Morphometric and Molecular Insights into Hepatozoon spp. in Wild and Synanthropic Rodents from Southern and Southeastern Brazil
Abstract
1. Introduction
2. Materials and Methods
2.1. Rodent Capture and Handling
2.2. Preparation of Blood Smears for Gametocytes Research and Analysis
2.3. Statistical Analysis
2.4. DNA Extraction
2.5. Molecular Analysis
2.6. Electrophoresis of Reactions, Purification of Amplified Products, and Sequencing
2.7. Phylogenetic Analysis
3. Results
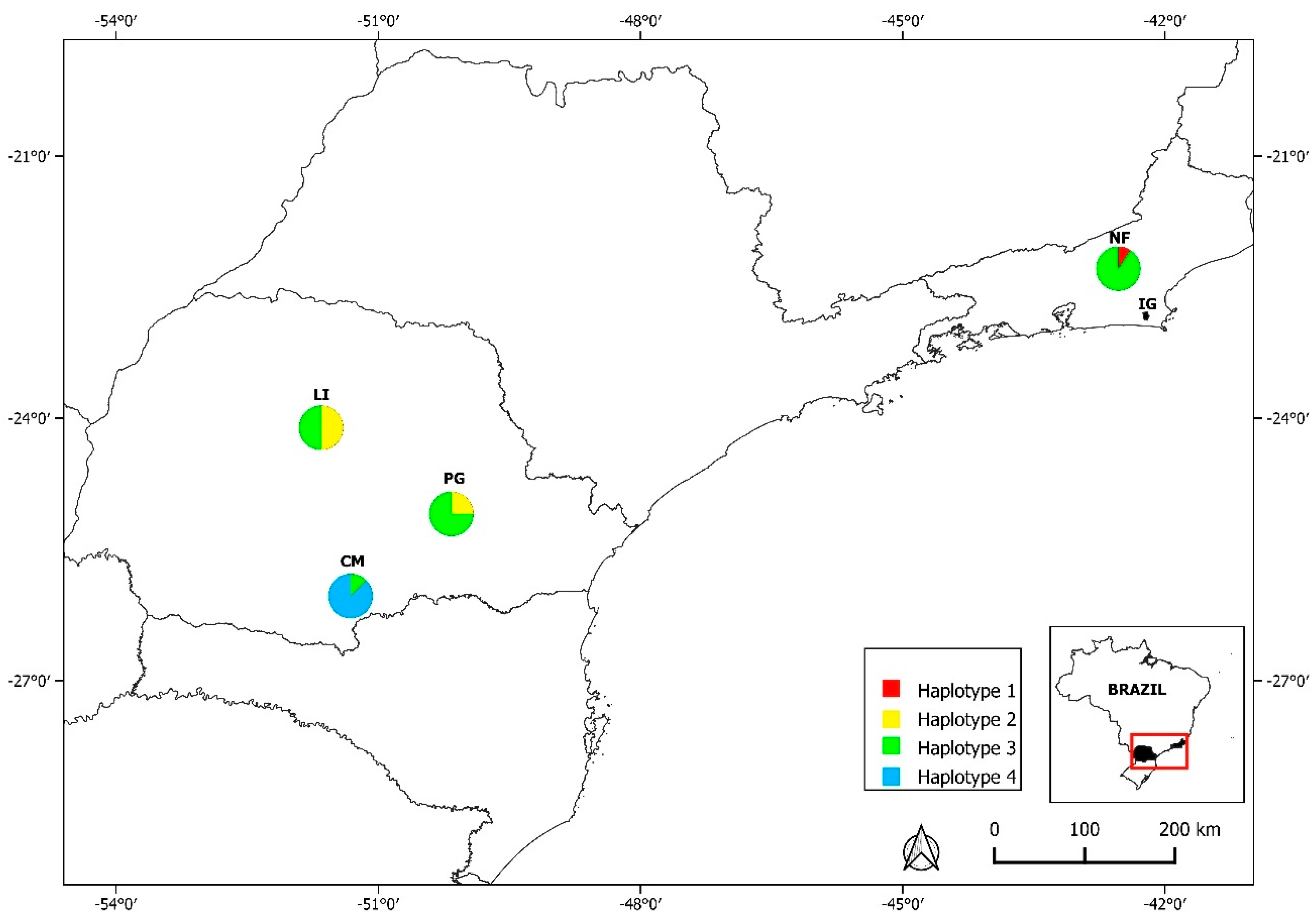
3.1. Distribution of Rodents
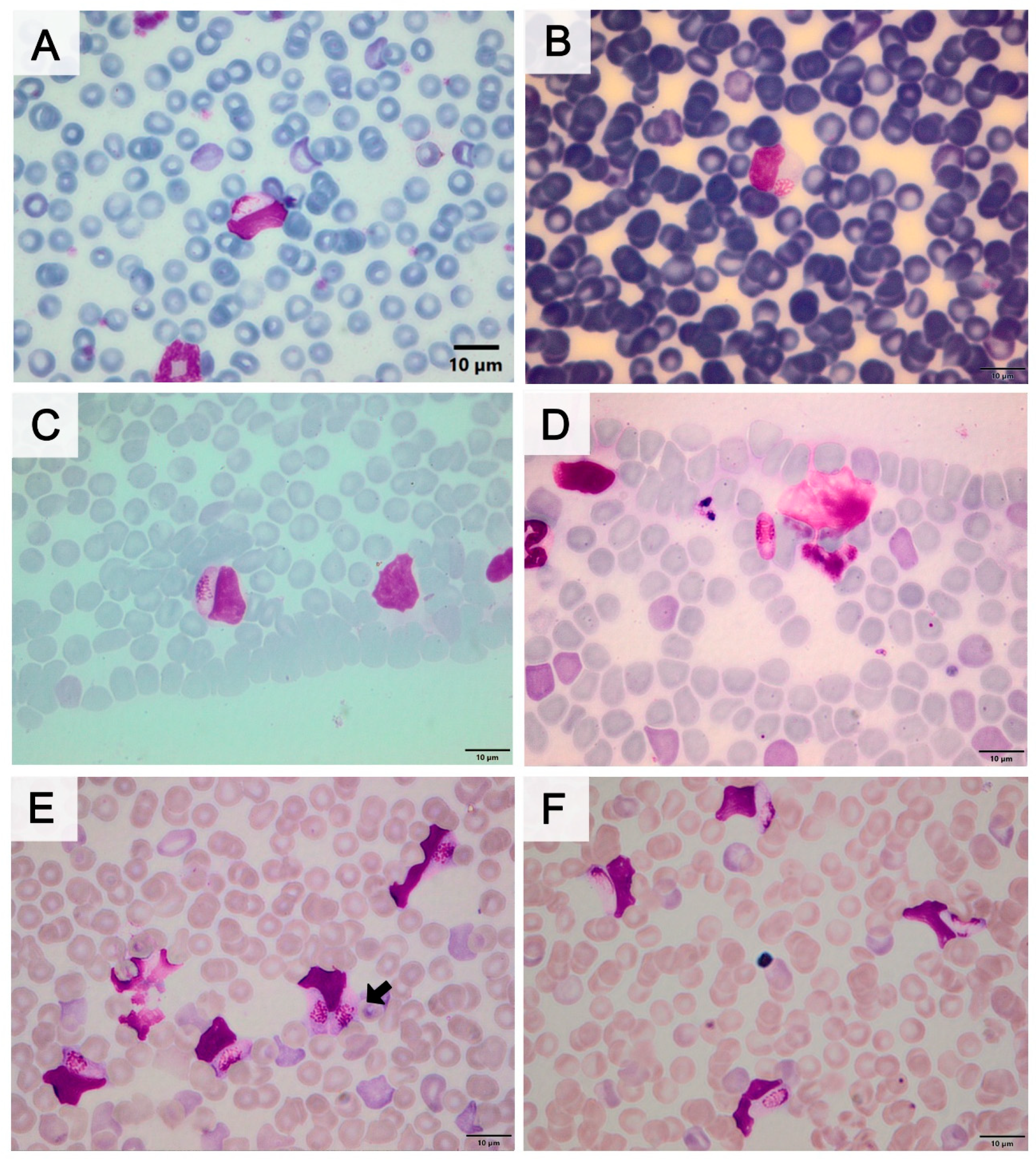
3.2. Morphometric Analysis of Gametocytes of Hepatozoon spp.
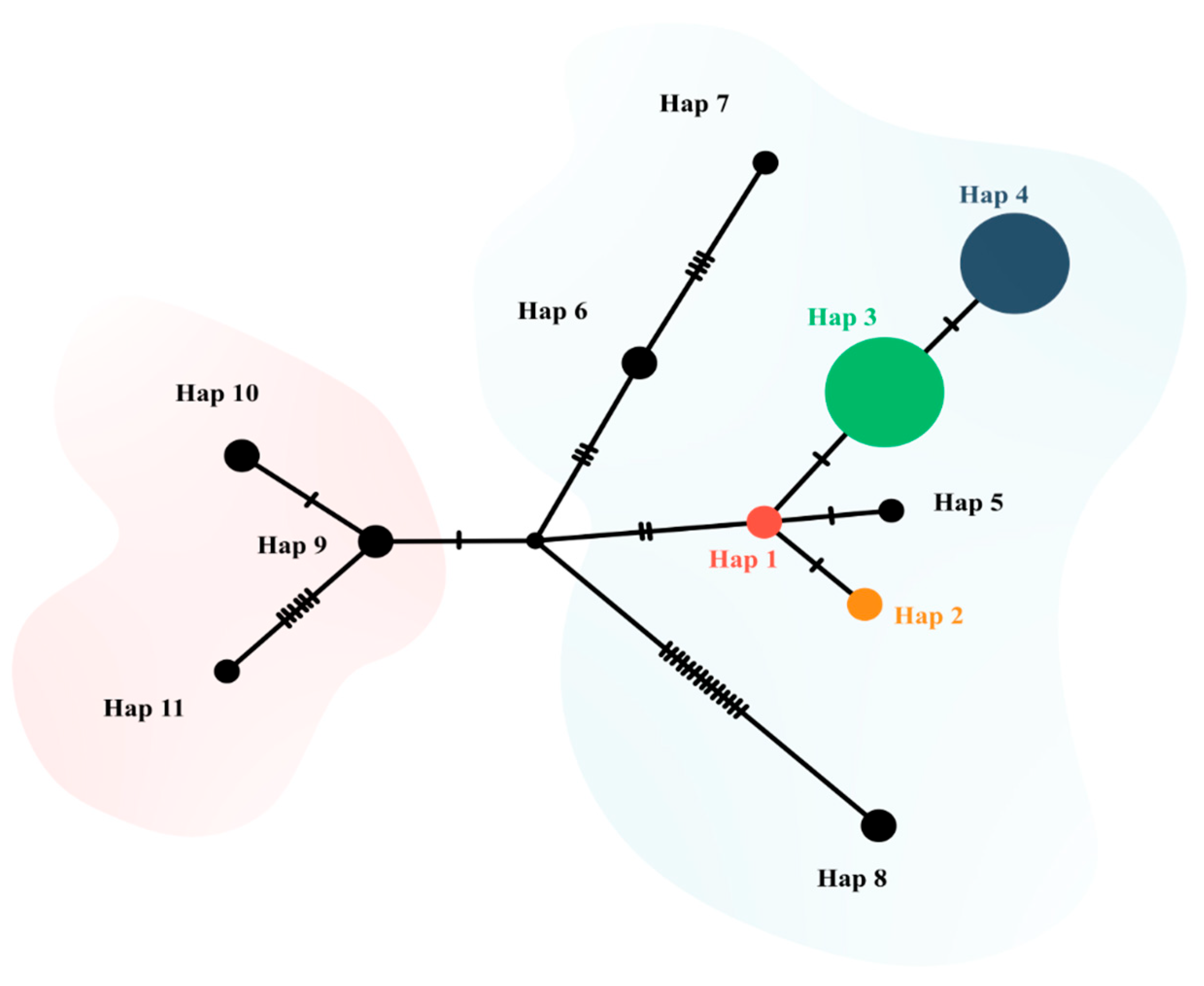
3.3. Detection and Phylogenetic Analysis of Hepatozoon spp.
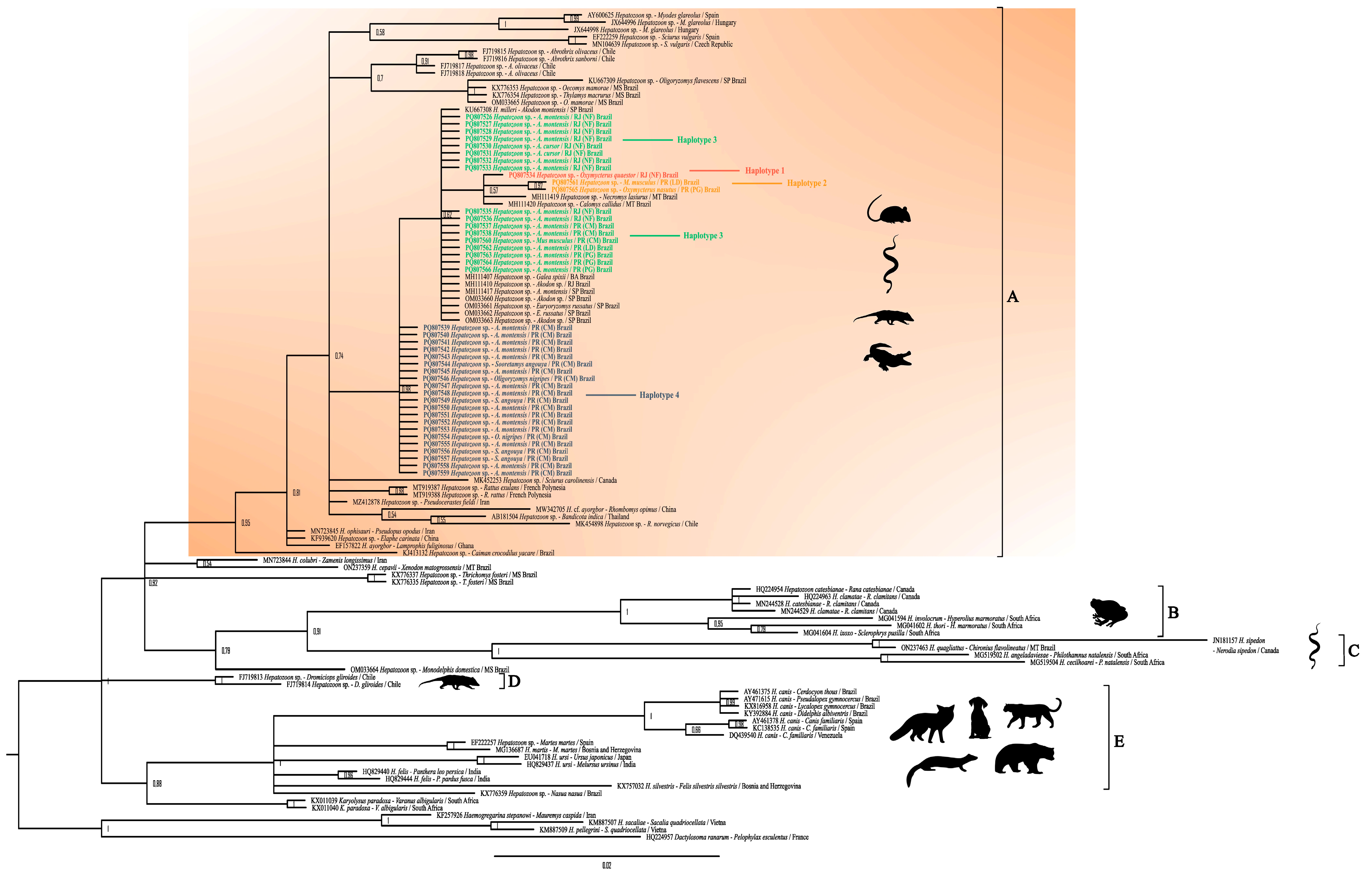
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DNA | Deoxyribonucleic acid |
| PCR | Polymerase chain reaction |
| Cytb | Cytochrome b |
| µM | Micrometer |
| SD | Standard deviation |
References
- Miller, W.W. Hepatozoon perniciosum (n. g., n. sp.); A haemogregarine pathogenic for white rats; With a description of the sexual cycle in the inter mediate host, a mite (Lelaps echidninus). Hygienic Lab. Bull. 1908, 46, 51–123. [Google Scholar]
- Smith, T.G. The genus Hepatozoon (apicomplexa: Adeleina). J. Parasitol. 1996, 82, 565–585. [Google Scholar] [CrossRef] [PubMed]
- Baneth, G. Perpectives on canine and feline hepatozoonosis. Vet. Parasitol. 2011, 181, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Maia, J.P.; Carranza, S.; Harris, D.J. Comments on the Systematic Revision of Adeleid Haemogregarines: Are More Data Needed? J. Parasitol. 2016, 102, 549–552. [Google Scholar] [CrossRef]
