Adenylate Cyclases of Trypanosoma brucei, Environmental Sensors and Controllers of Host Innate Immune Response
Abstract
1. Introduction
2. Role of cAMP in Innate and Adaptive Immunity and Pathogen Strategies to Counteract Immunity
3. T. brucei cAMP Signaling Pathway: From an Environmental Sensing Mechanism to an Innate Immune Evasion System
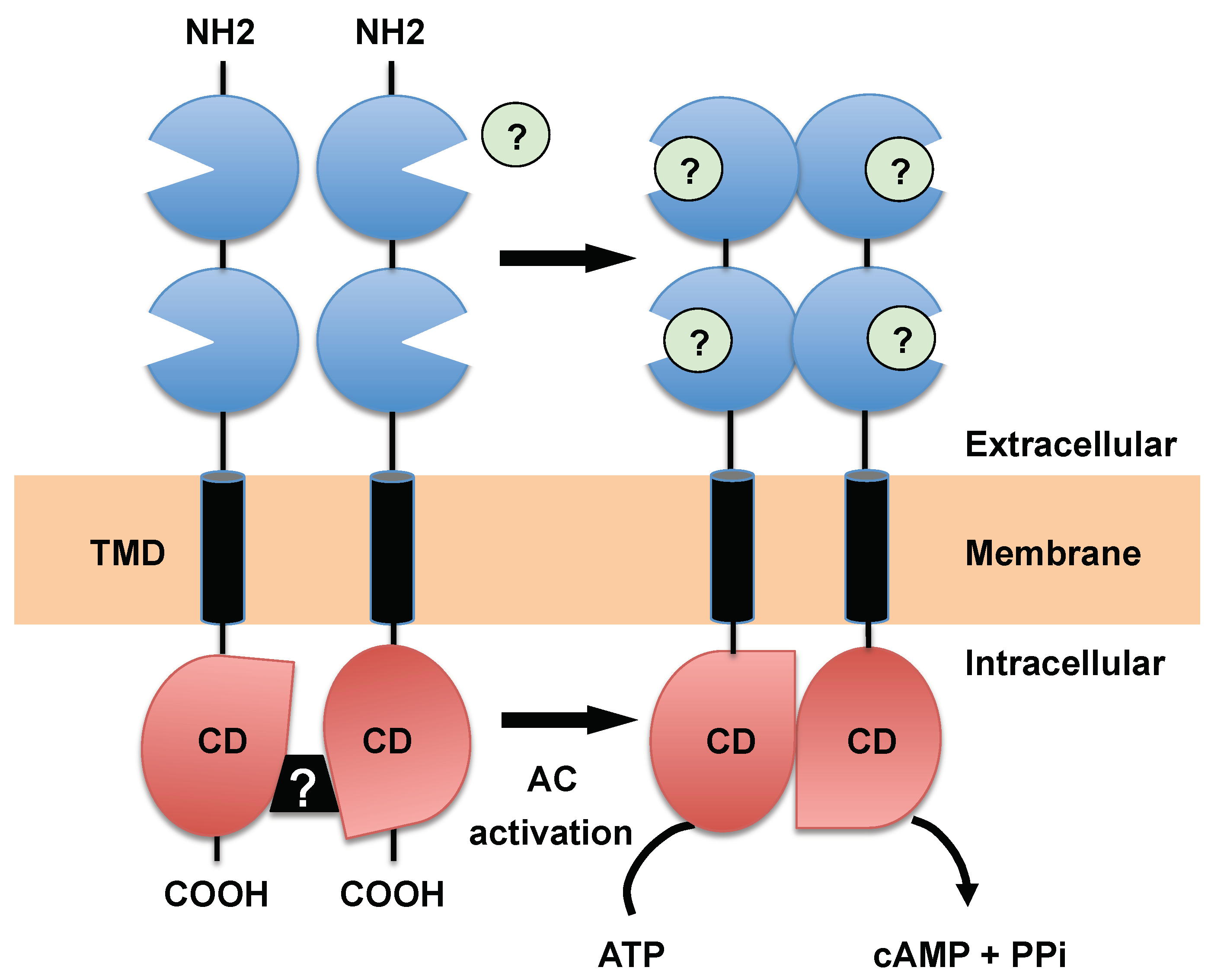
3.1. Receptor-Type ACs, a Hallmark of Trypanosomatids
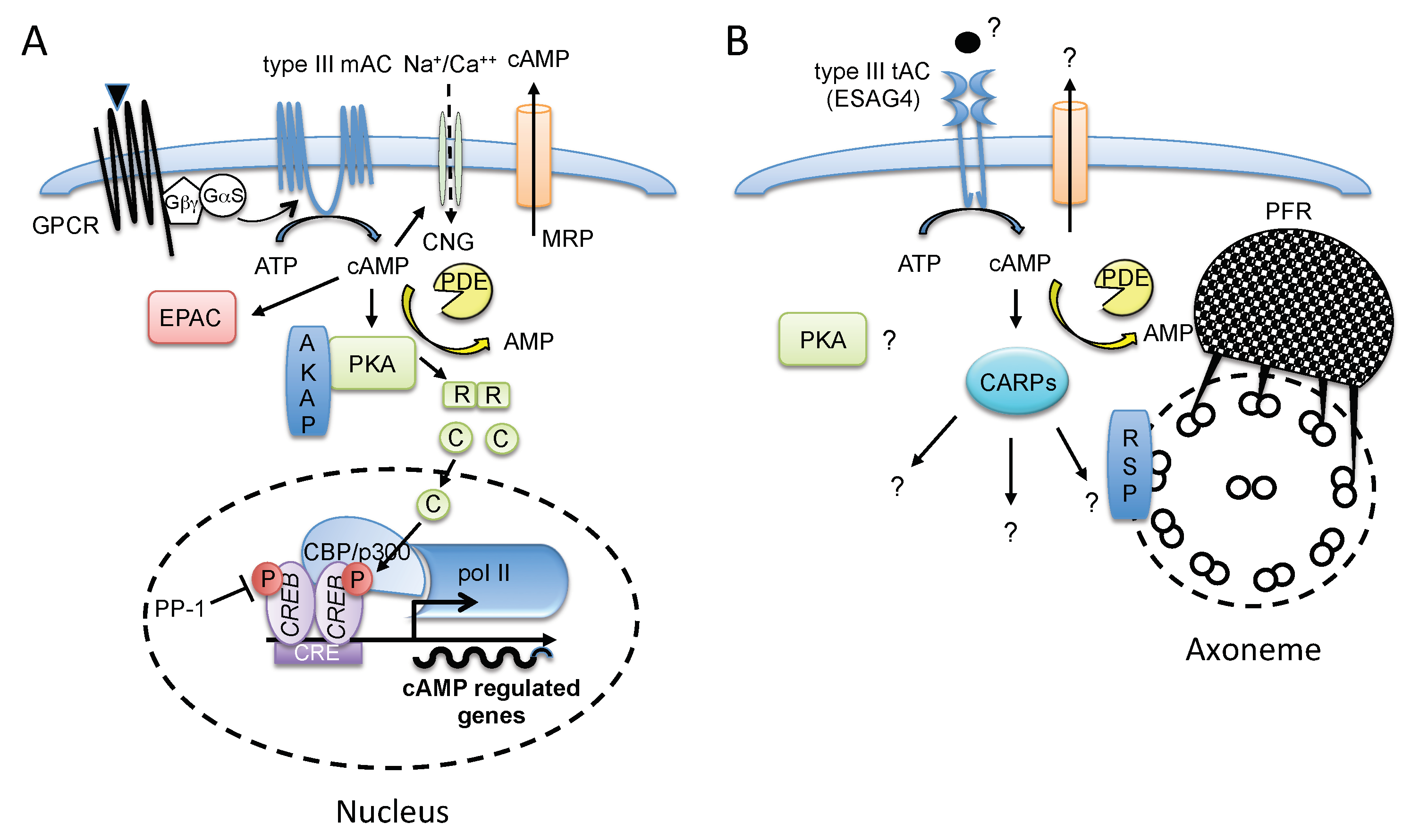
3.2. cAMP Signal Integration in the T. brucei Flagellum, a Complex Organ for Sensing the Environment
3.3. Intracellular cAMP Function and a Novel cAMP Pathway in T. brucei
3.4. Role of ESAG4, a Receptor-Type AC Specific for the Bloodstream Form
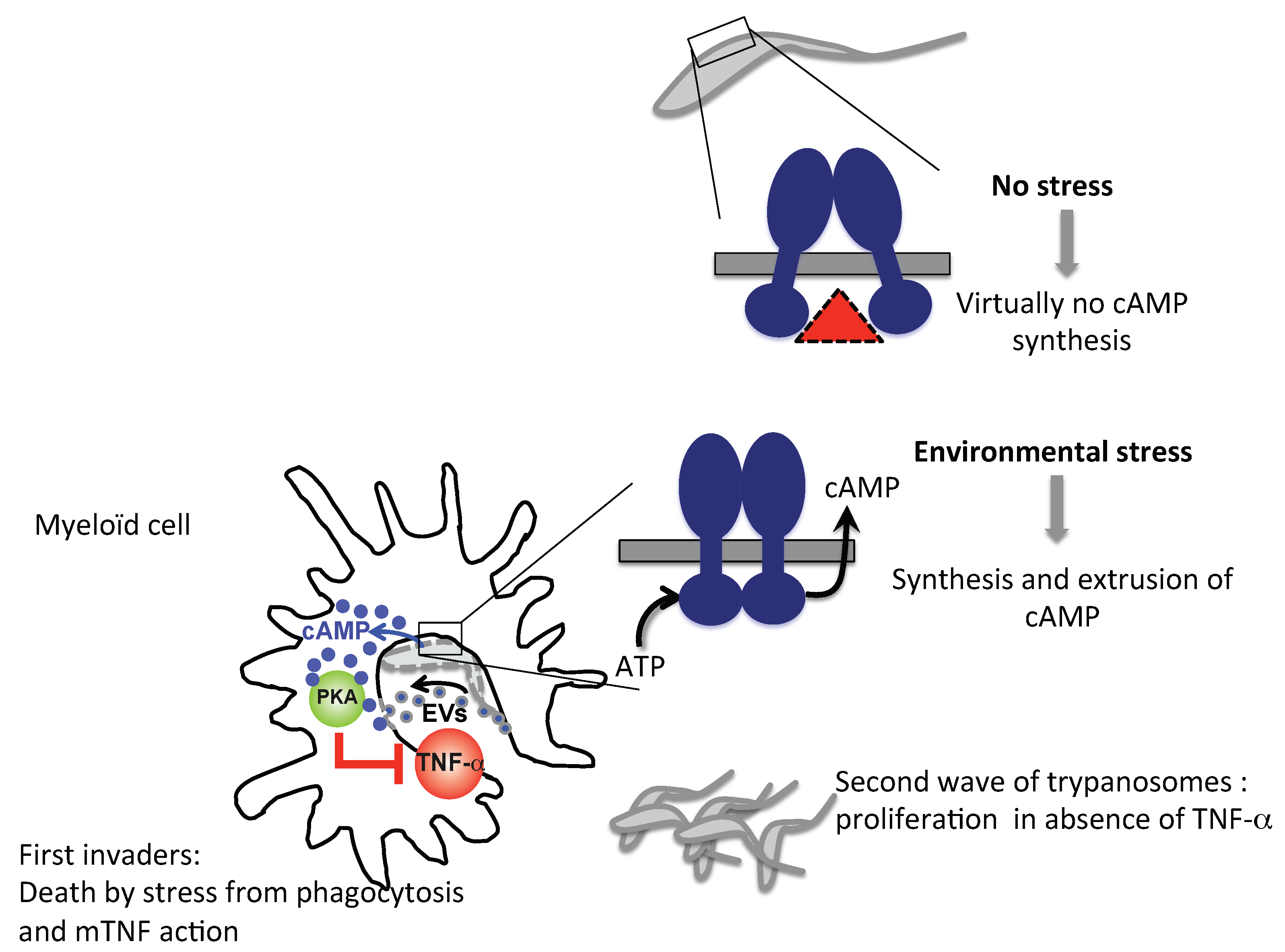
3.5. Trypanosomal ACs as a Tolerogenic Tool in Mammalian Host Innate Immunity
4. Concluding Remarks and Perspectives
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Agrebi, R.; Bellows, L.E.; Collet, J.F.; Kaever, V.; Grundling, A. Evolutionary adaptation of the essential trna methyltransferase trmd to the signaling molecule 3′,5′-camp in bacteria. J. Biol. Chem. 2017, 292, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Gorke, B.; Stulke, J. Carbon catabolite repression in bacteria: Many ways to make the most out of nutrients. Nat. Rev. Microbiol. 2008, 6, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Van Haastert, P.J. Transduction of the chemotactic camp signal across the plasma membrane of dictyostelium cells. Experientia 1995, 51, 1144–1154. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gancedo, J.M. Biological roles of camp: Variations on a theme in the different kingdoms of life. Biol. Rev. Camb. Philos. Soc. 2013, 88, 645–668. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, E.W., Jr.; Wosilait, W.D. Inactivation and activation of liver phosphorylase. Nature 1955, 175, 169–170. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, S.A. Earl sutherland (1915–1974) [corrected] and the discovery of cyclic amp. Perspect. Biol. Med. 2012, 55, 236–249. [Google Scholar] [CrossRef] [PubMed]
- Rodbell, M. The role of hormone receptors and gtp-regulatory proteins in membrane transduction. Nature 1980, 284, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Ross, E.M.; Maguire, M.E.; Sturgill, T.W.; Biltonen, R.L.; Gilman, A.G. Relationship between the beta-adrenergic receptor and adenylate cyclase. J. Biol. Chem. 1977, 252, 5761–5775. [Google Scholar] [PubMed]
- Soderling, T.R.; Hickenbottom, J.P.; Reimann, E.M.; Hunkeler, F.L.; Walsh, D.A.; Krebs, E.G. Inactivation of glycogen synthetase and activation of phosphorylase kinase by muscle adenosine 3′,5′-monophosphate-dependent protein kinases. J. Biol. Chem. 1970, 245, 6317–6328. [Google Scholar] [PubMed]
- Walsh, D.A.; Perkins, J.P.; Krebs, E.G. An adenosine 3′,5′-monophosphate-dependant protein kinase from rabbit skeletal muscle. J. Biol. Chem. 1968, 243, 3763–3765. [Google Scholar] [PubMed]
- De Rooij, J.; Zwartkruis, F.J.; Verheijen, M.H.; Cool, R.H.; Nijman, S.M.; Wittinghofer, A.; Bos, J.L. Epac is a rap1 guanine-nucleotide-exchange factor directly activated by cyclic amp. Nature 1998, 396, 474–477. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, H.; Springett, G.M.; Toki, S.; Canales, J.J.; Harlan, P.; Blumenstiel, J.P.; Chen, E.J.; Bany, I.A.; Mochizuki, N.; Ashbacher, A.; et al. A rap guanine nucleotide exchange factor enriched highly in the basal ganglia. Proc. Natl. Acad. Sci. USA 1998, 95, 13278–13283. [Google Scholar] [CrossRef] [PubMed]
- Fesenko, E.E.; Kolesnikov, S.S.; Lyubarsky, A.L. Induction by cyclic gmp of cationic conductance in plasma membrane of retinal rod outer segment. Nature 1985, 313, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Rall, T.W.; Sutherland, E.W. Formation of a cyclic adenine ribonucleotide by tissue particles. J. Biol. Chem. 1958, 232, 1065–1076. [Google Scholar] [PubMed]
- Weiss, B. Differential activation and inhibition of the multiple forms of cyclic nucleotide phosphodiesterase. Adv. Cycl. Nucleotide Res. 1975, 5, 195–211. [Google Scholar]
- Godinho, R.O.; Duarte, T.; Pacini, E.S. New perspectives in signaling mediated by receptors coupled to stimulatory g protein: The emerging significance of camp e ffl ux and extracellular camp-adenosine pathway. Front. Pharmacol. 2015, 6, 58. [Google Scholar] [CrossRef] [PubMed]
- Danchin, A. Phylogeny of adenylyl cyclases. Adv. Second Messenger Phosphoprot. Res. 1993, 27, 109–162. [Google Scholar]
- Sunahara, R.K.; Taussig, R. Isoforms of mammalian adenylyl cyclase: Multiplicities of signaling. Mol. Interv. 2002, 2, 168–184. [Google Scholar] [CrossRef] [PubMed]
