Listeria monocytogenes: The Impact of Cell Death on Infection and Immunity
Abstract
1. Introduction
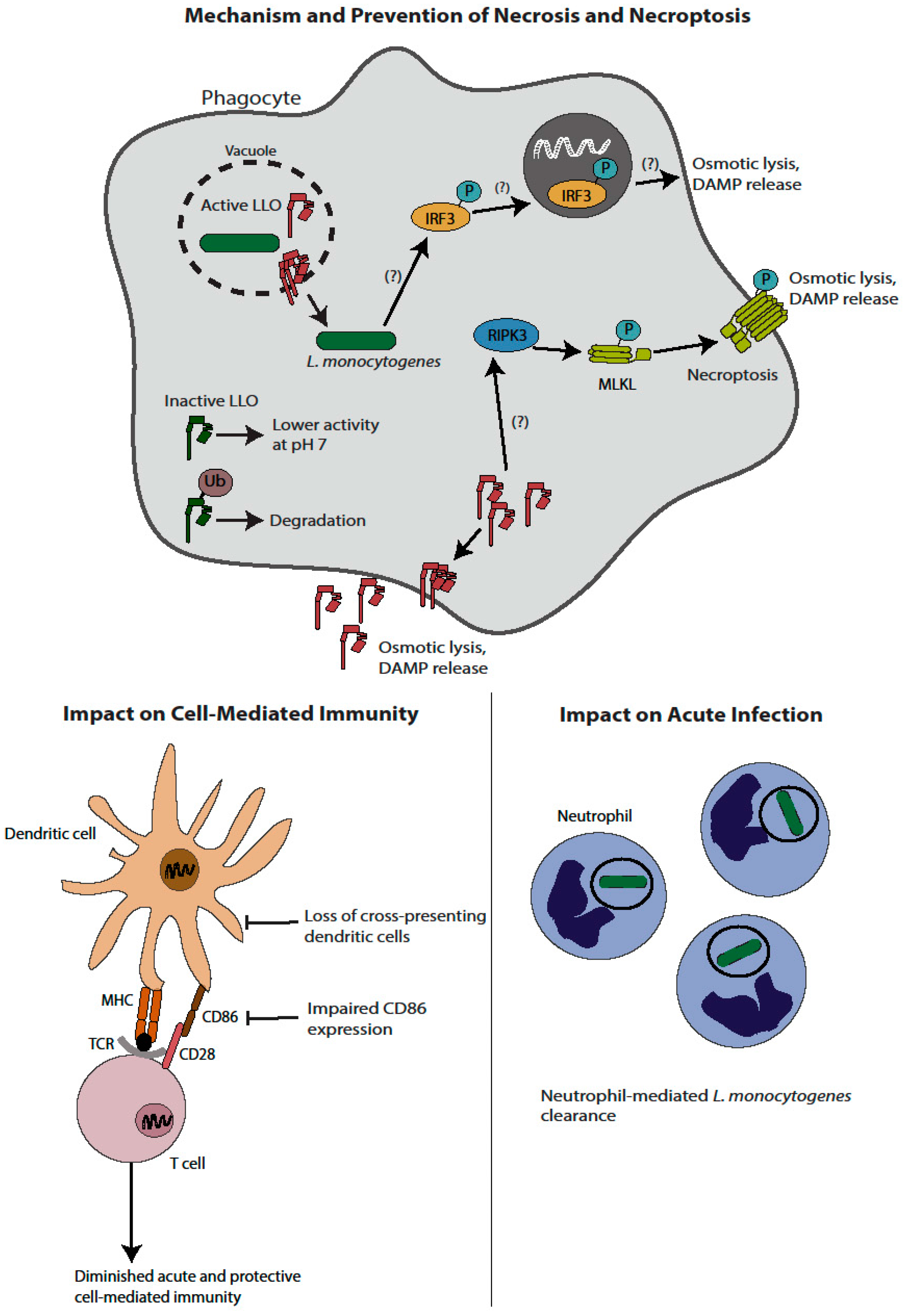
2. Necrosis and Necroptosis
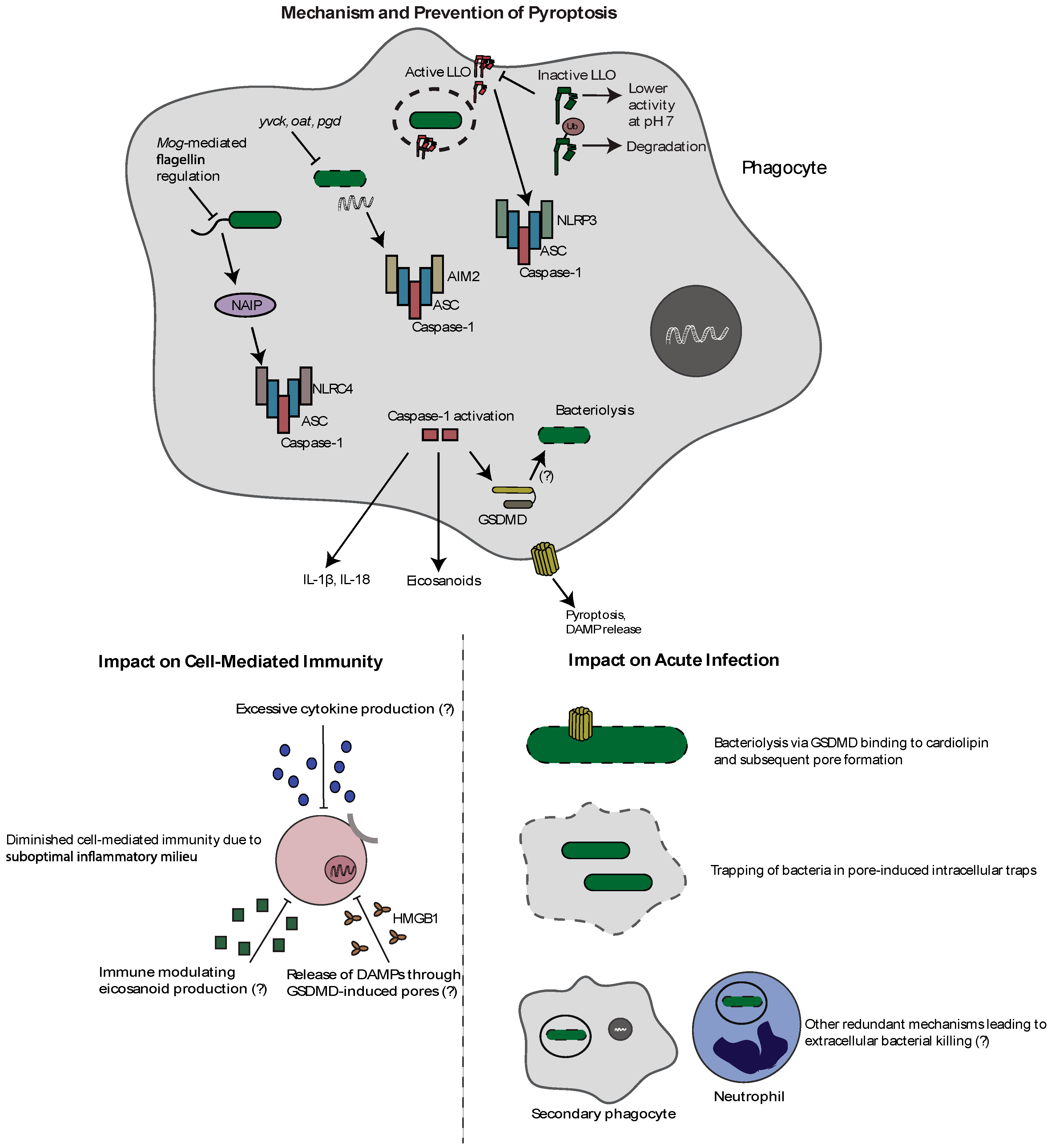
3. Pyroptosis
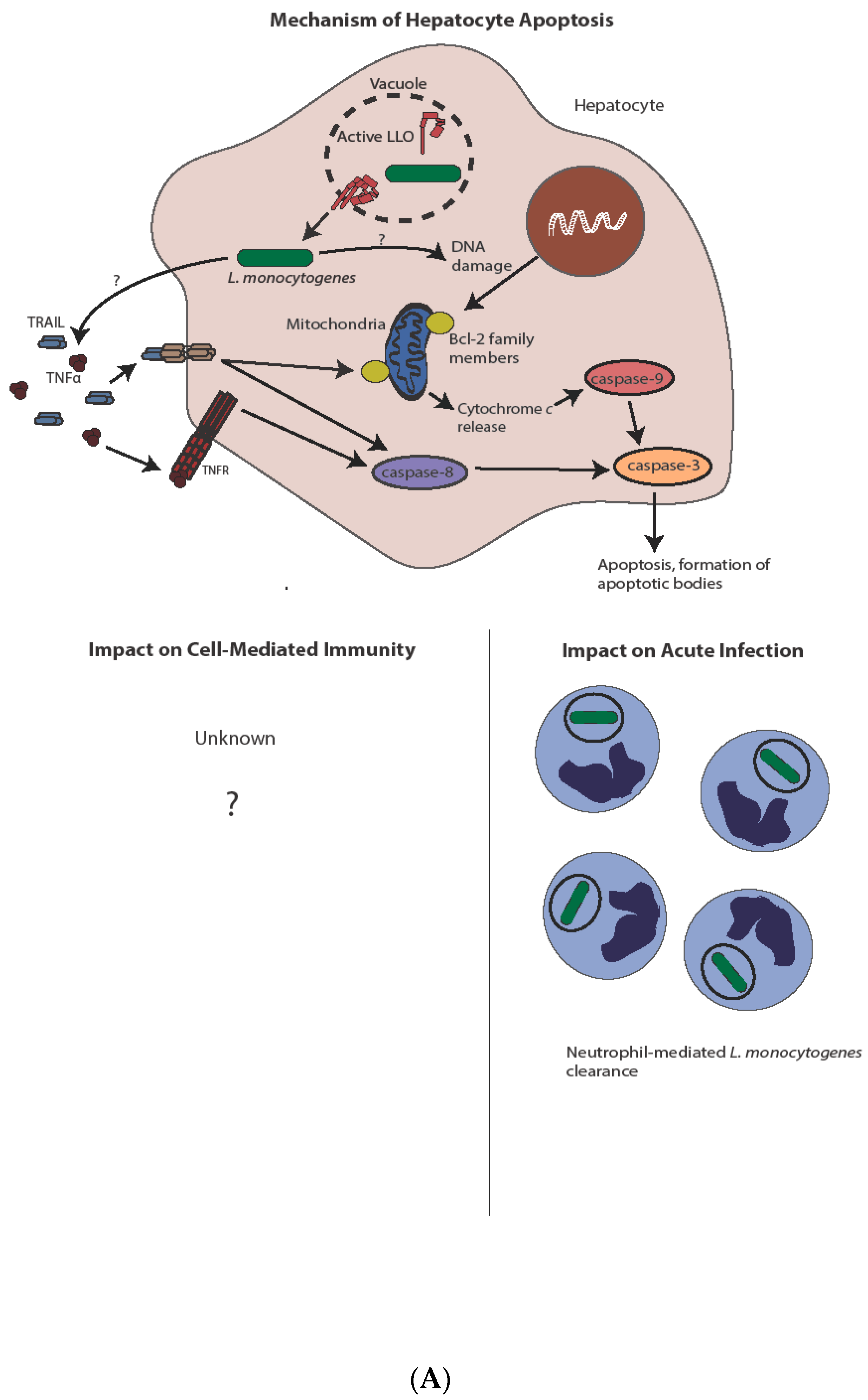
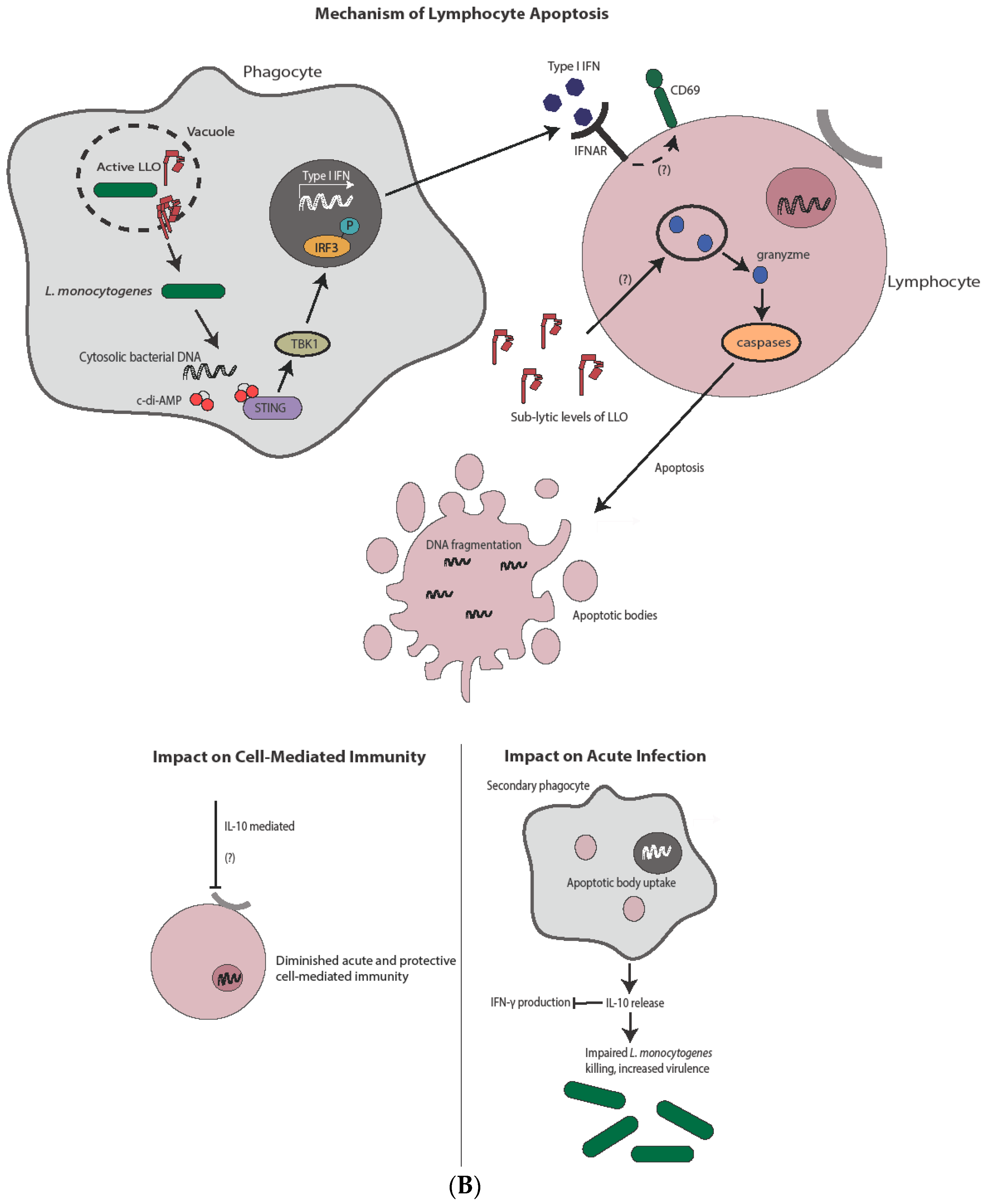
4. Apoptosis
5. Conclusions and Future Directions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ferreira, V.; Wiedmann, M.; Teixeira, P.; Stasiewicz, M.J. Listeria monocytogenes Persistence in Food-Associated Environments: Epidemiology, Strain Characteristics, and Implications for Public Health. J. Food Prot. 2014, 77, 150–170. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, B.; Gerner-Smidt, P. The epidemiology of human listeriosis. Microbes Infect. 2007, 9, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Mengaud, J.; Ohayon, H.; Gounon, P.; Mege, R.-M.; Cossart, P. E-cadherin is the receptor for internalin, a surface protein required for entry of L. monocytogenes into epithelial cells. Cell 1996, 84, 923–932. [Google Scholar] [CrossRef]
