Serological Data Shows Low Levels of Chikungunya Exposure in Senegalese Nomadic Pastoralists
Abstract
1. Introduction
2. Results
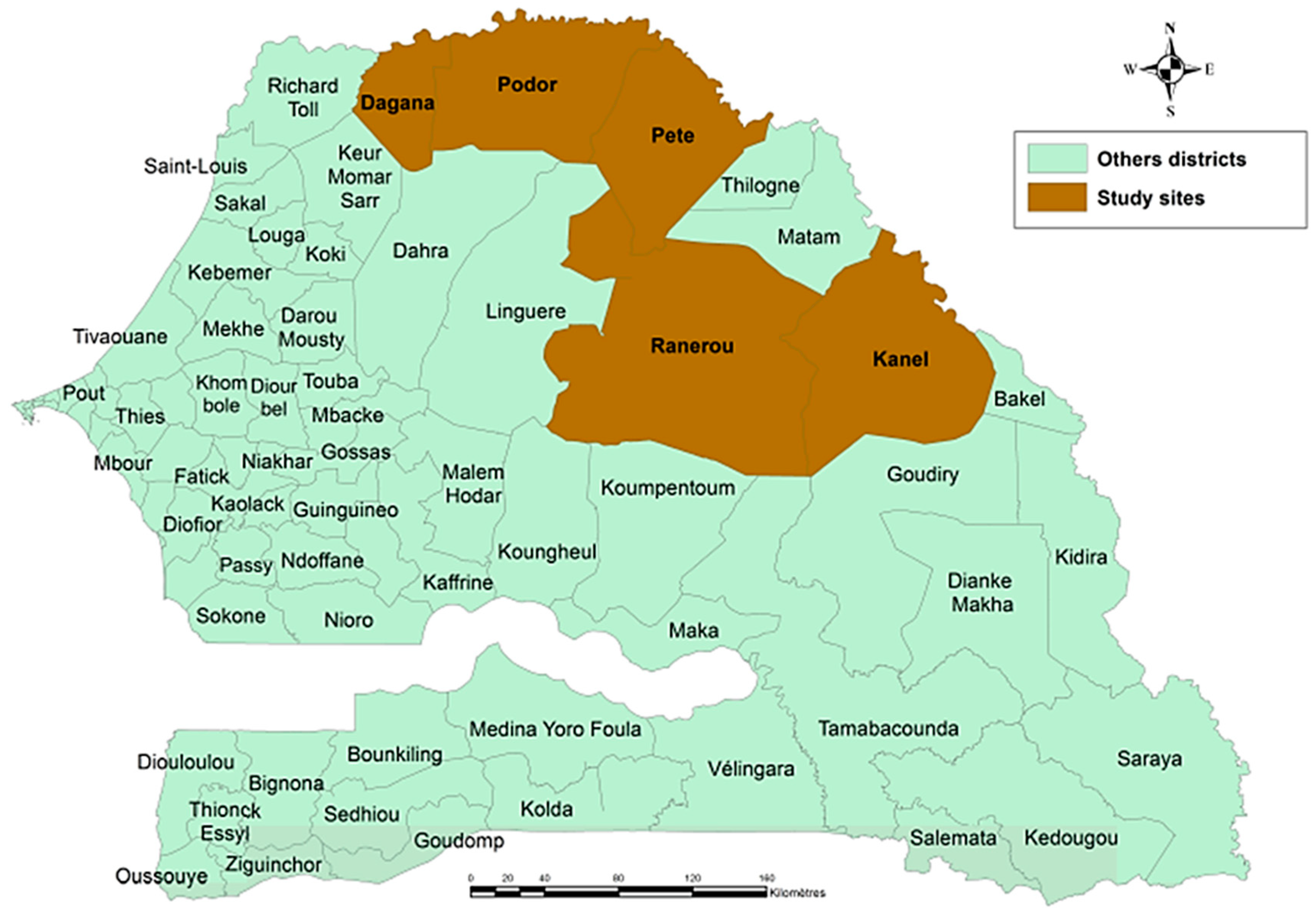
2.1. Study Population and Sampling Locations in Senegal
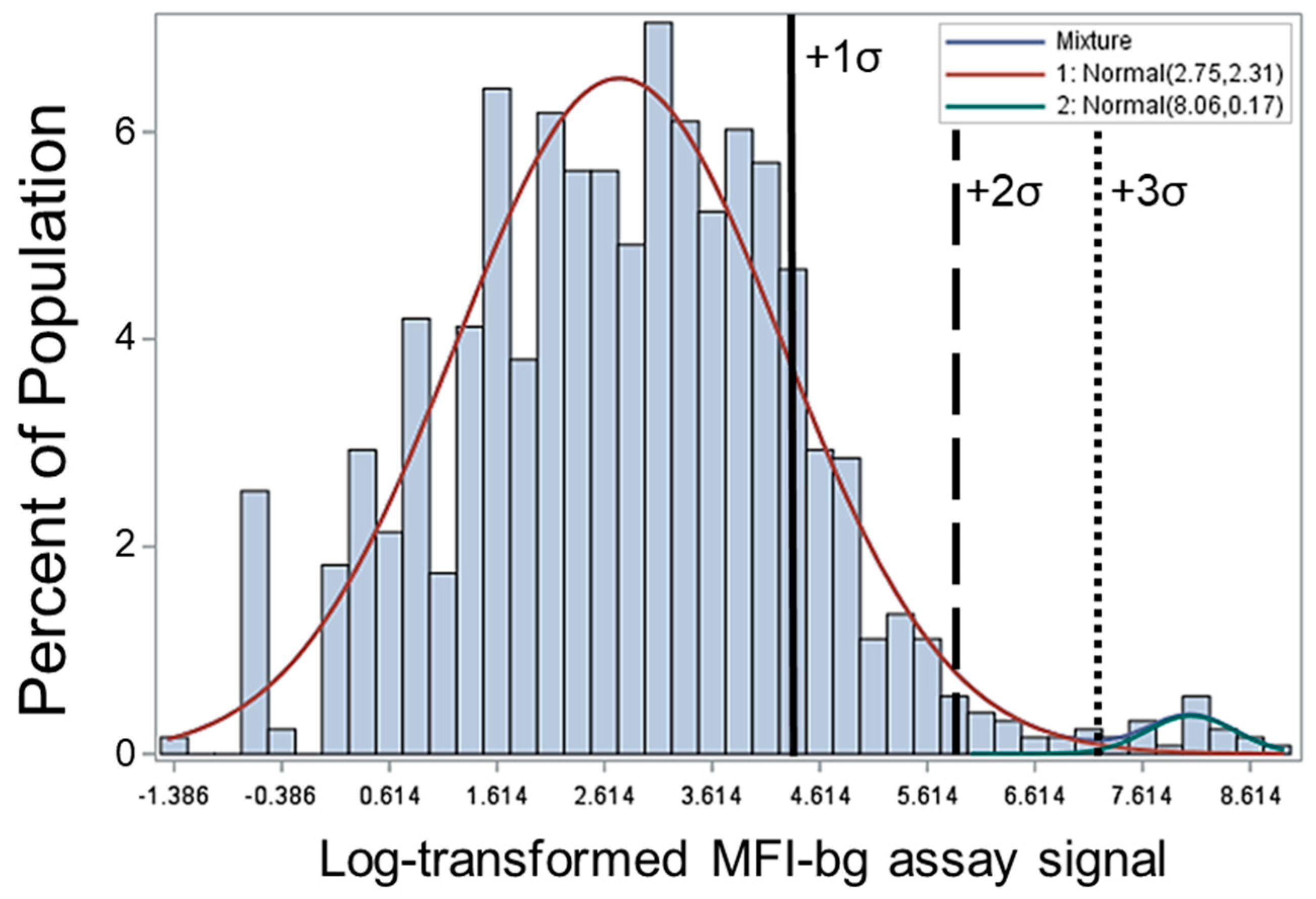
2.2. Range of IgG Responses to CHIKV E1 Antigen and Seropositivity Definition
2.3. Correlation of Seropositivity and MFI-bg Assay Signal with Age
2.4. Adjusted Odds Ratio (aOR) Estimates for Anti-CHIKV Seropositivity Based on Age, Gender, and Bednet Ownership and Usage
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Study Population, Survey, and Dried Blood Spot Sample Collection
4.3. CHIKV E1 Antigen Coupling to Beads
4.4. Sample Processing, Blood Elution, and Anti-CHIKV E1 IgG Immunoassay
4.5. Statistics
| Logit P (chikpos =1) = β0 + β1(age(years)) + β2(net ownership) + β3(net use previous night) + β4(net use every night) + β5(sex). |
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Tilston, N.; Skelly, C.; Weinstein, P. Pan-European Chikungunya surveillance: Designing risk stratified surveillance zones. Int. J. Health Geogr. 2009, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- Viennet, E.; Knopme, K.; Faddy, H.M.; Williams, C.R.; Harley, D. Assessing the threat of chikungunya virus emergence in Australia. Commun. Dis. Intell. Q. Rep. 2013, 37, E136–E143. [Google Scholar] [PubMed]
- Yamanishi, H.S.T.; Matsumura, T. Multiplication of chikungunya virus in male Culex pipiensmolestus. Jap. J. Sanit. Zool. 1980, 31, 69–70. [Google Scholar] [CrossRef][Green Version]
- Savage, H.M.L.J.; Yug, L.; Burkhalter, K.L.; Marfel, M.; Hancock, W.T. Incrimination of Aedes (Stegomyia) hensilli Farner as an epidemic vector of Chikungunya virus on Yap Island, Federated States of Micronesia, 2013. Am. J. Trop. Med. Hyg. 2015, 92, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Halstead, S.B. Reappearance of chikungunya, formerly called dengue, in the Americas. Emerg. Infect. Dis. 2015, 21, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Thiberville, S.D.; Moyen, N.; Dupuis-Maguiraga, L.; Nougairede, A.; Gould, E.A.; Roques, P.; de Lamballerie, X. Chikungunya fever: Epidemiology, clinical syndrome, pathogenesis and therapy. Antiviral Res. 2013, 99, 345–370. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.W. The Newala epidemic. III. The virus: Isolation, pathogenic properties and relationship to the epidemic. J. Hyg. (London) 1956, 54, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Fritz, M.; Taty Taty, R.; Portella, C.; Guimbi, C.; Mankou, M.; Leroy, E.M.; Becquart, P. Re-emergence of chikungunya in the Republic of the Congo in 2019 associated with a possible vector-host switch. Int. J. Infect. Dis. 2019, 84, 99–101. [Google Scholar] [CrossRef]
- Weetman, D.; Kamgang, B.; Badolo, A.; Moyes, C.L.; Shearer, F.M.; Coulibaly, M.; Pinto, J.; Lambrechts, L.; McCall, P.J. Aedes mosquitoes and aedes-borne arboviruses in Africa: Current and future threats. Int. J. Environ. Res. Public Health 2018, 15, 220. [Google Scholar] [CrossRef]
- Zeller, H.; Van Bortel, W.; Sudre, B. Chikungunya: Its history in Africa and Asia and its spread to new regions in 2013–2014. J. Infect. Dis. 2016, 214 (Suppl. 5), S436–S440. [Google Scholar] [CrossRef]
- Gudo, E.S.; Ali, S.; Antonio, V.S.; Chelene, I.R.; Chongo, I.; Demanou, M.; Guiliche, O.C.; Heinrich, N.; Monteiro, V.; Muianga, A.F.; et al. Seroepidemiological studies of arboviruses in Africa. Adv. Exp. Med. Biol. 2018, 1062, 361–371. [Google Scholar] [PubMed]
- Rogier, E.; Moss, D.; Mace, K.; Chang, M.; Jean, S.; Bullard, S.; Lammie, P.J.; Lemoine, F.J.; Udhayakumar, V. Use of bead-based serologic assay to evaluate chikungunya virus epidemic, Haiti. Emerg. Infect. Dis. J. 2018, 24. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, M.J.; de Souza, W.M.; Esposito, D.L.A.; Silva, A.; Romeiro, M.F.; Martinez, E.Z.; Lopes da Fonesca, B.A.; Figueiredo, L.T.M. Enzyme-linked immunosorbent assay using recombinant envelope protein 2 antigen for diagnosis of Chikungunya virus. Virol. J. 2018, 15, 112. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, M.J.; de Souza, W.M.; Romeiro, M.F.; de Souza Costa, M.C.; Slhessarenko, R.D.; Figueiredo, L.T.M. Development of an enzyme-linked immunosorbent assay to detect antibodies targeting recombinant envelope protein 2 of mayaro virus. J. Clin. Microbiol. 2019, 57, e01892-18. [Google Scholar] [CrossRef] [PubMed]