- Krupinski, J.; Coussen, F.; Bakalyar, H.A.; Tang, W.J.; Feinstein, P.G.; Orth, K.; Slaughter, C.; Reed, R.R.; Gilman, A.G. Adenylyl cyclase amino acid sequence: Possible channel- or transporter-like structure. Science 1989, 244, 1558–1564. [Google Scholar] [CrossRef] [PubMed]
- Buck, J.; Sinclair, M.L.; Schapal, L.; Cann, M.J.; Levin, L.R. Cytosolic adenylyl cyclase defines a unique signaling molecule in mammals. Proc. Natl. Acad. Sci. USA 1999, 96, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Omori, K.; Kotera, J. Overview of pdes and their regulation. Circ. Res. 2007, 100, 309–327. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.; Scott, J.D. Akap signalling complexes: Focal points in space and time. Nat. Rev. Mol. Cell Biol. 2004, 5, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Nikolaev, V.O.; Bunemann, M.; Hein, L.; Hannawacker, A.; Lohse, M.J. Novel single chain camp sensors for receptor-induced signal propagation. J. Biol. Chem. 2004, 279, 37215–37218. [Google Scholar] [CrossRef] [PubMed]
- Zaccolo, M.; Pozzan, T. Discrete microdomains with high concentration of camp in stimulated rat neonatal cardiac myocytes. Science 2002, 295, 1711–1715. [Google Scholar] [CrossRef] [PubMed]
- Rich, T.C.; Fagan, K.A.; Nakata, H.; Schaack, J.; Cooper, D.M.; Karpen, J.W. Cyclic nucleotide-gated channels colocalize with adenylyl cyclase in regions of restricted camp diffusion. J. Gen. Physiol. 2000, 116, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Skalhegg, B.S.; Tasken, K. Specificity in the camp/pka signaling pathway. Differential expression, regulation, and subcellular localization of subunits of pka. Front. Biosci. 1997, 2, d331–d342. [Google Scholar] [CrossRef] [PubMed]
- Sassone-Corsi, P. Transcription factors responsive to camp. Ann. Review Cell Dev. Biol. 1995, 11, 355–377. [Google Scholar] [CrossRef] [PubMed]
- Mayr, B.; Montminy, M. Transcriptional regulation by the phosphorylation-dependent factor creb. Nat. Rev. Mol. Cell Biol. 2001, 2, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, M.; Alberts, A.; Brindle, P.; Meinkoth, J.; Feramisco, J.; Deng, T.; Karin, M.; Shenolikar, S.; Montminy, M. Transcriptional attenuation following camp induction requires pp-1-mediated dephosphorylation of creb. Cell 1992, 70, 105–113. [Google Scholar] [CrossRef]
- Wadzinski, B.E.; Wheat, W.H.; Jaspers, S.; Peruski, L.F., Jr.; Lickteig, R.L.; Johnson, G.L.; Klemm, D.J. Nuclear protein phosphatase 2a dephosphorylates protein kinase a-phosphorylated creb and regulates creb transcriptional stimulation. Mol. Cell. Biol. 1993, 13, 2822–2834. [Google Scholar] [CrossRef] [PubMed]
- Clayton, C.E. Life without transcriptional control? From fly to man and back again. EMBO J. 2002, 21, 1881–1888. [Google Scholar] [CrossRef] [PubMed]
- Clayton, C.E. Networks of gene expression regulation in trypanosoma brucei. Mol. Biochem. Parasitol. 2014, 195, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Mony, B.M.; MacGregor, P.; Ivens, A.; Rojas, F.; Cowton, A.; Young, J.; Horn, D.; Matthews, K. Genome-wide dissection of the quorum sensing signalling pathway in trypanosoma brucei. Nature 2014, 505, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Sen Santara, S.; Roy, J.; Mukherjee, S.; Bose, M.; Saha, R.; Adak, S. Globin-coupled heme containing oxygen sensor soluble adenylate cyclase in leishmania prevents cell death during hypoxia. Proc. Natl. Acad. Sci. USA 2013, 110, 16790–16795. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, T.; Liniger, M.; Seebeck, T. The regulatory subunit of a cgmp-regulated protein kinase a of trypanosoma brucei. Eur. J. Biochem. 2001, 268, 6197–6206. [Google Scholar] [CrossRef] [PubMed]
- Gould, M.K.; Bachmaier, S.; Ali, J.A.; Alsford, S.; Tagoe, D.N.; Munday, J.C.; Schnaufer, A.C.; Horn, D.; Boshart, M.; de Koning, H.P. Cyclic amp effectors in african trypanosomes revealed by genome-scale rna interference library screening for resistance to the phosphodiesterase inhibitor cpda. Antimicrob. Agents Chemother. 2013, 57, 4882–4893. [Google Scholar] [CrossRef] [PubMed]
- Jager, A.V.; De Gaudenzi, J.G.; Mild, J.G.; Mc Cormack, B.; Pantano, S.; Altschuler, D.L.; Edreira, M.M. Identification of novel cyclic nucleotide binding proteins in trypanosoma cruzi. Mol. Biochem. Parasitol. 2014, 198, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Serezani, C.H.; Ballinger, M.N.; Aronoff, D.M.; Peters-Golden, M. Cyclic amp: Master regulator of innate immune cell function. Am. J. Respir. Cell Mol. Biol. 2008, 39, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Aronoff, D.M.; Carstens, J.K.; Chen, G.H.; Toews, G.B.; Peters-Golden, M. Short communication: Differences between macrophages and dendritic cells in the cyclic amp-dependent regulation of lipopolysaccharide-induced cytokine and chemokine synthesis. J. Interf. Cytokine Res. 2006, 26, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Van der Pouw Kraan, T.C.; Boeije, L.C.; Smeenk, R.J.; Wijdenes, J.; Aarden, L.A. Prostaglandin-e2 is a potent inhibitor of human interleukin 12 production. J. Exp. Med. 1995, 181, 775–779. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Jones, S.M.; Phare, S.M.; Coffey, M.J.; Peters-Golden, M.; Brock, T.G. Protein kinase a inhibits leukotriene synthesis by phosphorylation of 5-lipoxygenase on serine 523. J. Biol. Chem. 2004, 279, 41512–41520. [Google Scholar] [CrossRef] [PubMed]