- Shen, Y.; Naujokas, M.; Park, M.; Ireton, K. InIB-dependent internalization of Listeria is mediated by the Met receptor tyrosine kinase. Cell 2000, 103, 501–510. [Google Scholar] [CrossRef]
- Portnoy, D.A.; Jacks, P.S.; Hinrichs, D.J. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J. Exp. Med. 1988, 167, 1459–1471. [Google Scholar] [CrossRef] [PubMed]
- Hamon, M.A.; Ribet, D.; Stavru, F.; Cossart, P. Listeriolysin O: The Swiss army knife of Listeria. Trends Microbiol. 2012, 20, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Kocks, C.; Gouin, E.; Tabouret, M.; Berche, P.; Ohayon, H.; Cossart, P. L. monocytogenes-induced actin assembly requires the actA gene product, a surface protein. Cell 1992, 68, 521–531. [Google Scholar] [CrossRef]
- Carr, K.D.; Sieve, A.N.; Indramohan, M.; Break, T.J.; Lee, S.; Berg, R.E. Specific depletion reveals a novel role for neutrophil-mediated protection in the liver during Listeria monocytogenes infection. Eur. J. Immunol. 2011, 41, 2666–2676. [Google Scholar] [CrossRef] [PubMed]
- Baker, L.A.; Campbell, P.A.; Hollister, J.R. Chemotaxigenesis and complement fixation by Listeria monocytogenes cell wall fractions. J. Immunol. 1977, 119, 1723–1726. [Google Scholar] [PubMed]
- Shaughnessy, L.M.; Swanson, J.A. The role of the activated macrophage in clearing Listeria monocytogenes infection. Front. Biosci. 2007, 12, 2683–2692. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Dubenksy, T.W.; Brockstedt, D.G. Clinical development of Listeria monocytogenes-based immunotherapies. Semin. Oncol. 2012, 39, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Bahjat, K.S.; Liu, W.; Lemmens, E.E.; Schoenberger, S.P.; Portnoy, D.A.; Dubensky, T.W., Jr.; Brockstedt, D.G. Cytosolic entry controls CD8+-T-cell potency during bacterial infection. Infect. Immun. 2006, 74, 6387–6397. [Google Scholar] [CrossRef] [PubMed]
- Aduro BioTech. LADD Engineering Listeria Mononcytogenes Bacteria. 2017. Available online: http://www.aduro.com/technology/ladd/ (accessed on 17 May 2017).