- Baneth, G.; Samish, M.; Shkap, V. Life Cycle of Hepatozoon canis (Apicomplexa: Adeleorina: Hepatozoidae) in the Tick Rhipicephalus sanguineus and Domestic Dog (Canis familiaris). J. Parasitol. 2007, 93, 283–299. [Google Scholar] [CrossRef]
- Demoner, L.C.; Silva, M.R.L.; Magro, N.M.; O’Dwyer, L.H. Hepatozoon milleri sp. nov. (Adeleorina: Hepatozoidae) in Akodon montensis (Rodentia: Cricetidae: Sigmodntinae) from southeastern Brazil. Parasitology 2018, 146, 662–669. [Google Scholar] [CrossRef]
- Johnson, P.T.J.; Dobson, A.; Lafferty, K.D.; Marcogliese, D.J.; Memmott, J.; Orlofske, S.A.; Poulin, R.; Thieltges, D.W. When parasites become prey: Ecological and epidemiological significance of eating parasites. Trends Ecol. Evol. 2010, 25, 362–371. [Google Scholar] [CrossRef]
- Johnson, E.M.; Allen, K.E.; Breshears, M.A.; Panciera, R.J.; Little, S.E.; Ewing, S.A. Experimental transmission of Hepatozoon americanum to rodents. Vet. Parasitol. 2008, 151, 164–169. [Google Scholar] [CrossRef]
- Jonhson, E.M.; Panciera, R.J.; Allen, K.E.; Sheets, M.E.; Beal, J.D.; Ewing, S.A.; Little, S.E. Alternate Pathway of Infection with Hepatozoon americanum and the Epidemiologic Importance of Predation. J. Vet. Intern. Med. 2009, 23, 1315–1318. [Google Scholar] [CrossRef]
- Maia, J.P.; Álvares, F.; Boratynski, Z.; Brito, J.C.; Leite, J.V.; Harris, J. Molecular assessment of Hepatozoon (Apicomplexa: Adeleorina) infections in wild canids and rodents from North Africa, with implications for transmission dynamics across taxonomic groups. J. Wildl. Dis. 2014, 50, 837–848. [Google Scholar] [CrossRef]