- Wen, A.Y.; Sakamoto, K.M.; Miller, L.S. The role of the transcription factor creb in immune function. J. Immunol. 2010, 185, 6413–6419. [Google Scholar] [CrossRef] [PubMed]
- Raker, V.K.; Becker, C.; Steinbrink, K. The camp pathway as therapeutic target in autoimmune and inflammatory diseases. Front. Immunol. 2016, 7, 123. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, M.A.; Muscat, G.E. The nr4a subgroup: Immediate early response genes with pleiotropic physiological roles. Nuclear Recept. Signal. 2006, 4, e002. [Google Scholar] [CrossRef] [PubMed]
- Bystrom, J.; Evans, I.; Newson, J.; Stables, M.; Toor, I.; van Rooijen, N.; Crawford, M.; Colville-Nash, P.; Farrow, S.; Gilroy, D.W. Resolution-phase macrophages possess a unique inflammatory phenotype that is controlled by camp. Blood 2008, 112, 4117–4127. [Google Scholar] [CrossRef] [PubMed]
- Baumer, W.; Hoppmann, J.; Rundfeldt, C.; Kietzmann, M. Highly selective phosphodiesterase 4 inhibitors for the treatment of allergic skin diseases and psoriasis. Inflamm. Allergy Drug Targets 2007, 6, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Oger, S.; Mehats, C.; Dallot, E.; Cabrol, D.; Leroy, M.J. Evidence for a role of phosphodiesterase 4 in lipopolysaccharide-stimulated prostaglandin e2 production and matrix metalloproteinase-9 activity in human amniochorionic membranes. J. Immunol. 2005, 174, 8082–8089. [Google Scholar] [CrossRef] [PubMed]
- Mary, D.; Aussel, C.; Ferrua, B.; Fehlmann, M. Regulation of interleukin 2 synthesis by camp in human t cells. J. Immunol. 1987, 139, 1179–1184. [Google Scholar] [PubMed]
- Munoz, E.; Zubiaga, A.M.; Merrow, M.; Sauter, N.P.; Huber, B.T. Cholera toxin discriminates between t helper 1 and 2 cells in t cell receptor-mediated activation: Role of camp in t cell proliferation. J. Exp. Med. 1990, 172, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.; Bopp, T. Cyclic amp represents a crucial component of treg cell-mediated immune regulation. Front. Immunol. 2016, 7, 315. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Inflammation 2010: New adventures of an old flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; SuYang, H.; Erdjument-Bromage, H.; Tempst, P.; Ghosh, S. The transcriptional activity of nf-kappab is regulated by the ikappab-associated pkac subunit through a cyclic amp-independent mechanism. Cell 1997, 89, 413–424. [Google Scholar] [CrossRef]
- Christian, F.; Smith, E.L.; Carmody, R.J. The regulation of nf-kappab subunits by phosphorylation. Cells 2016, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Ollivier, V.; Parry, G.C.; Cobb, R.R.; de Prost, D.; Mackman, N. Elevated cyclic amp inhibits nf-kappab-mediated transcription in human monocytic cells and endothelial cells. J. Biol. Chem. 1996, 271, 20828–20835. [Google Scholar] [CrossRef] [PubMed]
- Parry, G.C.; Mackman, N. Role of cyclic amp response element-binding protein in cyclic amp inhibition of nf-kappab-mediated transcription. J. Immunol. 1997, 159, 5450–5456. [Google Scholar] [PubMed]
- Baker, D.A.; Kelly, J.M. Structure, function and evolution of microbial adenylyl and guanylyl cyclases. Mol. Microbiol. 2004, 52, 1229–1242. [Google Scholar] [CrossRef] [PubMed]
- Pezard, C.; Weber, M.; Sirard, J.C.; Berche, P.; Mock, M. Protective immunity induced by bacillus anthracis toxin-deficient strains. Infect. Immun. 1995, 63, 1369–1372. [Google Scholar] [PubMed]
- Agarwal, N.; Lamichhane, G.; Gupta, R.; Nolan, S.; Bishai, W.R. Cyclic amp intoxication of macrophages by a mycobacterium tuberculosis adenylate cyclase. Nature 2009, 460, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Yahr, T.L.; Vallis, A.J.; Hancock, M.K.; Barbieri, J.T.; Frank, D.W. Exoy, an adenylate cyclase secreted by the pseudomonas aeruginosa type iii system. Proc. Natl. Acad. Sci. USA 1998, 95, 13899–13904. [Google Scholar] [CrossRef] [PubMed]
- Sory, M.P.; Cornelis, G.R. Translocation of a hybrid yope-adenylate cyclase from yersinia enterocolitica into hela cells. Mol. Microbiol. 1994, 14, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Coote, J.G. Structural and functional relationships among the rtx toxin determinants of gram-negative bacteria. FEMS Microbiol. Rev. 1992, 8, 137–161. [Google Scholar] [CrossRef] [PubMed]