- Poirier, M.J.; Moss, D.M.; Feeser, K.R.; Streit, T.G.; Chang, G.J.; Whitney, M.; Russell, B.J.; Johnson, B.W.; Basile, A.J.; Goodman, C.H.; et al. Measuring Haitian children’s exposure to chikungunya, dengue and malaria. Bull. World Health Organ. 2016, 94, 817A–825A. [Google Scholar] [CrossRef] [PubMed]
- Roche, S.; Robin, Y. Human infections by Chikungunya virus in Rufisque (Senegal), October–November, 1966. Bull. Soc. Med. Afr. Noire Lang. Fr. 1967, 12, 490–496. [Google Scholar]
- Bres, P.; Camicas, J.L.; Cornet, M.; Robin, Y.; Taufflieb, R. Epidemiology of arbovirus diseases in Senegal. Bull. Soc. Pathol. Exot. Filiales 1969, 62, 253–259. [Google Scholar]
- Althouse, B.M.; Guerbois, M.; Cummings, D.A.T.; Diop, O.M.; Faye, O.; Faye, A.; Diallo, D.; Sadio, B.D.; Sow, A.; Faye, O.; et al. Role of monkeys in the sylvatic cycle of chikungunya virus in Senegal. Nat. Commun. 2018, 9, 1046. [Google Scholar] [CrossRef]
- Sow, A.; Faye, O.; Diallo, M.; Diallo, D.; Chen, R.; Faye, O.; Diagne, C.T.; Guerbois, M.; Weidmann, M.; Ndiaye, Y.; et al. Chikungunya outbreak in Kedougou, Southeastern Senegal in 2009–2010. Open Forum Infect. Dis. 2018, 5, ofx259. [Google Scholar] [CrossRef]
- Lopez-Jimena, B.; Wehner, S.; Harold, G.; Bakheit, M.; Frischmann, S.; Bekaert, M.; Faye, O.; Sall, A.A.; Weidmann, M. Development of a single-tube one-step RT-LAMP assay to detect the Chikungunya virus genome. PLoS Negl. Trop. Dis. 2018, 12, e0006448. [Google Scholar] [CrossRef]
- Richman, R.; Diallo, D.; Diallo, M.; Sall, A.A.; Faye, O.; Diagne, C.T.; Dia, I.; Weaver, S.C.; Hanley, K.A.; Buenemann, M. Ecological niche modeling of Aedes mosquito vectors of chikungunya virus in southeastern Senegal. Parasit Vectors 2018, 11, 255. [Google Scholar] [CrossRef] [PubMed]
- Okabayashi, T.; Sasaki, T.; Masrinoul, P.; Chantawat, N.; Yoksan, S.; Nitatpattana, N.; Chsuri, S.; Morales Vargas, R.E.; Grandadam, M.; Brey, P.T.; et al. Detection of chikungunya virus antigen by a novel rapid immunochromatographic test. J. Clin. Microbiol. 2015, 53, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Sow, A.; Loucoubar, C.; Diallo, D.; Faye, O.; Ndiaye, Y.; Senghor, C.S.; Dia, A.T.; Faye, O.; Weaver, S.C.; Dialo, M.; et al. Concurrent malaria and arbovirus infections in Kedougou, southeastern Senegal. Malar. J. 2016, 15, 47. [Google Scholar] [CrossRef] [PubMed]