- Murata, T.; Inoue, M.; Tateyama, S.; Taura, Y.; Nakama, S. Vertical transmission of Hepatozoon canis in dogs. J. Vet. Med. Sci. 1993, 55, 867–868. [Google Scholar] [CrossRef] [PubMed]
- Tolkacz, K.; Kowalec, M.; Alsarraf, M.; Grzybek, M.; Dwuznik-Sazare, D.; Behnke, J.M.; Bajer, A. Candidatus Neoehrlichia mikurensis and Hepatozoon sp. in voles (Microtus spp.): Occurrence and evidence for vertical transmission. Sci. Rep. 2023, 13, 1733. [Google Scholar] [CrossRef] [PubMed]
- Demoner, L.C.; Magro, N.M.; Silva, M.R.L.; Antunes, J.A.P.; Calabuig, C.I.P.; O’Dwyer, L.H. Hepatozoon spp. infections in wild rodents in an area of endemic canine hepatozoonosis in southeastern Brazil. Tick Tick-Borne Dis. 2016, 7, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Wolf, R.W.; Aragona, M.; Muñoz-Leal, S.; Pinto, L.B.; Melo, A.L.T.; Braga, I.A.; Costa, J.S.; Martins, T.F.; Marcili, A.; Pacheco, R.C.; et al. Novel Babesia and Hepatozoon agents infecting non-volant small mammals in the Brazilian Pantanal, with the first record of the tick Ornithodoros guaporensis in Brazil. Tick Tick-Borne Dis. 2016, 7, 449–456. [Google Scholar] [CrossRef]
- Gomes, L.A.; Moraes, L.A.; Aguiar, D.C.F.; Dias, H.L.T.; Ribeiro, A.S.S.; Rocha, H.P.C.; Nunes, M.R.T.; Gonçalves, E.C. Genetic diversity of Hepatozoon spp. in Hydrochoerus hydrochaeris and Pecari tajacu from eastern Amazon. Ticks Tick-Borne Dis. 2018, 9, 314–318. [Google Scholar] [CrossRef]
- Sousa, K.C.M.; Fernandes, M.P.; Herrera, H.M.; Benevenute, J.L.; Santos, F.M.; Barreto, W.T.G.; Macedo, G.C.; Campos, J.B.; Martins, T.F.; Pinto, P.C.E.A.; et al. Molecular detection of Hepatozoon spp. in domestic dogs and wild mammals in southern Pantanal, Brazil with implications in the transmission route. Vet. Parasitol. 2017, 237, 37–46. [Google Scholar] [CrossRef]