- Krueger, K.M.; Barbieri, J.T. The family of bacterial adp-ribosylating exotoxins. Clin. Microbiol. Rev. 1995, 8, 34–47. [Google Scholar] [PubMed]
- Paindavoine, P.; Rolin, S.; Van Assel, S.; Geuskens, M.; Jauniaux, J.C.; Dinsart, C.; Huet, G.; Pays, E. A gene from the variant surface glycoprotein expression site encodes one of several transmembrane adenylate cyclases located on the flagellum of trypanosoma brucei. Mol. Cell. Biol. 1992, 12, 1218–1225. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Salmon, D.; Bachmaier, S.; Krumbholz, C.; Kador, M.; Gossmann, J.A.; Uzureau, P.; Pays, E.; Boshart, M. Cytokinesis of trypanosoma brucei bloodstream forms depends on expression of adenylyl cyclases of the esag4 or esag4-like subfamily. Mol. Microbiol. 2012, 84, 225–242. [Google Scholar] [CrossRef] [PubMed]
- Salmon, D.; Vanwalleghem, G.; Morias, Y.; Denoeud, J.; Krumbholz, C.; Lhomme, F.; Bachmaier, S.; Kador, M.; Gossmann, J.; Dias, F.B.; et al. Adenylate cyclases of trypanosoma brucei inhibit the innate immune response of the host. Science 2012, 337, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Magez, S.; Geuskens, M.; Beschin, A.; del Favero, H.; Verschueren, H.; Lucas, R.; Pays, E.; de Baetselier, P. Specific uptake of tumor necrosis factor-alpha is involved in growth control of trypanosoma brucei. J. Cell Biol. 1997, 137, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Lucas, R.; Magez, S.; De Leys, R.; Fransen, L.; Scheerlinck, J.P.; Rampelberg, M.; Sablon, E.; De Baetselier, P. Mapping the lectin-like activity of tumor necrosis factor. Science 1994, 263, 814–817. [Google Scholar] [CrossRef] [PubMed]
- McDonough, K.A.; Rodriguez, A. The myriad roles of cyclic amp in microbial pathogens: From signal to sword. Nat. Rev. Microbiol. 2011, 10, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Langousis, G.; Hill, K.L. Motility and more: The flagellum of trypanosoma brucei. Nat. Rev. Microbiol. 2014, 12, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.Y.; Wang, C.; Yuan, Y.A.; He, C.Y. An intracellular membrane junction consisting of flagellum adhesion glycoproteins links flagellum biogenesis to cell morphogenesis in trypanosoma brucei. J. Cell Sci. 2013, 126, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Ralston, K.S.; Lerner, A.G.; Diener, D.R.; Hill, K.L. Flagellar motility contributes to cytokinesis in trypanosoma brucei and is modulated by an evolutionarily conserved dynein regulatory system. Eukaryotic Cell 2006, 5, 696–711. [Google Scholar] [CrossRef] [PubMed]
- Parsons, M.; Worthey, E.A.; Ward, P.N.; Mottram, J.C. Comparative analysis of the kinomes of three pathogenic trypanosomatids: Leishmania major, trypanosoma brucei and trypanosoma cruzi. BMC Genom. 2005, 6, 127. [Google Scholar] [CrossRef] [PubMed]
- Nett, I.R.; Martin, D.M.; Miranda-Saavedra, D.; Lamont, D.; Barber, J.D.; Mehlert, A.; Ferguson, M.A. The phosphoproteome of bloodstream form trypanosoma brucei, causative agent of african sleeping sickness. Mol. Cell. Proteom. 2009, 8, 1527–1538. [Google Scholar] [CrossRef] [PubMed]
- Parsons, M.; Ruben, L. Pathways involved in environmental sensing in trypanosomatids. Parasitol. Today 2000, 16, 56–62. [Google Scholar] [CrossRef]
- Garbers, D.L.; Koesling, D.; Schultz, G. Guanylyl cyclase receptors. Mol. Biol. Cell 1994, 5, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Linder, J.U. Class iii adenylyl cyclases: Molecular mechanisms of catalysis and regulation. Cell. Mol. Life Sci. 2006, 63, 1736–1751. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.C.; Sprang, S.R. Structures, mechanism, regulation and evolution of class iii nucleotidyl cyclases. Revi. Physiol. Biochem. Pharmacol. 2006, 157, 105–140. [Google Scholar]
- Zhang, G.; Liu, Y.; Ruoho, A.E.; Hurley, J.H. Structure of the adenylyl cyclase catalytic core. Nature 1997, 386, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Tesmer, J.J.; Sprang, S.R. The structure, catalytic mechanism and regulation of adenylyl cyclase. Curr. Opin. Struct. Biol. 1998, 8, 713–719. [Google Scholar] [CrossRef]
- Tesmer, J.J.; Sunahara, R.K.; Johnson, R.A.; Gosselin, G.; Gilman, A.G.; Sprang, S.R. Two-metal-ion catalysis in adenylyl cyclase. Science 1999, 285, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Bieger, B.; Essen, L.O. Structural analysis of adenylate cyclases from trypanosoma brucei in their monomeric state. EMBO J. 2001, 20, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Rolin, S.; Hanocq-Quertier, J.; Paturiaux-Hanocq, F.; Nolan, D.; Salmon, D.; Webb, H.; Carrington, M.; Voorheis, P.; Pays, E. Simultaneous but independent activation of adenylate cyclase and glycosylphosphatidylinositol-phospholipase c under stress conditions in trypanosoma brucei. J. Biol. Chem. 1996, 271, 10844–10852. [Google Scholar] [CrossRef] [PubMed]