- Diallo, D.; Sall, A.A.; Buenemann, M.; Chen, R.; Faye, O.; Diagne, C.T.; Faye, O.; Ba, Y.; Dias, I.; Watts, D.; et al. Landscape ecology of sylvatic chikungunya virus and mosquito vectors in southeastern Senegal. PLoS Negl. Trop. Dis. 2012, 6, e1649. [Google Scholar] [CrossRef] [PubMed]
- Seck, M.C.; Thwing, J.; Fall, F.B.; Gomis, J.F.; Deme, A.; Ndiaye, Y.D.; Daniels, R.; Volkman, S.K.; Ndiop, M.; Ba, M.; et al. Malaria prevalence, prevention and treatment seeking practices among nomadic pastoralists in northern Senegal. Malar. J. 2017, 16, 413. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Kaneko, S.; Nzou, S.M.; Mwau, M.; Njenga, S.M.; Tanigawa, C.; Kimptho, J.; Mwangi, A.W.; Kiche, I.; Matsumoto, S.; et al. Serological surveillance development for tropical infectious diseases using simultaneous microsphere-based multiplex assays and finite mixture models. PLoS Negl. Trop. Dis. 2014, 8, e3040. [Google Scholar] [CrossRef] [PubMed]
- Mason, P.J.; Haddow, A.J. An epidemic of virus disease in Southern Province, Tanganyika Territory, in 1952-53; an additional note on Chikungunya virus isolations and serum antibodies. Trans. R. Soc. Trop. Med. Hyg. 1957, 51, 238–240. [Google Scholar] [CrossRef]
- Weinbren, M.P.; Haddow, A.J.; Williams, M.C. The occurrence of Chikungunya virus in Uganda. I. Isolation from mosquitoes. Trans. R. Soc. Trop. Med. Hyg. 1958, 52, 253–257. [Google Scholar] [CrossRef]
- Osterrieth, P.; Blanes-Ridaura, G. Research on the Chikungunya virus in the Belgian Congo. I. Isolation of the virus in upper Uele. Ann. Soc. Belg. Med. Trop. (1920) 1960, 40, 199–203. [Google Scholar]
- Weaver, S.C.; Lecuit, M. Chikungunya virus and the global spread of a mosquito-borne disease. N. Engl. J. Med. 2015, 372, 1231–1239. [Google Scholar] [CrossRef]
- Silva, J.V.J., Jr.; Ludwig-Begall, L.F.; Oliveira-Filhom, E.F.; Oliveira, R.A.S.; Duraes-Carvalho, R.; Lopes, T.R.R.; Silva, D.E.A.; Gil, L.H.V.G. A scoping review of Chikungunya virus infection: Epidemiology, clinical characteristics, viral co-circulation complications, and control. Acta Trop. 2018, 188, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, I.S.B.; Tanabe, E.L.L.; Santos, E.C.; Martins, W.V.; Araujo, I.; Cavalcante, M.C.A.; Lima, A.R.V.; Camara, N.O.S.; Anderson, L.; Yunusov, D.; et al. Cellular and Molecular Immune Response to Chikungunya Virus Infection. Front. Cell. Infect. Microbiol. 2018, 8, 345. [Google Scholar] [CrossRef] [PubMed]