- Lm Technology—Advaxis. 2017. Available online: https://www.advaxis.com/lm-technology/ (accessed on 15 November 2017).
- Blériot, C.; Lecuit, M. The interplay between regulated necrosis and bacterial infection. Cell. Mol. Life Sci. 2016, 73, 2369–2378. [Google Scholar] [CrossRef] [PubMed]
- Pasparakis, M.; Vandenabeele, P. Necroptosis and its role in inflammation. Nature 2015, 517, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Vanden Berghe, T.; Kaiser, W.J.; Bertrand, M.J.; Vandenabeele, P. Molecular crosstalk between apoptosis, necroptosis, and survival signaling. Mol. Cell. Oncol. 2015, 2, e975093. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Galluzzi, L.; Vandenabeele, P.; Abrams, J.; Alnemri, E.S.; Baehrecke, E.H.; Blagosklonny, M.V.; El-Deiry, W.S.; Golstein, P.; Green, D.R.; et al. Classification of cell death: Recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009, 16, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Kokkola, R.; Andersson, A.; Mullins, G.; Ostberg, T.; Treutiger, C.-J.; Arnold, B.; Nawroth, P.; Andersson, U.; Harris, R.A.; Harris, H.E. RAGE is the Major Receptor for the Proinflammatory Activity of HMGB1 in Rodent Macrophages. Scand. J. Immunol. 2005, 61, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Scaffidi, P.; Misteli, T.; Bianchi, M.E. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 2002, 418, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Seveau, S. Multifaceted activity of listeriolysin O, the cholesterol-dependent cytolysin of Listeria monocytogenes. Subcell. Biochem. 2014, 80, 161–195. [Google Scholar] [PubMed]
- Barsig, J.; Kaufmann, S.H. The mechanism of cell death in Listeria monocytogenes-infected murine macrophages is distinct from apoptosis. Infect. Immun. 1997, 65, 4075–4081. [Google Scholar] [PubMed]
- González-Juarbe, N.; Gilley, R.P.; Hinojosa, C.A.; Bradley, K.M.; Kamei, A.; Gao, G.; Dube, P.H.; Bergman, M.A.; Orihuela, C.J. Pore-Forming Toxins Induce Macrophage Necroptosis during Acute Bacterial Pneumonia. PLoS Pathog. 2015, 11, e1005337. [Google Scholar] [CrossRef] [PubMed]
- Geoffroy, C.; Gaillard, J.-L.; Alouf, J.E.; Berche, P. Purification, Characterization, and Toxicity of the Sulfhydryl-Activated Hemolysin Listeriolysin 0 from Listeria monocytogenes. Infect. Immun. 1987, 55, 1641–1646. [Google Scholar] [PubMed]
- Glomski, I.J.; Decatur, A.L.; Portnoy, D.A. Listeria monocytogenes mutants that fail to compartmentalize listerolysin O activity are cytotoxic, avirulent, and unable to evade host extracellular defenses. Infect. Immun. 2003, 71, 6754–6765. [Google Scholar] [CrossRef] [PubMed]
- Glomski, I.J.; Gedde, M.M.; Tsang, A.W.; Swanson, J.A.; Portnoy, D.A. The Listeria monocytogenes hemolysin has an acidic pH optimum to compartmentalize activity and prevent damage to infected host cells. J. Cell Biol. 2002, 156, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Schnupf, P.; Portnoy, D.A.; Decatur, A.L. Phosphorylation, ubiquitination and degradation of listeriolysin O in mammalian cells: Role of the PEST-like sequence. Cell Microbiol. 2006, 8, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Schuerch, D.W.; Wilson-Kubalek, E.M.; Tweten, R.K. Molecular basis of listeriolysin O pH dependence. Proc. Natl. Acad. Sci. USA 2005, 102, 12537–12542. [Google Scholar] [CrossRef] [PubMed]
- Schnupf, P.; Hofmann, J.; Norseen, J.; Glomski, I.J.; Schwartzstein, H.; Decatur, A.L. Regulated translation of listeriolysin O controls virulence of Listeria monocytogenes. Mol. Microbiol. 2006, 61, 999–1012. [Google Scholar] [CrossRef] [PubMed]
- Bavdek, A.; Gekara, N.O.; Priselac, D.; Gutiérrez Aguirre, I.; Darji, A.; Chakraborty, T.; Maček, P.; Lakey, J.H.; Weiss, S.; Anderluh, G. Sterol and pH Interdependence in the Binding, Oligomerization, and Pore Formation of Listeriolysin O. Biochemistry 2007, 46, 4425–4437. [Google Scholar] [CrossRef] [PubMed]
- Blériot, C.; Dupuis, T.; Jouvion, G.; Eberl, G.; Disson, O.; Lecuit, M. Liver-resident macrophage necroptosis orchestrates type 1 microbicidal inflammation and type-2-mediated tissue repair during bacterial infection. Immunity 2015, 42, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Di Paolo, N.C.; Doronin, K.; Baldwin, L.K.; Papayannopoulou, T.; Shayakhmetov, D.M. The transcription factor IRF3 triggers defensive suicide necrosis in response to viral and bacterial pathogens. Cell Rep. 2013, 3, 1840–1846. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, R.M.; Saha, S.K.; Vaidya, S.A.; Bruhn, K.W.; Miranda, G.A.; Zarnegar, B.; Perry, A.K.; Nguyen, B.O.; Lane, T.F.; Taniguchi, T.; et al. Type I Interferon Production Enhances Susceptibility to Listeria monocytogenes Infection. J. Exp. Med. 2004, 200, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Auerbuch, V.; Brockstedt, D.G.; Meyer-Morse, N.; O’Riordan, M.; Portnoy, D.A. Mice lacking the type I interferon receptor are resistant to Listeria monocytogenes. J. Exp. Med. 2004, 200, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, A.; Nishimura, K.; Nakaima, Y.; Oh, T.; Noguchi, S.; Taniguchi, T.; Tamura, T. Cell type-dependent proapoptotic role of Bcl2L12 revealed by a mutation concomitant with the disruption of the juxtaposed Irf3 gene. Proc. Natl. Acad. Sci. USA 2009, 106, 12448–12452. [Google Scholar] [CrossRef] [PubMed]