- Perles, L.; Roque, A.L.R.; D’Andrea, P.S.; Lemos, E.R.S.; Santos, A.F.; Morales, A.C.; Machado, R.Z.; André, M.R. Genetic diversity of Hepatozoon spp. in rodents from Brazil. Sci. Rep. 2019, 9, 10122. [Google Scholar] [CrossRef]
- Santos, F.M.; Sousa, K.C.M.; Sano, N.Y.; Nantes, W.A.G.; Liberal, S.C.; Machado, R.Z.; André, M.R.; Herrera, H.M. Relationships between vector-borne parasites and free-living mammals at the Brazilian Pantanal. Parasitol. Res. 2021, 120, 1003–1010. [Google Scholar] [CrossRef]
- Weck, B.C.; Serpa, M.C.A.; Ramos, V.N.; Luz, H.R.; Ramirez, D.G.; Benatti, H.R.; Piovezan, U.; Szabó, M.P.J.; Marcili, A.; Krawczak, F.S.; et al. Novel genotypes of Hepatozoon spp. in small mammals, Brazil. Parasite Vector 2022, 15, 87. [Google Scholar] [CrossRef]
- Merino, S.; Vásquez, R.A.; Martínez, J.; Celis-Diez, J.L.; Gutiérrez-Jimenes, L.; Ippi, S.; Sánchez-Monsalvez, I.; La Puente, J.M. Molecular characterization of an ancient Hepatozoon species parasitizing the ‘living fossil’ marsupial ‘Monito del Monte’ Dromiciops gliroides from Chile. Biol. J. Linn. Soc. 2009, 98, 568–576. [Google Scholar] [CrossRef]
- Alabi, A.S.; Monti, G.; Otth, C.; Sepulveda-Garcia, P.; Perles, L.; Machado, R.Z.; André, M.R.; Bittencourt, P.; Muller, A. Genetic diversity of Hepatozoon spp. in rodents from Chile. Braz. J. Vet. Parasitol. 2021, 30, e012721. [Google Scholar] [CrossRef] [PubMed]