- Delynn, M.; Moss, M.T.W.; Chard, A.N.; Trinies, V.; Doumbia, S.; Goodman, C.H.; Bullard, S.; Wiegand, R.E.; Freeman, M.C.; Lammie, P.J.; et al. Serological Evidence of Dengue and Chikungunya Exposures in Malian children by multiplex bead assay. Int. J. Trop. Dis. 2018, 1, 1–8. [Google Scholar] [CrossRef]
- Yathi, K.K.; Joseph, J.M.; Bhasker, S.; Kumar, R.; Chinnamma, M. Recombinant CHIK virus E1 coat protein of 11 KDa with antigenic domains for the detection of Chikungunya. J. Immunol. Methods 2011, 372, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Yap, G.; Pok, K.Y.; Lai, Y.L.; Hapuarachchi, H.C.; Chow, A.; Leo, Y.S.; Li, K.-T.; Lee, C.N. Evaluation of Chikungunya diagnostic assays: Differences in sensitivity of serology assays in two independent outbreaks. PLoS Negl. Trop. Dis. 2010, 4, e753. [Google Scholar] [CrossRef] [PubMed]
- Rogier, E.; Wiegand, R.; Moss, D.; Priest, J.; Angov, E.; Dutta, S.; Journel, I.; Jean, S.E.; Mace, K.; Chang, M.; et al. Multiple comparisons analysis of serological data from an area of low Plasmodium falciparum transmission. Malar. J. 2015, 14, 436. [Google Scholar] [CrossRef] [PubMed]
- Mint Lekweiry, K.; Ould Ahmedou Salem, M.S.; Ould Brahim, K.; Ould Lemrabott, M.A.; Brengues, C.; Faye, O.; Simard, F.; Mohamed, A.O.; Boukhary, S. Aedes aegypti (Diptera: Culicidae) in Mauritania: First report on the presence of the arbovirus mosquito vector in Nouakchott. J. Med. Entomol. 2015, 52, 730–733. [Google Scholar] [CrossRef]
- Zahouli, J.B.Z.; Koudou, B.G.; Muller, P.; Malone, D.; Tano, Y.; Utzinger, J. Effect of land-use changes on the abundance, distribution, and host-seeking behavior of Aedes arbovirus vectors in oil palm-dominated landscapes, southeastern Cote d’Ivoire. PLoS ONE 2017, 12, e0189082. [Google Scholar] [CrossRef]
- Casas Martinez, M.; Orozco Bonilla, A.; Munoz Reyes, M.; Ulloa Garcia, A.; Bond, J.G.; Valle Mora, J.; Weber, M.; Rojas, J.C. A new tent trap for monitoring the daily activity of Aedes aegypti and Aedes albopictus. J. Vector. Ecol. 2013, 38, 277–288. [Google Scholar] [CrossRef]
