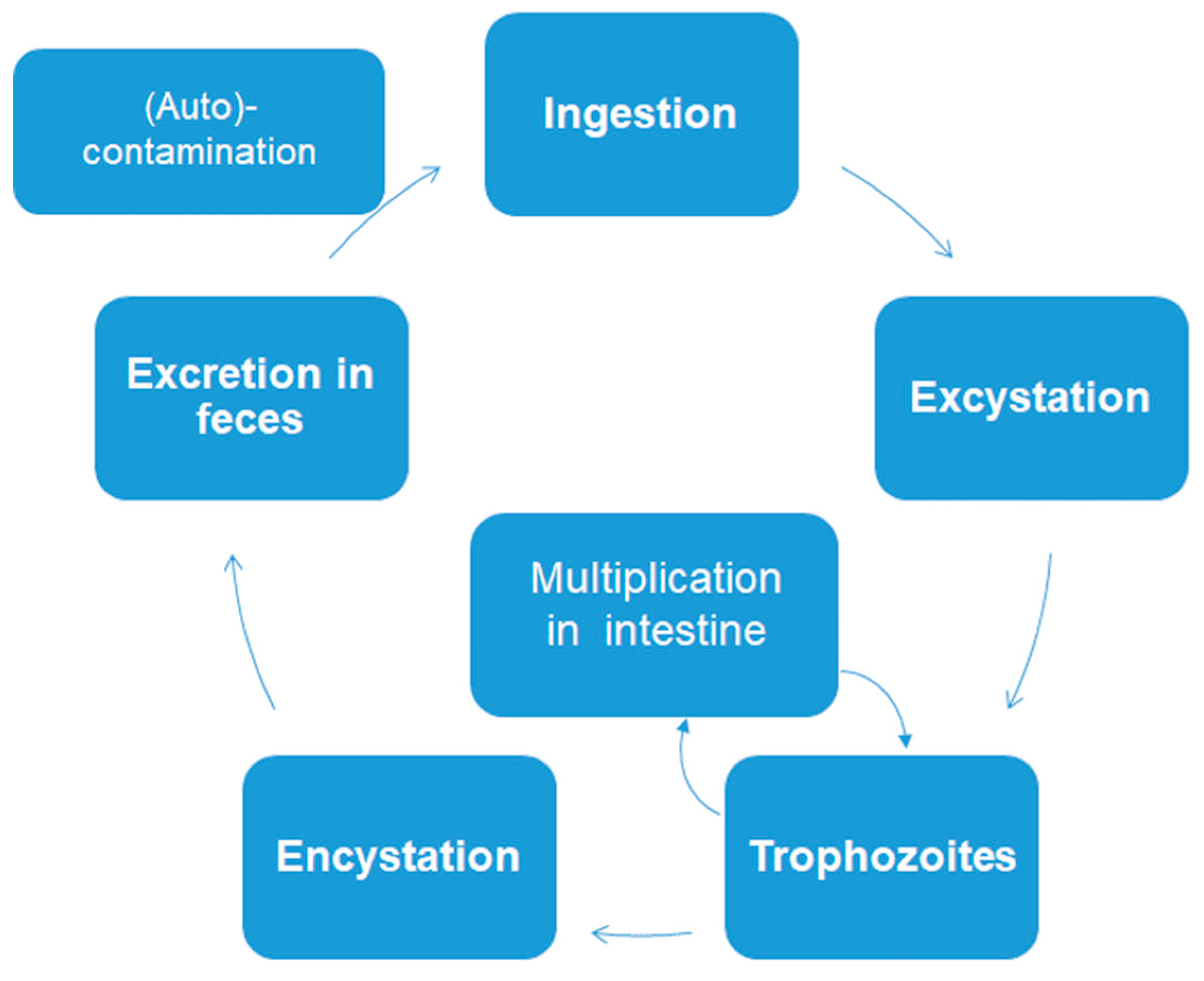
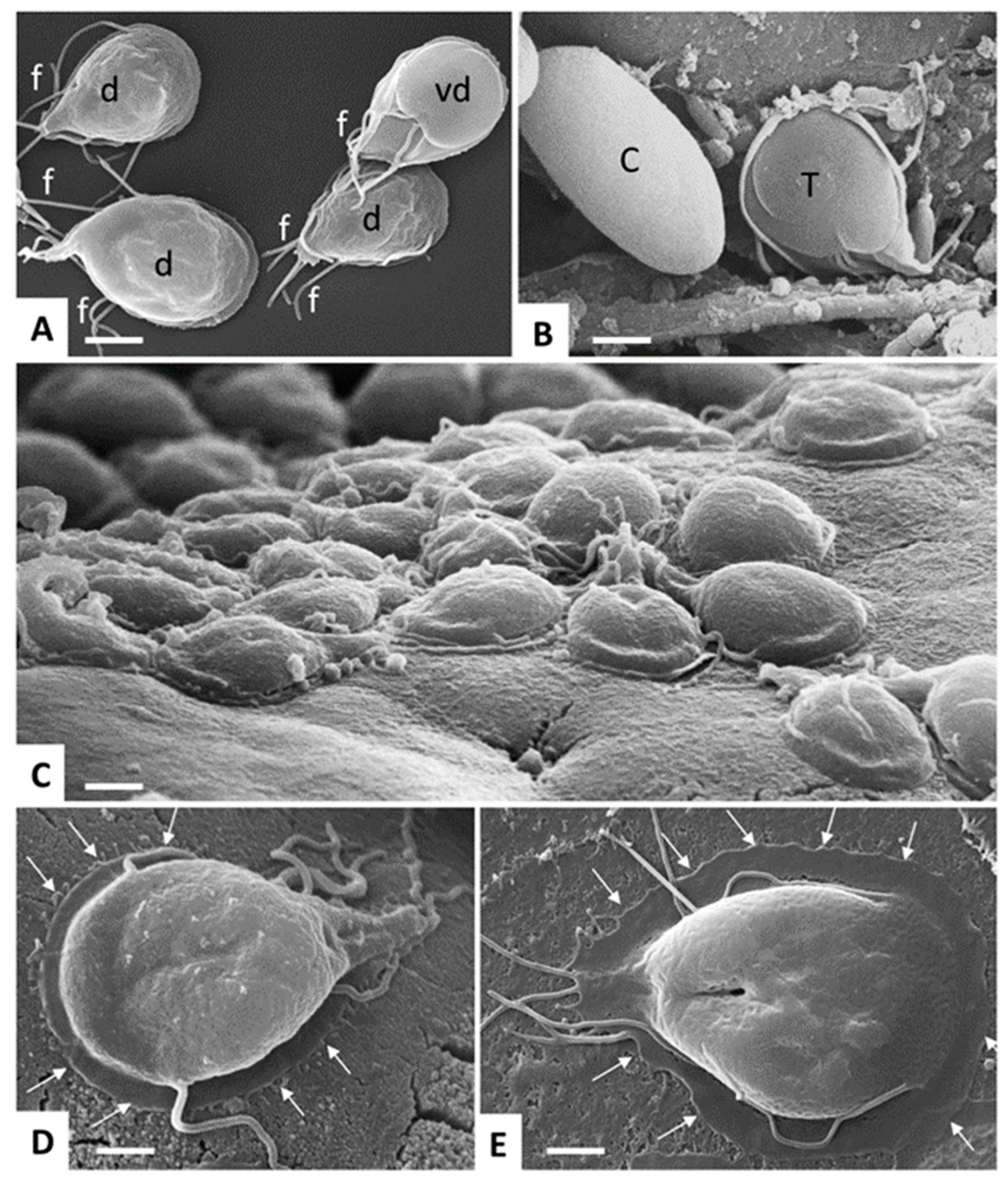
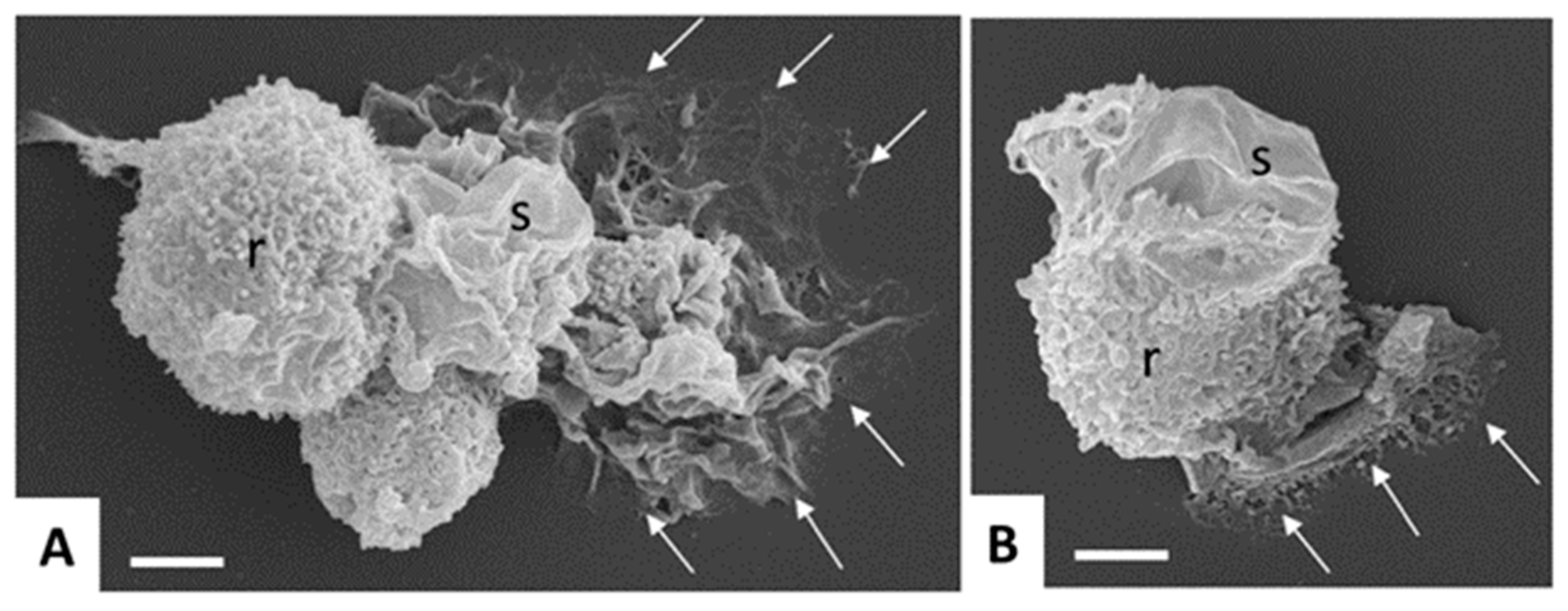
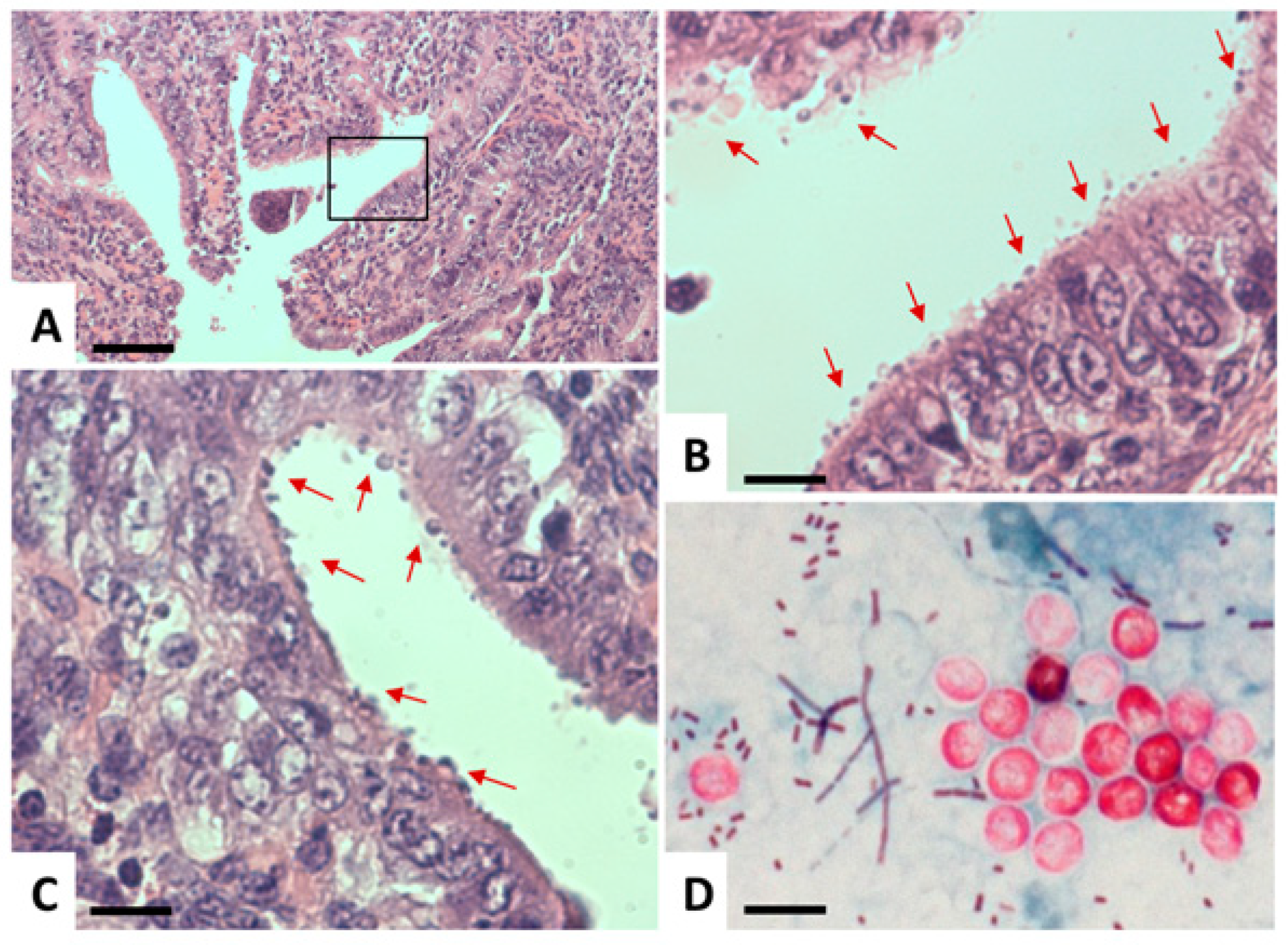
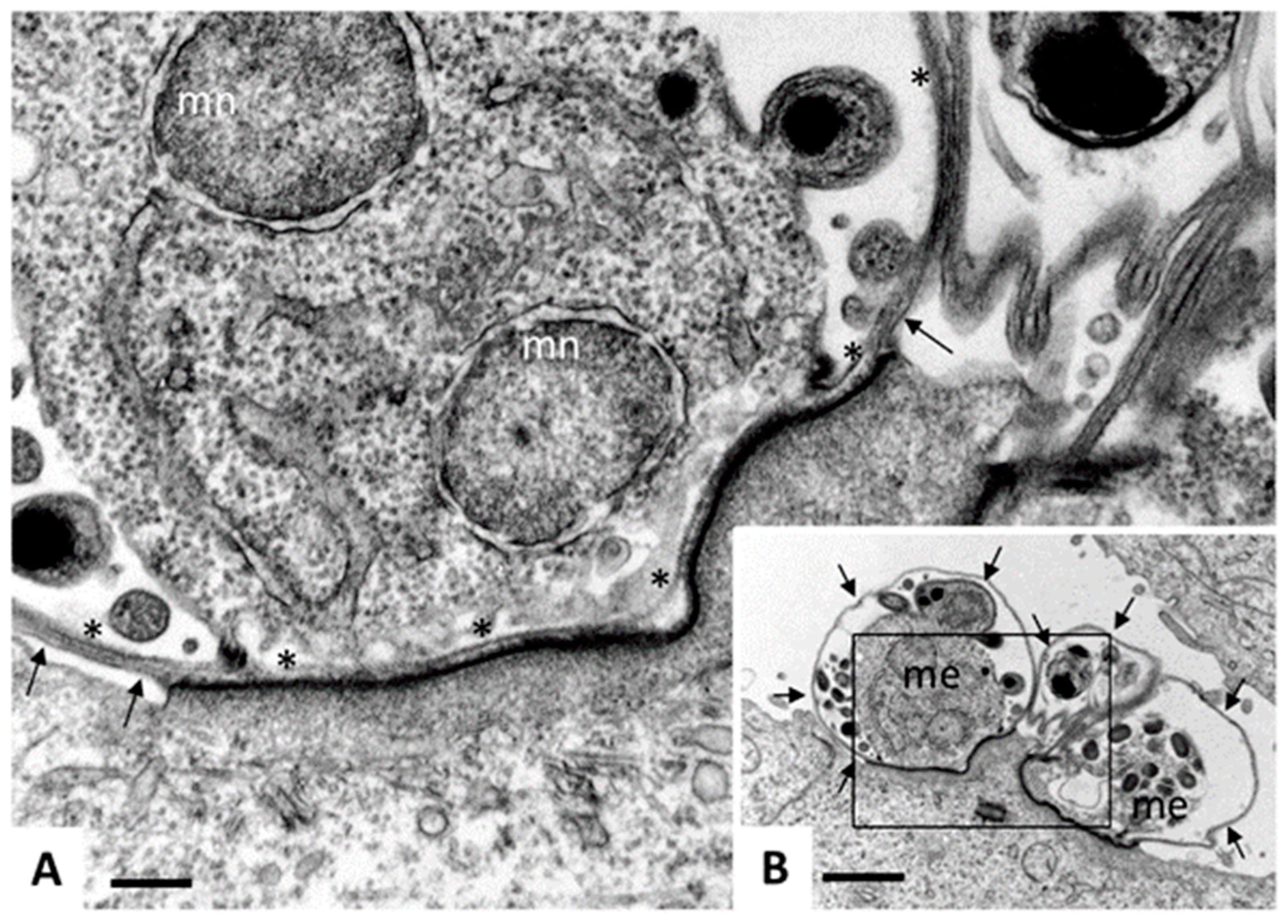
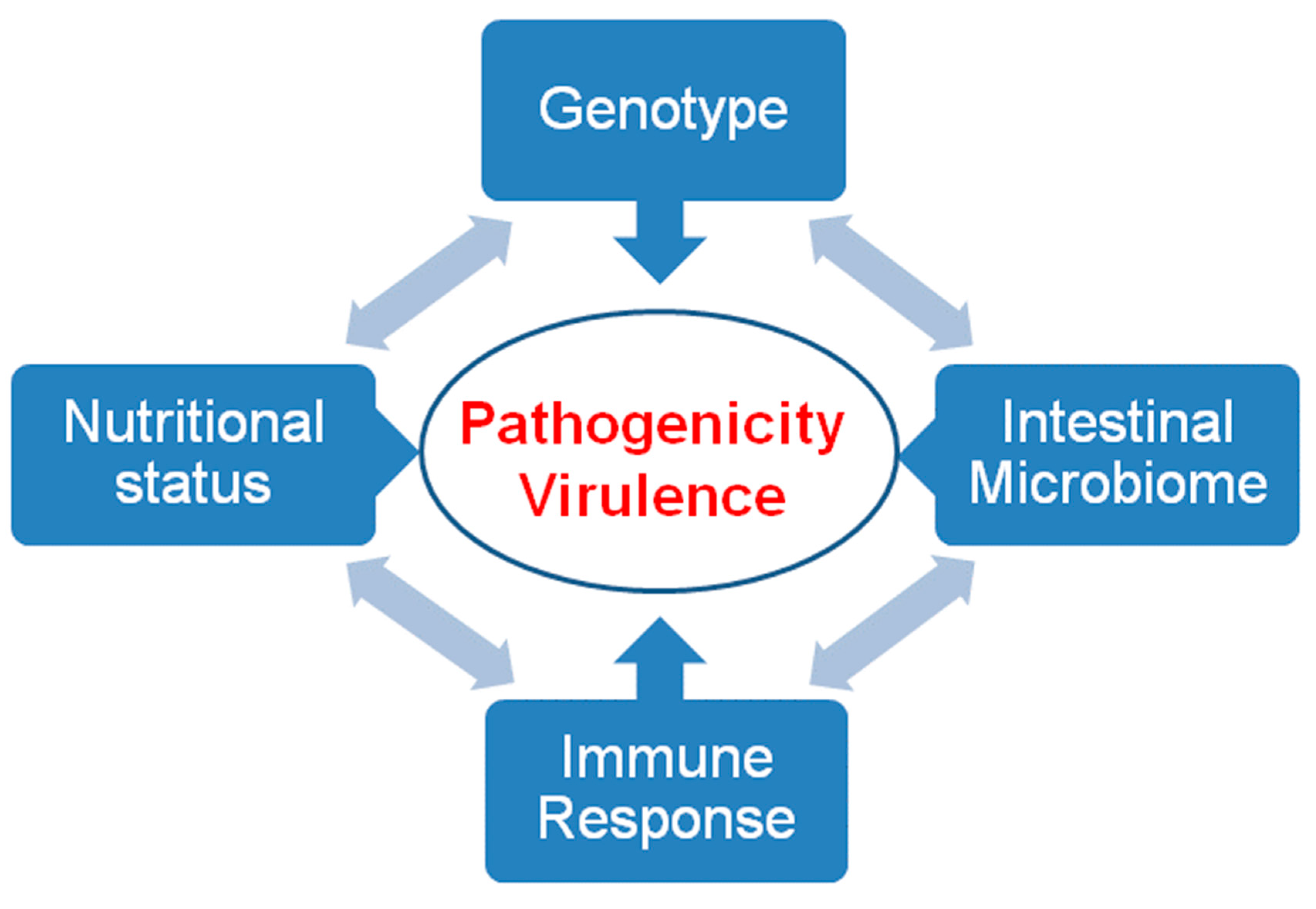
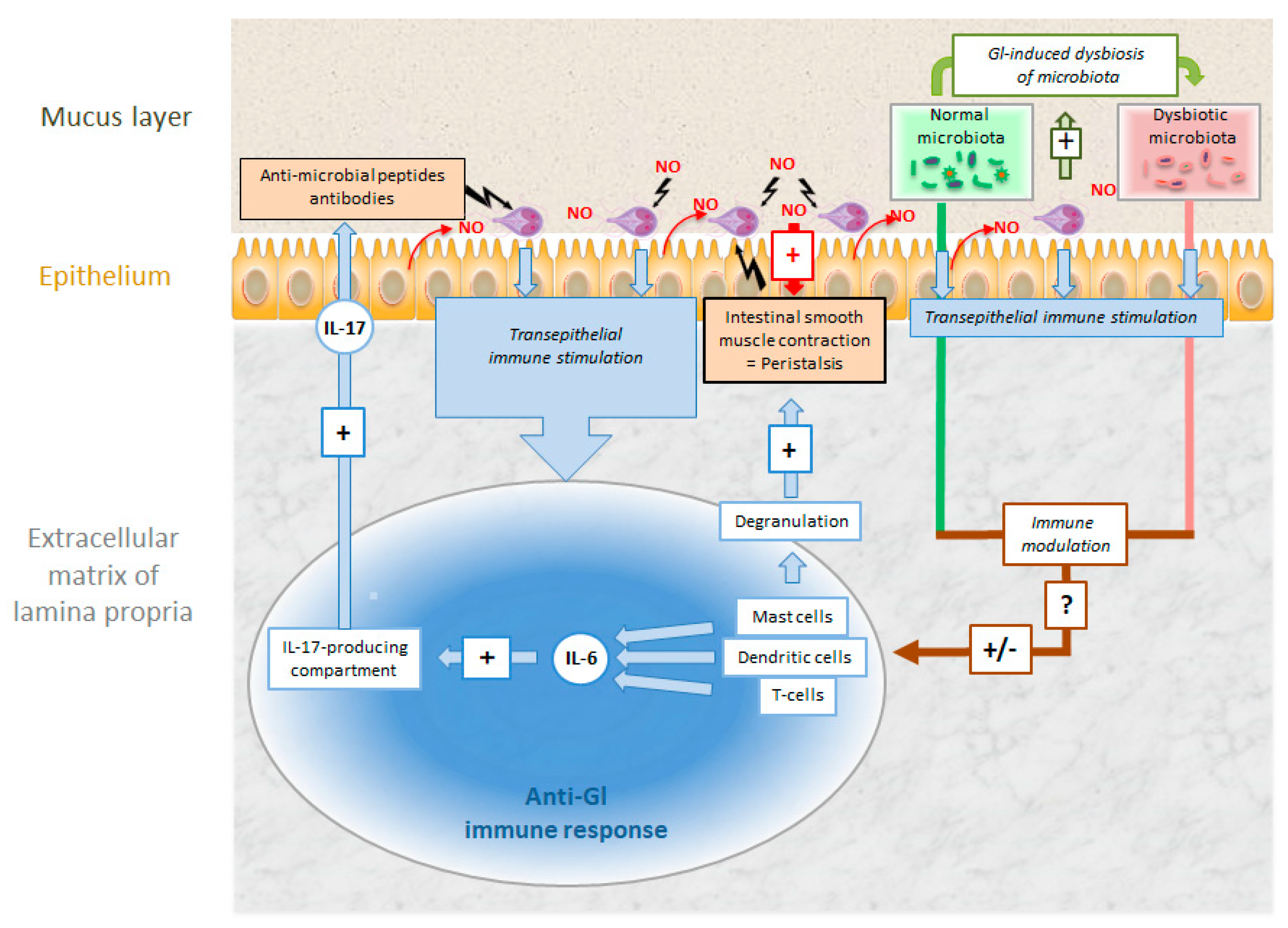
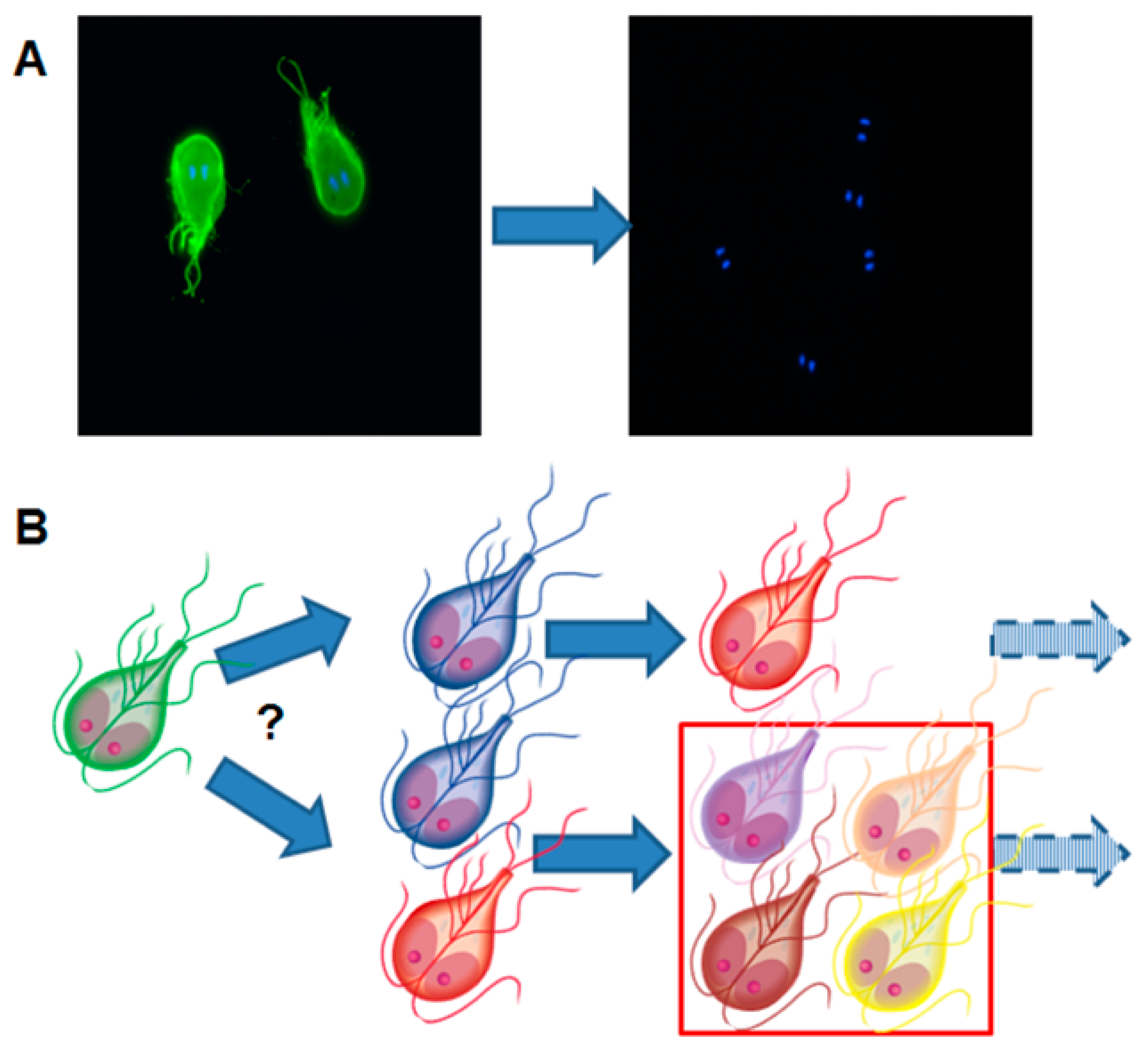
| Species | Classification (Super Groups) | Incidence | Pathogenicity | Localization | ||
| World 1 | US 2 | EU 3 | ||||