- Santodomingo, A.M.; Thomas, R.S.; Quintero-Galvis, J.F.; Echeverry-Berrio, D.M.; Silva-de la Fuente, M.C.; Moreno-Salas, L.; Muñoz-Leal, S. Apicomplexans in small mammals from Chile, with the first report of the Babesia microti group in South American rodents. Parasitol. Res. 2022, 121, 1009–1020. [Google Scholar] [CrossRef] [PubMed]
- Jonhson, E.M.; Allen, K.E.; Panciera, R.J.; Ewing, S.A.; Little, S.E.; Reichard, M.V. Field survey of rodents for Hepatozoon infections in na endemic focus of American canine hepatozoonosis. Vet. Parasitol. 2007, 150, 27–32. [Google Scholar] [CrossRef]
- Ljaz, M.; Khan, A.U.; Ali, M.; Naeem, M.; Ibenmoussa, S.; Dawoud, R.M.; Khan, A.; Said, M.B.; Iqbal, F. First report of Hepatozoon and Lankesterella spp. infections in wild rodents from Pakistan, and their potential impact on blood parameters and oxidative stress markers in vital organs. Vet. Res. Commun. 2025, 49, 45. [Google Scholar]
- Carini, A.; Maciel, J. Sur une hémogrégarine et um trypanosome d’un Muridé (Akodon fuliginosus). Bull. Soc. Pathol. Exot. 1915, 8, 165–169. [Google Scholar]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World Map of the Köppen-Geiger climate classification updated. Metereol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef]
- Peel, M.C.; Finlayson, B.L.; Mcmahon, T.A. Update world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- Mills, J.N.; Childs, J.E.; Ksiazek, T.G.; Velleca, W.M. Methods for Trapping and Sampling Small Mammals for Virologic Testing. Department of Health & Human Services; Centers for Disease Control and Prevention: Atlanta, GA, USA, 1995; pp. 1–61. [Google Scholar]
- Lemos, E.R.S.; D’Andrea, P.S. Trabalho de Campo com Animais: Procedimentos, Riscos e Biossegurança, 1st ed.; Fiocruz: Rio de Janeiro, Brazil, 2014; 180p. [Google Scholar]