- Tesmer, J.J.; Dessauer, C.W.; Sunahara, R.K.; Murray, L.D.; Johnson, R.A.; Gilman, A.G.; Sprang, S.R. Molecular basis for p-site inhibition of adenylyl cyclase. Biochemistry 2000, 39, 14464–14471. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.A.; Shoshani, I. Inhibition of bordetella pertussis and bacillus anthracis adenylyl cyclases by polyadenylate and “p”-site agonists. J. Biol. Chem. 1990, 265, 19035–19039. [Google Scholar] [PubMed]
- Voorheis, H.P.; Martin, B.R. Characteristics of the calcium-mediated mechanism activating adenylate cyclase in trypanosoma brucei. Eur. J. Biochem. 1981, 116, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Rolin, S.; Halleux, S.; Van Sande, J.; Dumont, J.; Pays, E.; Steinert, M. Stage-specific adenylate cyclase activity in trypanosoma brucei. Exp. Parasitol. 1990, 71, 350–352. [Google Scholar] [CrossRef]
- Hamedi, A.; Botelho, L.; Britto, C.; Fragoso, S.P.; Umaki, A.C.; Goldenberg, S.; Bottu, G.; Salmon, D. In vitro metacyclogenesis of trypanosoma cruzi induced by starvation correlates with a transient adenylyl cyclase stimulation as well as with a constitutive upregulation of adenylyl cyclase expression. Mol. Biochem. Parasitol. 2015, 200, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Amorim, J.C.; Batista, M.; da Cunha, E.S.; Lucena, A.C.R.; Lima, C.V.P.; Sousa, K.; Krieger, M.A.; Marchini, F.K. Quantitative proteome and phosphoproteome analyses highlight the adherent population during trypanosoma cruzi metacyclogenesis. Sci. Rep. 2017, 7, 9899. [Google Scholar] [CrossRef] [PubMed]
- Potter, L.R.; Hunter, T. Phosphorylation of the kinase homology domain is essential for activation of the a-type natriuretic peptide receptor. Mol. Cell. Biol. 1998, 18, 2164–2172. [Google Scholar] [CrossRef] [PubMed]
- Naula, C.; Schaub, R.; Leech, V.; Melville, S.; Seebeck, T. Spontaneous dimerization and leucine-zipper induced activation of the recombinant catalytic domain of a new adenylyl cyclase of trypanosoma brucei, gresag4.4b. Mol. Biochem. Parasitol. 2001, 112, 19–28. [Google Scholar] [CrossRef]
- Gould, M.K.; de Koning, H.P. Cyclic-nucleotide signalling in protozoa. FEMS Microbiol. Rev. 2011, 35, 515–541. [Google Scholar] [CrossRef] [PubMed]
- Nolan, D.P.; Rolin, S.; Rodriguez, J.R.; Van Den Abbeele, J.; Pays, E. Slender and stumpy bloodstream forms of trypanosoma brucei display a differential response to extracellular acidic and proteolytic stress. Eur. J. Biochem. 2000, 267, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Saada, E.A.; Kabututu, Z.P.; Lopez, M.; Shimogawa, M.M.; Langousis, G.; Oberholzer, M.; Riestra, A.; Jonsson, Z.O.; Wohlschlegel, J.A.; Hill, K.L. Insect stage-specific receptor adenylate cyclases are localized to distinct subdomains of the trypanosoma brucei flagellar membrane. Eukaryot Cell 2014, 13, 1064–1076. [Google Scholar] [CrossRef] [PubMed]
- Garbers, D.L.; Chrisman, T.D.; Wiegn, P.; Katafuchi, T.; Albanesi, J.P.; Bielinski, V.; Barylko, B.; Redfield, M.M.; Burnett, J.C., Jr. Membrane guanylyl cyclase receptors: An update. Trends Endocrinol. Metab. 2006, 17, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, S.; Paindavoine, P.; Tebabi, P.; Pays, A.; Halleux, S.; Steinert, M.; Pays, E. Differential expression of a family of putative adenylate/guanylate cyclase genes in trypanosoma brucei. Mol. Biochem. Parasitol. 1990, 43, 279–288. [Google Scholar] [CrossRef]
- Tam, R.; Saier, M.H., Jr. Structural, functional, and evolutionary relationships among extracellular solute-binding receptors of bacteria. Microbiol. Rev. 1993, 57, 320–346. [Google Scholar] [PubMed]
- Felder, C.B.; Graul, R.C.; Lee, A.Y.; Merkle, H.P.; Sadee, W. The venus flytrap of periplasmic binding proteins: An ancient protein module present in multiple drug receptors. AAPS PharmSci 1999, 1, E2. [Google Scholar] [CrossRef] [PubMed]
- Herrou, J.; Bompard, C.; Wintjens, R.; Dupre, E.; Willery, E.; Villeret, V.; Locht, C.; Antoine, R.; Jacob-Dubuisson, F. Periplasmic domain of the sensor-kinase bvgs reveals a new paradigm for the venus flytrap mechanism. Proc. Natl. Acad. Sci. USA 2010, 107, 17351–17355. [Google Scholar] [CrossRef] [PubMed]
- Pin, J.P.; Kniazeff, J.; Liu, J.; Binet, V.; Goudet, C.; Rondard, P.; Prezeau, L. Allosteric functioning of dimeric class c g-protein-coupled receptors. FEBS J. 2005, 272, 2947–2955. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Chow, D.; Martick, M.M.; Garcia, K.C. Allosteric activation of a spring-loaded natriuretic peptide receptor dimer by hormone. Science 2001, 293, 1657–1662. [Google Scholar] [CrossRef] [PubMed]