| Balantidium coli | Cliliata (Diaphoretickes) | Rare | colon | |||
| Blastocystis sp. | Stramenopile (Diaphoretickes) | Very high | opportunistic (?) | colon | ||
| Cryptosporidium parvum | Apicomplexa (Diaphoretickes) | nk | 8–9 | 7 | obligate | duodenum, jejunum, ileum |
| Dientamoeba fragilis | Trichomonadina (Excavata) | Common | unclear, most likely same as G. lamblia | colon | ||
| Entamoeba histolytica | Amoebozoa (Amorphea) | 100 | rare | rare | obligate | colon, liver |
| Giardia lamblia | Diplomonadida (Excavata) | 250 | 15 | 18 | obligate | duodenum, jejunum, ileum |
| Microsporidia sp. | Fungi (Amorphea) | Very high | opportunistic | colon | ||
| Giardiasis | Amebiasis | Cryptosporidiosis | |
|---|---|---|---|
| Pathogen | Giardia lamblia | Entamoeba histolytica | Cryptosporidium parvum |
| Transmission | Via (Oo)cysts in feces | ||
| Symptoms | |||
| Acute | Persistent diarrhea (>1 w), malabsorption. | Diarrhea, abdominal pain. | Mild-to-acute diarrhea, nausea, abdominal pain, low-grade fever. |
| Chronic | Malabsorption, loose stools, gassiness, cramping, fatigue, liver or pancreatic inflammations. | Fever, sepsis, liver abscesses, skin lesions. | Severe diarrhea, vomiting, malabsorption, volume depletion and wasting, biliary and respiratory involvement in immunodeficient persons. |
| Diagnosis | |||