- D’Andrea, P.S.; Teixeira, B.R.; Gonçalves-Oliveira, J.; Dias, D.; Vilela, R.V.; Lúcio, C.S.; Santos, F.O.; Costa, G.C.; Carvalhaes, J.G.; Santos, M.M.; et al. The Mammal Collection of the Laboratory of Biology and Parasitology of Reservoir Wild Mammals—Oswaldo Cruz Foundation. Braz. J. Mammal. 2021, 90, e90202119. [Google Scholar] [CrossRef]
- Ayres, M.; Ayres, J.M.; Aures, D.L.; Santos, A.D.A. Aplicações Estatísticas nas Áreas das Ciências Bio-Médicas; Instituto Mamirauá: Belém, Brazil, 2007; Volume 364. [Google Scholar]
- Smith, M.F.; Patton, J.L. Phylogenetic relationships and the radiation of sigmodontine rodents in South America: Evidence from Cytochrome b. J. Mamm. Evol. 1999, 6, 89–128. [Google Scholar] [CrossRef]
- Smith, M.F.; Patton, J.L. The diversification of South American murid rodents: Evidence from mitochondrial DNA sequence data for the akodontine tribe. Biol. J. Linn. Soc. 1993, 50, 149–177. [Google Scholar] [CrossRef]
- Ujvari, B.; Madsen, T.; Olsson, M. High prevalence Hepatozoon spp. (Apicomplexa, Hepatozoidae) infection in water pythons (Liasis fuscus) from tropical Australia. J. Parasitol. 2004, 90, 670–672. [Google Scholar] [CrossRef] [PubMed]
- Criado-Fornelio, A.; Ruas, J.L.; Casado, N.; Farias, N.A.R.; Soares, M.P.; Müller, G.; Brum, J.G.W.; Berne, M.E.A.; Buling-Saraña, A.; Barba-Carretero, J.C. New molecular data on mammalian Hepatozoon species (Apicomplexa: Adeleorina) from Brazil and Spain. J. Parasitol. 2006, 92, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Hrazdilová, K.; Cervená, B.; Blanvillain, C.; Foronda, P.; Modrý, D. Quest for the type species of the genus Hepatozoon—phylogenetic position of hemogregarines of rats and consequences for taxonomy. Syst. Biodivers. 2021, 19, 622–631. [Google Scholar] [CrossRef]