- Van den Akker, F. Structural insights into the ligand binding domains of membrane bound guanylyl cyclases and natriuretic peptide receptors. J. Mol. Biol. 2001, 311, 923–937. [Google Scholar] [CrossRef] [PubMed]
- Beschin, A.; Van Den Abbeele, J.; De Baetselier, P.; Pays, E. African trypanosome control in the insect vector and mammalian host. Trends Parasitol. 2014, 30, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Szurmant, H.; Ordal, G.W. Diversity in chemotaxis mechanisms among the bacteria and archaea. Microbiol. Mol. Biol. Rev. 2004, 68, 301–319. [Google Scholar] [CrossRef] [PubMed]
- Swaney, K.F.; Huang, C.H.; Devreotes, P.N. Eukaryotic chemotaxis: A network of signaling pathways controls motility, directional sensing, and polarity. Ann. Rev. Biophys. 2010, 39, 265–289. [Google Scholar] [CrossRef] [PubMed]
- Ralston, K.S.; Kabututu, Z.P.; Melehani, J.H.; Oberholzer, M.; Hill, K.L. The trypanosoma brucei flagellum: Moving parasites in new directions. Ann. Rev. Microbiol. 2009, 63, 335–362. [Google Scholar] [CrossRef] [PubMed]
- Tyler, K.M.; Fridberg, A.; Toriello, K.M.; Olson, C.L.; Cieslak, J.A.; Hazlett, T.L.; Engman, D.M. Flagellar membrane localization via association with lipid rafts. J. Cell Sci. 2009, 122, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.I.; Olson, C.L.; Engman, D.M. The lipid raft proteome of african trypanosomes contains many flagellar proteins. Pathogens 2017, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Hertz-Fowler, C.; Ersfeld, K.; Gull, K. Cap5.5, a life-cycle-regulated, cytoskeleton-associated protein is a member of a novel family of calpain-related proteins in trypanosoma brucei. Mol. Biochem. Parasitol. 2001, 116, 25–34. [Google Scholar] [CrossRef]
- Liu, W.; Apagyi, K.; McLeavy, L.; Ersfeld, K. Expression and cellular localisation of calpain-like proteins in trypanosoma brucei. Mol. Biochem. Parasitol. 2010, 169, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Pagano, M.; Clynes, M.A.; Masada, N.; Ciruela, A.; Ayling, L.J.; Wachten, S.; Cooper, D.M. Insights into the residence in lipid rafts of adenylyl cyclase ac8 and its regulation by capacitative calcium entry. Am. J. Physiol. Cell Physiol. 2009, 296, C607–C619. [Google Scholar] [CrossRef] [PubMed]
- Oberholzer, M.; Marti, G.; Baresic, M.; Kunz, S.; Hemphill, A.; Seebeck, T. The trypanosoma brucei camp phosphodiesterases tbrpdeb1 and tbrpdeb2: Flagellar enzymes that are essential for parasite virulence. FASEB J. 2007, 21, 720–731. [Google Scholar] [CrossRef] [PubMed]
- Baron, D.M.; Ralston, K.S.; Kabututu, Z.P.; Hill, K.L. Functional genomics in trypanosoma brucei identifies evolutionarily conserved components of motile flagella. J. Cell Sci. 2007, 120, 478–491. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, A.R.; Diener, D.R.; Rosenbaum, J.L.; Sale, W.S. Flagellar radial spoke protein 3 is an a-kinase anchoring protein (akap). J. Cell Biol. 2001, 153, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Kramer, S. Characterization of a Pka-Like Kinase from Trypanosoma Brucei. Ph.D. Thesis, LMU München, München, Germany, 2005. [Google Scholar]
- Oberholzer, M.; Langousis, G.; Nguyen, H.T.; Saada, E.A.; Shimogawa, M.M.; Jonsson, Z.O.; Nguyen, S.M.; Wohlschlegel, J.A.; Hill, K.L. Independent analysis of the flagellum surface and matrix proteomes provides insight into flagellum signaling in mammalian-infectious trypanosoma brucei. Mol. Cell. Proteom. 2011, 10. [Google Scholar] [CrossRef] [PubMed]
- Engstler, M.; Boshart, M. Cold shock and regulation of surface protein trafficking convey sensitization to inducers of stage differentiation in trypanosoma brucei. Genes Dev. 2004, 18, 2798–2811. [Google Scholar] [CrossRef] [PubMed]
- Bridges, D.J.; Pitt, A.R.; Hanrahan, O.; Brennan, K.; Voorheis, H.P.; Herzyk, P.; de Koning, H.P.; Burchmore, R.J. Characterisation of the plasma membrane subproteome of bloodstream form trypanosoma brucei. Proteomics 2008, 8, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Broadhead, R.; Dawe, H.R.; Farr, H.; Griffiths, S.; Hart, S.R.; Portman, N.; Shaw, M.K.; Ginger, M.L.; Gaskell, S.J.; McKean, P.G.; et al. Flagellar motility is required for the viability of the bloodstream trypanosome. Nature 2006, 440, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Merchant, S.S.; Prochnik, S.E.; Vallon, O.; Harris, E.H.; Karpowicz, S.J.; Witman, G.B.; Terry, A.; Salamov, A.; Fritz-Laylin, L.K.; Marechal-Drouard, L.; et al. The chlamydomonas genome reveals the evolution of key animal and plant functions. Science 2007, 318, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Mancini, P.E.; Patton, C.L. Cyclic 3′,5′-adenosine monophosphate levels during the developmental cycle of trypanosoma brucei brucei in the rat. Mol. Biochem. Parasitol. 1981, 3, 19–31. [Google Scholar] [CrossRef]