| Feces Biopsy material | Microscopy (cysts), coproantigen test, PCR. | Microscopy (trophozoites, cysts), coproantigen test and PCR. | Microscopy, coproantigen test, PCR, enzyme-immunoassays. |
| Serology | Positive in the case of extraintestinal infection. | ||
| Differential Diagnosis | Cryptosporidiosis, IBS, celiac. | IBD, cancer, bacterial infections. | Giardiasis, Rotavirus, Cyclospora cayetanensis, Clostridium difficile. Microsporidia, IBS, celiac. |
| Management | |||
| First line treatment | Metronidazole (500 to 750 mg p.o. t.i.d., 10 d) | Immunocompetent: NTZ (nitazoxanide)100–500 mg p.o. twice daily, 3 d. HIV: Antiretroviral therapy, possibly combined with NTZ or paromomycin/azithromycin. Other immunodeficiencies: NTZ 500 mg twice daily, 14 d. | |
| Prevention | Personal hygiene, water treatment, appropriate cleaning and storage of vegetables. | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hemphill, A.; Müller, N.; Müller, J. Comparative Pathobiology of the Intestinal Protozoan Parasites Giardia lamblia, Entamoeba histolytica, and Cryptosporidium parvum. Pathogens 2019, 8, 116. https://doi.org/10.3390/pathogens8030116
Hemphill A, Müller N, Müller J. Comparative Pathobiology of the Intestinal Protozoan Parasites Giardia lamblia, Entamoeba histolytica, and Cryptosporidium parvum. Pathogens. 2019; 8(3):116. https://doi.org/10.3390/pathogens8030116
Chicago/Turabian StyleHemphill, Andrew, Norbert Müller, and Joachim Müller. 2019. "Comparative Pathobiology of the Intestinal Protozoan Parasites Giardia lamblia, Entamoeba histolytica, and Cryptosporidium parvum" Pathogens 8, no. 3: 116. https://doi.org/10.3390/pathogens8030116
APA StyleHemphill, A., Müller, N., & Müller, J. (2019). Comparative Pathobiology of the Intestinal Protozoan Parasites Giardia lamblia, Entamoeba histolytica, and Cryptosporidium parvum. Pathogens, 8(3), 116. https://doi.org/10.3390/pathogens8030116