- Kvicerová, J.; Pakandl, M.; Hypsa, V. Phylogenetic relationships among Eimeria spp. (Apicomplexa, Eimeriidae) infecting rabbits: Evolutionary significance of biological and morphological features. Parasitolology 2008, 135, 443–452. [Google Scholar] [CrossRef]
- Quilfeldt, P.; Romeike, T.; Masello, J.F.; Reiner, G.; Willems, H.; Bedolla-Guzmán, Y. Molecular survey of coccidian infections of the side-blotched lizard Uta stansburiana on San Benito Oeste Island, Mexico. Parasite 2018, 25, 43. [Google Scholar] [CrossRef]
- Benson, D.A.; Karsch-Mizrachi, I.; Lipman, D.J.; Ostell, J.; Rapp, B.A.; Wheeler, D.L. GenBank. Nucleic Acids Res. 2000, 28, 15–18. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Maddison, W.P.; Maddison, D.R. Mesquite: A Modular System for Evolutionary Analysis; Version 3.81; University of California: Davis, CA, USA, 2023. [Google Scholar]
- Ronques, F.; Teslenko, M.; Mark, V.; Darling, A.; Höhna, S.; Larget, B.; Suchard, A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010; pp. 1–8. [Google Scholar]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarisation in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef]
- Rozas, J.; Ferrer-Mat, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Garcia, A. DnaSP v6: DNA Sequence Polymorphism Analysis of Large Datasets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef]