- Barker, R.N.; Erwig, L.-P.; Pearce, W.P.; Devine, A.; Rees, A.J. Differential Effects of Necrotic or Apoptotic Cell Uptake on Antigen Presentation by Macrophages. Pathobiology 1999, 67, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Festjens, N.; Vanden Berghe, T.; Vandenabeele, P. Necrosis, a well-orchestrated form of cell demise: Signalling cascades, important mediators and concomitant immune response. Biochim. Biophys. Acta Bioenerg. 2006, 1757, 1371–1387. [Google Scholar] [CrossRef] [PubMed]
- Sauter, B.; Albert, M.L.; Francisco, L.; Larsson, M.; Somersan, S.; Bhardwaj, N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J. Exp. Med. 2000, 191, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Theisen, E.; Sauer, J.-D. Listeria monocytogenes-Induced Cell Death Inhibits the Generation of Cell-Mediated Immunity. Infect. Immun. 2017, 85, e00733-16. [Google Scholar] [CrossRef] [PubMed]
- Janda, J.; Schoneberger, P.; Skoberne, M.; Messerle, M.; Russmann, H.; Geginat, G. Cross-presentation of Listeria-derived CD8 T cell epitopes requires unstable bacterial translation products. J. Immunol. 2004, 173, 5644–5651. [Google Scholar] [CrossRef] [PubMed]
- Reinicke, A.T.; Omilusik, K.D.; Basha, G.; Jefferies, W.A. Dendritic cell cross-priming is essential for immune responses to Listeria monocytogenes. PLoS ONE 2009, 4, e7210. [Google Scholar] [CrossRef] [PubMed]
- Von Moltke, J.; Ayres, J.S.; Kofoed, E.M.; Chavarria-Smith, J.; Vance, R.E. Recognition of bacteria by inflammasomes. Annu. Rev. Immunol. 2013, 31, 73–106. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F.; Mayor, A.; Tschopp, J. The inflammasomes: Guardians of the body. Annu. Rev. Immunol. 2009, 27, 229–265. [Google Scholar] [CrossRef] [PubMed]
- DeYoung, K.L.; Ray, M.E.; Su, Y.A.; Anzick, S.L.; Johnstone, R.W.; Trapani, J.A.; Meltzer, P.S.; Trent, J.M. Cloning a novel member of the human interferon-inducible gene family associated with control of tumorigenicity in a model of human melanoma. Oncogene 1997, 15, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Inohara; Chamaillard; McDonald, C.; Nunez, G.; Inohara, N.; Chamaillard, M.; McDonald, C.; Nunez, G. NOD-LRR proteins: role in host-microbial interactions and inflammatory disease. Annu. Rev. Biochem. 2004, 74, 355–383. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F.; Hofmann, K.; Tschopp, J. The pyrin domain: A possible member of the death domain-fold family implicated in apoptosis and inflammation. Curr. Biol. 2001, 11, R118–R120. [Google Scholar] [CrossRef]
- Masumoto, J.; Taniguchi, S.; Ayukawa, K.; Sarvotham, H.; Kishino, T.; Niikawa, N.; Hidaka, E.; Katsuyama, T.; Higuchi, T.; Sagara, J. ASC, a novel 22-kDa protein, aggregates during apoptosis of human promyelocytic leukemia HL-60 cells. J. Biol. Chem. 1999, 274, 33835–33838. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.; Magupalli, V.G.; Ruan, J.; Yin, Q.; Atianand, M.K.; Vos, M.R.; Schröder, G.F.; Fitzgerald, K.A.; Wu, H.; Egelman, E.H. Unified Polymerization Mechanism for the Assembly of ASC-Dependent Inflammasomes. Cell 2014, 156, 1193–1206. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yang, J.; Shi, J.; Gong, Y.-N.; Lu, Q.; Xu, H.; Liu, L.; Shao, F. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature 2011, 477, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Hornung, V.; Ablasser, A.; Charrel-Dennis, M.; Bauernfeind, F.; Horvath, G.; Caffrey, D.R.; Latz, E.; Fitzgerald, K.A. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 2009, 458, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Fernandes-Alnemri, T.; Yu, J.W.; Datta, P.; Wu, J.; Alnemri, E.S. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature 2009, 458, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Mariathasan, S.; Weiss, D.S.; Newton, K.; McBride, J.; O’Rourke, K.; Roose-Girma, M.; Lee, W.P.; Weinrauch, Y.; Monack, D.M.; Dixit, V.M. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 2006, 440, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F.; Burns, K.; Tschopp, J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell 2002, 10, 417–426. [Google Scholar] [CrossRef]