- Rolin, S.; Paindavoine, P.; Hanocq-Quertier, J.; Hanocq, F.; Claes, Y.; Le Ray, D.; Overath, P.; Pays, E. Transient adenylate cyclase activation accompanies differentiation of trypanosoma brucei from bloodstream to procyclic forms. Mol. Biochem. Parasitol. 1993, 61, 115–125. [Google Scholar] [CrossRef]
- Gonzales-Perdomo, M.; Romero, P.; Goldenberg, S. Cyclic amp and adenylate cyclase activators stimulate trypanosoma cruzi differentiation. Exp. Parasitol. 1988, 66, 205–212. [Google Scholar] [CrossRef]
- Rangel-Aldao, R.; Triana, F.; Fernandez, V.; Comach, G.; Abate, T.; Montoreano, R. Cyclic amp as an inducer of the cell differentiation of trypanosoma cruzi. Biochem. Int. 1988, 17, 337–344. [Google Scholar] [PubMed]
- Vassella, E.; Reuner, B.; Yutzy, B.; Boshart, M. Differentiation of african trypanosomes is controlled by a density sensing mechanism which signals cell cycle arrest via the camp pathway. J. Cell Sci. 1997, 110 Pt 21, 2661–2671. [Google Scholar] [PubMed]
- Bhattacharya, A.; Biswas, A.; Das, P.K. Role of intracellular camp in differentiation-coupled induction of resistance against oxidative damage in leishmania donovani. Free Radic. Biol. Med. 2008, 44, 779–794. [Google Scholar] [CrossRef] [PubMed]
- Laxman, S.; Riechers, A.; Sadilek, M.; Schwede, F.; Beavo, J.A. Hydrolysis products of camp analogs cause transformation of trypanosoma brucei from slender to stumpy-like forms. Proc. Natl. Acad. Sci. USA 2006, 103, 19194–19199. [Google Scholar] [CrossRef] [PubMed]
- Oberholzer, M.; Lopez, M.A.; McLelland, B.T.; Hill, K.L. Social motility in african trypanosomes. PLoS Pathog. 2010, 6, e1000739. [Google Scholar] [CrossRef] [PubMed]
- Imhof, S.; Knusel, S.; Gunasekera, K.; Vu, X.L.; Roditi, I. Social motility of african trypanosomes is a property of a distinct life-cycle stage that occurs early in tsetse fly transmission. PLoS Pathog. 2014, 10, e1004493. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.A.; Saada, E.A.; Hill, K.L. Insect stage-specific adenylate cyclases regulate social motility in african trypanosomes. Eukaryotic Cell 2015, 14, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Oberholzer, M.; Saada, E.A.; Hill, K.L. Cyclic amp regulates social behavior in african trypanosomes. mBio 2015, 6, e01954-14. [Google Scholar] [CrossRef] [PubMed]
- Saada, E.A.; DeMarco, S.F.; Shimogawa, M.M.; Hill, K.L. “With a little help from my friends”-social motility in trypanosoma brucei. PLoS Pathog. 2015, 11, e1005272. [Google Scholar] [CrossRef] [PubMed]
- Eliaz, D.; Kannan, S.; Shaked, H.; Arvatz, G.; Tkacz, I.D.; Binder, L.; Waldman Ben-Asher, H.; Okalang, U.; Chikne, V.; Cohen-Chalamish, S.; et al. Exosome secretion affects social motility in trypanosoma brucei. PLoS Pathog. 2017, 13, e1006245. [Google Scholar] [CrossRef] [PubMed]
- Imhof, S.; Vu, X.L.; Butikofer, P.; Roditi, I. A glycosylation mutant of trypanosoma brucei links social motility defects in vitro to impaired colonization of tsetse flies in vivo. Eukaryotic Cell 2015, 14, 588–592. [Google Scholar] [CrossRef] [PubMed]
- Pays, E.; Tebabi, P.; Pays, A.; Coquelet, H.; Revelard, P.; Salmon, D.; Steinert, M. The genes and transcripts of an antigen gene expression site from t. Brucei. Cell 1989, 57, 835–845. [Google Scholar] [CrossRef]
- Alexandre, S.; Paindavoine, P.; Hanocq-Quertier, J.; Paturiaux-Hanocq, F.; Tebabi, P.; Pays, E. Families of adenylate cyclase genes in trypanosoma brucei. Mol. Biochem. Parasitol. 1996, 77, 173–182. [Google Scholar] [CrossRef]
- Ross, D.T.; Raibaud, A.; Florent, I.C.; Sather, S.; Gross, M.K.; Storm, D.R.; Eisen, H. The trypanosome vsg expression site encodes adenylate cyclase and a leucine-rich putative regulatory gene. EMBO J. 1991, 10, 2047–2053. [Google Scholar] [PubMed]
- Palmer, G.H.; Bankhead, T.; Seifert, H.S. Antigenic variation in bacterial pathogens. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Dempsey, W.L.; Mansfield, J.M. Lymphocyte function in experimental african trypanosomiasis. V. Role of antibody and the mononuclear phagocyte system in variant-specific immunity. J. Immunol. 1983, 130, 405–411. [Google Scholar] [PubMed]
- Hertz, C.J.; Filutowicz, H.; Mansfield, J.M. Resistance to the african trypanosomes is ifn-gamma dependent. J. Immunol. 1998, 161, 6775–6783. [Google Scholar] [PubMed]
- De Muylder, G.; Daulouede, S.; Lecordier, L.; Uzureau, P.; Morias, Y.; Van Den Abbeele, J.; Caljon, G.; Herin, M.; Holzmuller, P.; Semballa, S.; et al. A trypanosoma brucei kinesin heavy chain promotes parasite growth by triggering host arginase activity. PLoS Pathog. 2013, 9, e1003731. [Google Scholar] [CrossRef] [PubMed]