- Che-Mendoza, A.; Medina-Barreiro, A.; Koyoc-Cardena, E.; Uc-Puc, V.; Contreras-Perera, Y.; Herrera-Bojorquez, J.; Dzul-Manyanilla, F.; Morales-Correa, F.; Ranson, H.; Lenhart, A.; et al. House screening with insecticide-treated netting provides sustained reductions in domestic populations of Aedes aegypti in Merida, Mexico. PLoS Negl. Trop. Dis. 2018, 12, e0006283. [Google Scholar] [CrossRef]
- Gopalakrishnan, R.; Sukumaran, D.; Thakare, V.B.; Garg, P.; Singh, R. A review on test methods for insecticidal fabrics and the need for standardisation. Parasitol. Res. 2018, 117, 3067–3080. [Google Scholar] [CrossRef] [PubMed]


| Age Category | Number Enrolled | Number Seropositive (%; 95% Confidence Interval) |
|---|---|---|
| 0–4 | 112 | 0 (0.0%; −2.9–1.3%) |
| 5–10 | 188 | 0 (0.0%; −1.5–1.9%) |
| 11–15 | 172 | 2 (1.2%; −0.1–2.7%) |
| 16–20 | 190 | 5 (2.6%; 1.1–3.5%) |
| 21–30 | 295 | 3 (1.0%; 2.2–4.6%) |
| 31–40 | 186 | 11 (5.9%; 3.1–5.8%) |
| 41–50 | 133 | 6 (4.5%; 3.8–7.2%) |
| >50 | 158 | 12 (7.6%; 4.4–8.6%) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seck, M.C.; Badiane, A.S.; Thwing, J.; Moss, D.; Fall, F.B.; Gomis, J.F.; Deme, A.B.; Diongue, K.; Sy, M.; Mbaye, A.; et al. Serological Data Shows Low Levels of Chikungunya Exposure in Senegalese Nomadic Pastoralists. Pathogens 2019, 8, 113. https://doi.org/10.3390/pathogens8030113
Seck MC, Badiane AS, Thwing J, Moss D, Fall FB, Gomis JF, Deme AB, Diongue K, Sy M, Mbaye A, et al. Serological Data Shows Low Levels of Chikungunya Exposure in Senegalese Nomadic Pastoralists. Pathogens. 2019; 8(3):113. https://doi.org/10.3390/pathogens8030113
Chicago/Turabian StyleSeck, Mame Cheikh, Aida Sadikh Badiane, Julie Thwing, Delynn Moss, Fatou Ba Fall, Jules Francois Gomis, Awa Bineta Deme, Khadim Diongue, Mohamed Sy, Aminata Mbaye, and et al. 2019. "Serological Data Shows Low Levels of Chikungunya Exposure in Senegalese Nomadic Pastoralists" Pathogens 8, no. 3: 113. https://doi.org/10.3390/pathogens8030113
APA StyleSeck, M. C., Badiane, A. S., Thwing, J., Moss, D., Fall, F. B., Gomis, J. F., Deme, A. B., Diongue, K., Sy, M., Mbaye, A., Ndiaye, T., Gaye, A., Ndiaye, Y. D., Diallo, M. A., Ndiaye, D., & Rogier, E. (2019). Serological Data Shows Low Levels of Chikungunya Exposure in Senegalese Nomadic Pastoralists. Pathogens, 8(3), 113. https://doi.org/10.3390/pathogens8030113