- Von Moltke, J.; Trinidad, N.J.; Moayeri, M.; Kintzer, A.F.; Wang, S.B.; van Rooijen, N.; Brown, C.R.; Krantz, B.A.; Leppla, S.H.; Gronert, K.; et al. Rapid induction of inflammatory lipid mediators by the inflammasome in vivo. Nature 2012, 490, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhao, Y.; Wang, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015, 526, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Kayagaki, N.; Stowe, I.B.; Lee, B.L.; O’Rourke, K.; Anderson, K.; Warming, S.; Cuellar, T.; Haley, B.; Roose-Girma, M.; Phung, Q.T.; et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 2015, 526, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Lamkanfi, M.; Sarkar, A.; Vande Walle, L.; Vitari, A.C.; Amer, A.O.; Wewers, M.D.; Tracey, K.J.; Kanneganti, T.-D.; Dixit, V.M. Inflammasome-dependent release of the alarmin HMGB1 in endotoxemia. J. Immunol. 2010, 185, 4385–4392. [Google Scholar] [CrossRef] [PubMed]
- Fink, S.L.; Cookson, B.T. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell. Microbiol. 2006, 8, 1812–1825. [Google Scholar] [CrossRef] [PubMed]
- Mascarenhas, D.P.A.; Cerqueira, D.M.; Pereira, M.S.F.; Castanheira, F.V.S.; Fernandes, T.D.; Manin, G.Z.; Cunha, L.D.; Zamboni, D.S. Inhibition of caspase-1 or gasdermin-D enable caspase-8 activation in the Naip5/NLRC4/ASC inflammasome. PLOS Pathog. 2017, 13, e1006502. [Google Scholar] [CrossRef] [PubMed]
- Lamkanfi, M.; Dixit, V.M. Mechanisms and Functions of Inflammasomes. Cell 2014, 157, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, I.; Zhang, Y.; Krantz, B.A.; Miao, E.A. Pyroptosis triggers pore-induced intracellular traps (PITs) that capture bacteria and lead to their clearance by efferocytosis. J. Exp. Med. 2016, 213, 2113–2128. [Google Scholar] [CrossRef] [PubMed]
- Miao, E.A.; Leaf, I.A.; Treuting, P.M.; Mao, D.P.; Dors, M.; Sarkar, A.; Warren, S.E.; Wewers, M.D.; Aderem, A. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat. Immunol. 2010, 11, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- Sauer, J.; Pereyre, S.; Archer, K.A.; Burke, T.P.; Hanson, B.; Lauer, P.; Portnoy, D.A. Listeria monocytogenes engineered to activate the Nlrc4 inflammasome are severely attenuated and are poor inducers of protective immunity. Proc. Natl. Acad. Sci. USA 2011, 108, 12419–12424. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Z.; Ruan, J.; Pan, Y.; Magupalli, V.G.; Wu, H.; Lieberman, J. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 2016, 535, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Shen, A.; Higgins, D.E. The MogR transcriptional repressor regulates nonhierarchal expression of flagellar motility genes and virulence in Listeria monocytogenes. PLoS Pathog. 2006, 2, e30. [Google Scholar] [CrossRef] [PubMed]
- Peel, M.; Donachie, W.; Shaw, A. Temperature-dependent Expression of Flagella of Listeria manocytogenes Studied by Electron Microscopy, SDS-PAGE and Western Blotting. Microbiology 1988, 134, 2171–2178. [Google Scholar] [CrossRef] [PubMed]
- Warren, S.E.; Mao, D.P.; Rodriguez, A.E.; Miao, E.A.; Aderem, A. Multiple Nod-like receptors activate caspase 1 during Listeria monocytogenes infection. J. Immunol. 2008, 180, 7558–7564. [Google Scholar] [CrossRef] [PubMed]
- Warren, S.E.; Duong, H.; Mao, D.P.; Armstrong, A.; Rajan, J.; Miao, E.A.; Aderem, A. Generation of a Listeria vaccine strain by enhanced caspase-1 activation. Eur. J. Immunol. 2011, 41, 1934–1940. [Google Scholar] [CrossRef] [PubMed]
- Sauer, J.D.; Witte, C.E.; Zemansky, J.; Hanson, B.; Lauer, P.; Portnoy, D.A. Listeria monocytogenes triggers AIM2-mediated pyroptosis upon infrequent bacteriolysis in the macrophage cytosol. Cell Host Microbe 2010, 7, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.W.; Kayagaki, N.; Broz, P.; Henry, T.; Newton, K.; O’Rourke, K.; Chan, S.; Dong, J.; Qu, Y.; Roose-Girma, M.; et al. Absent in melanoma 2 is required for innate immune recognition of Francisella tularensis. Proc. Natl. Acad. Sci. USA 2010, 107, 9771–9776. [Google Scholar] [CrossRef] [PubMed]
- Pensinger, D.A.; Boldon, K.M.; Chen, G.Y.; Vincent, W.J.B.; Sherman, K.; Xiong, M.; Schaenzer, A.J.; Forster, E.R.; Coers, J.; Striker, R.; et al. The Listeria monocytogenes PASTA Kinase PrkA and Its Substrate YvcK Are Required for Cell Wall Homeostasis, Metabolism, and Virulence. PLOS Pathog. 2016, 12, e1006001. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Y.; McDougal, C.E.; D’Antonio, M.A.; Portman, J.L.; Sauer, J.-D. A Genetic Screen Reveals that Synthesis of 1,4-Dihydroxy-2-Naphthoate (DHNA), but Not Full-Length Menaquinone, Is Required for Listeria monocytogenes Cytosolic Survival. MBio 2017, 8, e00119-17. [Google Scholar] [CrossRef] [PubMed]