- Stijlemans, B.; Caljon, G.; Van Den Abbeele, J.; Van Ginderachter, J.A.; Magez, S.; De Trez, C. Immune evasion strategies of trypanosoma brucei within the mammalian host: Progression to pathogenicity. Front. Immunol. 2016, 7, 233. [Google Scholar] [CrossRef] [PubMed]
- Vanwalleghem, G.; Morias, Y.; Beschin, A.; Szymkowski, D.E.; Pays, E. Trypanosoma brucei growth control by tnf in mammalian host is independent of the soluble form of the cytokine. Sci. Rep. 2017, 7, 6165. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Bull, H.; Zhou, X.; Tabel, H. Intradermal infections of mice by low numbers of african trypanosomes are controlled by innate resistance but enhance susceptibility to reinfection. J. Infect. Dis. 2011, 203, 418–429. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Szempruch, A.J.; Sykes, S.E.; Kieft, R.; Dennison, L.; Becker, A.C.; Gartrell, A.; Martin, W.J.; Nakayasu, E.S.; Almeida, I.C.; Hajduk, S.L.; et al. Extracellular vesicles from trypanosoma brucei mediate virulence factor transfer and cause host anemia. Cell 2016, 164, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Wall, E.A.; Zavzavadjian, J.R.; Chang, M.S.; Randhawa, B.; Zhu, X.; Hsueh, R.C.; Liu, J.; Driver, A.; Bao, X.R.; Sternweis, P.C.; et al. Suppression of lps-induced tnf-alpha production in macrophages by camp is mediated by pka-akap95-p105. Sci. Signal. 2009, 2, ra28. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Das, S.; Smith, T.F.; Samuelson, J. Trichomonas transmembrane cyclases result from massive gene duplication and concomitant development of pseudogenes. PLoS Negl. Trop. Dis. 2010, 4, e782. [Google Scholar] [CrossRef] [PubMed]
- Ratier, L.; Urrutia, M.; Paris, G.; Zarebski, L.; Frasch, A.C.; Goldbaum, F.A. Relevance of the diversity among members of the trypanosoma cruzi trans-sialidase family analyzed with camelids single-domain antibodies. PLoS ONE 2008, 3, e3524. [Google Scholar] [CrossRef] [PubMed]
- Mayer, D.C.; Mu, J.B.; Feng, X.; Su, X.Z.; Miller, L.H. Polymorphism in a plasmodium falciparum erythrocyte-binding ligand changes its receptor specificity. J. Exp. Med. 2002, 196, 1523–1528. [Google Scholar] [CrossRef] [PubMed]
- Bitter, W.; Gerrits, H.; Kieft, R.; Borst, P. The role of transferrin-receptor variation in the host range of trypanosoma brucei. Nature 1998, 391, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Pays, E.; Lips, S.; Nolan, D.; Vanhamme, L.; Perez-Morga, D. The vsg expression sites of trypanosoma brucei: Multipurpose tools for the adaptation of the parasite to mammalian hosts. Mol. Biochem. Parasitol. 2001, 114, 1–16. [Google Scholar] [CrossRef]
- Jansen, C.; Wang, H.; Kooistra, A.J.; de Graaf, C.; Orrling, K.M.; Tenor, H.; Seebeck, T.; Bailey, D.; de Esch, I.J.; Ke, H.; et al. Discovery of novel trypanosoma brucei phosphodiesterase b1 inhibitors by virtual screening against the unliganded tbrpdeb1 crystal structure. J. Med. Chem. 2013, 56, 2087–2096. [Google Scholar] [CrossRef] [PubMed]
- Makin, L.; Gluenz, E. Camp signalling in trypanosomatids: Role in pathogenesis and as a drug target. Trends Parasitol. 2015, 31, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, D.; Cooper, D.M. Live-cell imaging of camp dynamics. Nat. Methods 2008, 5, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Karpen, J.W.; Rich, T.C. High-resolution measurements of cyclic adenosine monophosphate signals in 3d microdomains. Methods Mol. Biol. 2005, 307, 15–26. [Google Scholar] [PubMed]
- Voorheis, H.P.; Martin, B.R. ‘Swell dialysis‘ demonstrates that adenylate cyclase in trypanosoma brucei is regulated by calcium ions. Eur. J. Biochem. 1980, 113, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Jansen, V.; Alvarez, L.; Balbach, M.; Strunker, T.; Hegemann, P.; Kaupp, U.B.; Wachten, D. Controlling fertilization and camp signaling in sperm by optogenetics. eLife 2015, 4. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salmon, D. Adenylate Cyclases of Trypanosoma brucei, Environmental Sensors and Controllers of Host Innate Immune Response. Pathogens 2018, 7, 48. https://doi.org/10.3390/pathogens7020048
Salmon D. Adenylate Cyclases of Trypanosoma brucei, Environmental Sensors and Controllers of Host Innate Immune Response. Pathogens. 2018; 7(2):48. https://doi.org/10.3390/pathogens7020048
Chicago/Turabian StyleSalmon, Didier. 2018. "Adenylate Cyclases of Trypanosoma brucei, Environmental Sensors and Controllers of Host Innate Immune Response" Pathogens 7, no. 2: 48. https://doi.org/10.3390/pathogens7020048
APA StyleSalmon, D. (2018). Adenylate Cyclases of Trypanosoma brucei, Environmental Sensors and Controllers of Host Innate Immune Response. Pathogens, 7(2), 48. https://doi.org/10.3390/pathogens7020048