- Rae, C.S.; Geissler, A.; Adamson, P.C.; Portnoy, D.A. Mutations of the Listeria monocytogenes Peptidoglycan N-Deacetylase and O-Acetylase Result in Enhanced Lysozyme Sensitivity, Bacteriolysis, and Hyperinduction of Innate Immune Pathways. Infect. Immun. 2011, 79, 3596–3606. [Google Scholar] [CrossRef] [PubMed]
- Meixenberger, K.; Pache, F.; Eitel, J.; Schmeck, B.; Hippenstiel, S.; Slevogt, H.; Guessan, P.N.; Witzenrath, M.; Netea, M.G.; Chakraborty, T.; et al. Listeria monocytogenes-Infected Human Peripheral Blood Mononuclear Cells Produce IL-1{beta}, Depending on Listeriolysin O and NLRP3. J. Immunol. 2009, 184, 922–930. [Google Scholar]
- Sakhon, O.S.; Victor, K.A.; Choy, A.; Tsuchiya, T.; Eulgem, T.; Pedra, J.H.F. NSD1 Mitigates Caspase-1 Activation by Listeriolysin O in Macrophages. PLoS ONE 2013, 8, e75911. [Google Scholar] [CrossRef] [PubMed]
- Hamon, M.A.; Cossart, P. K+ Efflux Is Required for Histone H3 Dephosphorylation by Listeria monocytogenes Listeriolysin O and Other Pore-Forming Toxins. Infect. Immun. 2011, 79, 2839–2846. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.R.; Dustin, M.L.; Sauer, J.-D. Inflammasome-Mediated Inhibition of Listeria monocytogenes-Stimulated Immunity Is Independent of Myelomonocytic Function. PLoS ONE 2013, 8, e83191. [Google Scholar] [CrossRef] [PubMed]
- Mayer-Barber, K.D.; Andrade, B.B.; Oland, S.D.; Amaral, E.P.; Barber, D.L.; Gonzales, J.; Derrick, S.C.; Shi, R.; Kumar, N.P.; Wei, W.; et al. Host-directed therapy of tuberculosis based on interleukin-1 and type I interferon crosstalk. Nature 2014, 511, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Perry, C.J.; Tsui, Y.-C.; Staron, M.M.; Parish, I.A.; Dominguez, C.X.; Rosenberg, D.W.; Kaech, S.M. Prostaglandin E2 and programmed cell death 1 signaling coordinately impair CTL function and survival during chronic viral infection. Nat. Med. 2015, 21, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Nataraj, C.; Thomas, D.W.; Tilley, S.L.; Nguyen, M.T.; Mannon, R.; Koller, B.H.; Coffman, T.M. Receptors for prostaglandin E2 that regulate cellular immune responses in the mouse. J. Clin. Investig. 2001, 108, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Maltez, V.I.; Tubbs, A.L.; Cook, K.D.; Aachoui, Y.; Falcone, E.L.; Holland, S.M.; Whitmire, J.K.; Miao, E.A. Inflammasomes Coordinate Pyroptosis and Natural Killer Cell Cytotoxicity to Clear Infection by a Ubiquitous Environmental Bacterium. Immunity 2015, 43, 987–997. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.F.; Wyllie, A.H.; Currie, A.R. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Thorburn, A. Death receptor-induced cell killing. Cell Signal. 2004, 16, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Riedl, S.J.; Salvesen, G.S. The apoptosome: Signalling platform of cell death. Nat. Rev. Mol. Cell Biol. 2007, 8, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Weinrauch, Y.; Zychlinsky, A. The Induction of Apoptosis by Bacterial Pathogens. Annu. Rev. Microbiol. 1999, 53, 155–187. [Google Scholar] [CrossRef] [PubMed]
- Rogers, H.W.; Callery, M.P.; Deck, B.; Unanue, E.R.; Rogers, H.W.; Gallery, M.P.; Deck, B.; Unanue2, E.R. Listeria monocytogenes induces apoptosis of infected hepatocytes. J. Immunol. 1996, 156, 679–684. [Google Scholar] [PubMed]
- Dos Santos, S.A.; de Andrade Júnior, D.R.; de Andrade, D.R. TNF-α production and apoptosis in hepatocytes after Listeria monocytogenes and Salmonella Typhimurium invasion. Rev. Inst. Med. Trop. Sao Paulo 2011, 53, 107–112. [Google Scholar] [CrossRef]
- Dos Santos, S.A.; de Andrade, D.R.; Andrade, J.D.R. Rat hepatocyte invasion by Listeria monocytogenes and analysis of TNF-alpha role in apoptosis. Rev. Inst. Med. Trop. Sao Paulo 2005, 47, 73–80. [Google Scholar] [CrossRef]
- Margaroli, C.; Oberle, S.; Lavanchy, C.; Scherer, S.; Rosa, M.; Strasser, A.; Pellegrini, M.; Zehn, D.; Acha-Orbea, H.; Ehirchiou, D. Role of proapoptotic BH3-only proteins in Listeria monocytogenes infection. Eur. J. Immunol. 2016, 46, 1427–1437. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.-J.; Jiang, J.; Shen, H.; Chen, Y.H. Reduced apoptosis and ameliorated listeriosis in TRAIL-null mice. J. Immunol. 2004, 173, 5652–5658. [Google Scholar] [CrossRef] [PubMed]
- Mandel, T.E.; Cheers, C. Resistance and Susceptibility of Mice to Bacterial Infection: Histopathology of Listeriosis in Resistant and Susceptible Strains. Infect. Immun. 1980, 30, 851–861. [Google Scholar] [PubMed]
- Merrick, J.C.; Edelson, B.T.; Bhardwaj, V.; Swanson, P.E.; Unanue, E.R. Lymphocyte apoptosis during early phase of Listeria infection in mice. Am. J. Pathol. 1997, 151, 785–792. [Google Scholar] [PubMed]
- Carrero, J.A.; Calderon, B.; Unanue, E.R. Lymphocytes are detrimental during the early innate immune response against Listeria monocytogenes. J. Exp. Med. 2006, 203, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.A.; Vivanco-Cid, H.; Unanue, E.R. Granzymes drive a rapid listeriolysin O-induced T cell apoptosis. J. Immunol. 2008, 181, 1365–1374. [Google Scholar] [CrossRef] [PubMed]
- Browne, K.A.; Blink, E.; Sutton, V.R.; Froelich, C.J.; Jans, D.A.; Trapani, J.A. Cytosolic delivery of granzyme B by bacterial toxins: evidence that endosomal disruption, in addition to transmembrane pore formation, is an important function of perforin. Mol. Cell. Biol. 1999, 19, 8604–8615. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.A.; Calderon, B.; Vivanco-Cid, H.; Unanue, E.R. Recombinant Listeria monocytogenes Expressing a Cell Wall-Associated Listeriolysin O Is Weakly Virulent but Immunogenic. Infect. Immun. 2009, 77, 4371–4382. [Google Scholar] [CrossRef] [PubMed]
- Sauer, J.-D.; Sotelo-Troha, K.; von Moltke, J.; Monroe, K.M.; Rae, C.S.; Brubaker, S.W.; Hyodo, M.; Hayakawa, Y.; Woodward, J.J.; Portnoy, D.A.; et al. The N-Ethyl-N-Nitrosourea-Induced Goldenticket Mouse Mutant Reveals an Essential Function of Sting in the In Vivo Interferon Response to Listeria monocytogenes and Cyclic Dinucleotides. Infect. Immun. 2011, 79, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Woodward, J.J.; Iavarone, A.T.; Portnoy, D.A. c-di-AMP Secreted by Intracellular Listeria monocytogenes Activates a Host Type I Interferon Response. Science 2010, 328, 1703–1705. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.; Prabakaran, T.; Laustsen, A.; Jørgensen, S.E.; Rahbæk, S.H.; Jensen, S.B.; Nielsen, R.; Leber, J.H.; Decker, T.; Horan, K.A.; et al. Listeria monocytogenes induces IFNβ expression through an IFI16-, cGAS- and STING-dependent pathway. EMBO J. 2014, 33, 1654–1666. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.A.; Calderon, B.; Unanue, E.R. Type I interferon sensitizes lymphocytes to apoptosis and reduces resistance to Listeria infection. J. Exp. Med. 2004, 200, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Voll, R.E.; Herrmann, M.; Roth, E.A.; Stach, C.; Kalden, J.R.; Girkontaite, I. Immunosuppressive effects of apoptotic cells. Nature 1997, 390, 350–351. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.J.; Köhler, G.; Brombacher, F. Both innate and acquired immunity to Listeria monocytogenes infection are increased in IL-10-deficient mice. J. Immunol. 1997, 158, 2259–2267. [Google Scholar] [PubMed]
- Bancroft, G.J.; Bosma, M.J.; Bosma, G.C.; Unanue, E.R. Regulation of macrophage Ia expression in mice with severe combined immunodeficiency: Induction of Ia expression by a T cell-independent mechanism. J. Immunol. 1986, 137, 4–9. [Google Scholar] [PubMed]
- Bhardwaj, V.; Kanagawa, O.; Swanson, P.E.; Unanue, E.R. Chronic Listeria infection in SCID mice: Requirements for the carrier state and the dual role of T cells in transferring protection or suppression. J. Immunol. 1998, 160, 376–384. [Google Scholar] [PubMed]
- Archer, K.A.; Durack, J.; Portnoy, D.A. STING-dependent type I IFN production inhibits cell-mediated immunity to Listeria monocytogenes. PLoS Pathog. 2014, 10, e1003861. [Google Scholar] [CrossRef] [PubMed]
- Pattabiraman, G.; Palasiewicz, K.; Visvabharathy, L.; Freitag, N.E.; Ucker, D.S. Apoptotic cells enhance pathogenesis of Listeria monocytogenes. Microb. Pathog. 2017, 105, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Gougeon, M.L.; Lecoeur, H.; Dulioust, A.; Enouf, M.G.; Crouvoiser, M.; Goujard, C.; Debord, T.; Montagnier, L. Programmed cell death in peripheral lymphocytes from HIV-infected persons: increased susceptibility to apoptosis of CD4 and CD8 T cells correlates with lymphocyte activation and with disease progression. J. Immunol. 1996, 156, 3509–3520. [Google Scholar] [PubMed]
- Early, J.; Fischer, K.; Bermudez, L.E. Mycobacterium avium uses apoptotic macrophages as tools for spreading. Microb. Pathog. 2011, 50, 132–139. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McDougal, C.E.; Sauer, J.-D. Listeria monocytogenes: The Impact of Cell Death on Infection and Immunity. Pathogens 2018, 7, 8. https://doi.org/10.3390/pathogens7010008
McDougal CE, Sauer J-D. Listeria monocytogenes: The Impact of Cell Death on Infection and Immunity. Pathogens. 2018; 7(1):8. https://doi.org/10.3390/pathogens7010008
Chicago/Turabian StyleMcDougal, Courtney E., and John-Demian Sauer. 2018. "Listeria monocytogenes: The Impact of Cell Death on Infection and Immunity" Pathogens 7, no. 1: 8. https://doi.org/10.3390/pathogens7010008
APA StyleMcDougal, C. E., & Sauer, J.-D. (2018). Listeria monocytogenes: The Impact of Cell Death on Infection and Immunity. Pathogens, 7(1), 8. https://doi.org/10.3390/pathogens7010008