- Excoffier, L.; Smouse, P.E.; Quattro, P.E. Analysis of molecular variance inferred from metric distances among DNA haplotypes: Application to human mitochondrial DNA restriction data. Genetics 1992, 131, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Wright, S. Evolution and the Genetics of Populations. Variability Within and Among Natural Populations; University of Chicago Press: Chicaco, IL, USA, 1978; Volume 4. [Google Scholar]
- Excoffier, L.; Lischer, H.E.L. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 2010, 10, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Karbowiak, G.; Rychlik, L.; Nowakowski, W.; Wita, I. Natural infections of small mammals with blood parasites on the borderland of boreal and temperature forest zones. Acta Theriol. 2005, 50, 31–42. [Google Scholar] [CrossRef]
- Leveille, N.A.; Skhawy, N.E.; Barta, J.R. Multilocus sequencing of Hepatozoon cf. griseisciuri infections in Ontario Eastern gray squirrels (Sciurus carolinensis) uncovers two genotypically distinct sympatric parasite species. Parasitol. Res. 2020, 119, 713–724. [Google Scholar]
- Sloboda, M.; Kamler, M.; Bulantová, J.; Votýpka, J.; Modrý, D. A new species of Hepatozoon (Apicomplexa: Adeleorina) from Python regius (Serpentes: Pythonidae) and its experimental transmission by a mosquito vector. J. Parasitol. 2007, 93, 1189–1198. [Google Scholar] [CrossRef]
- Poulin, R.; Keeney, D.B. Host specificity under molecular and experimental scrutiny. Trends Parasitol. 2008, 24, 24–28. [Google Scholar] [CrossRef]
- Pardiñas, U.F.J.; Teta, P.; Alvarado-Serrano, D.; Geise, L.; Jayat, J.P.; Ortiz, P.E.; Gonçalves, P.E.; Della, G. Genus Akodon. In Mammals of South America, 1st ed.; Patton, J.L., Pardiñas, U.F.J., D’Elía, G., et al., Eds.; The University of Chicago Press: Chicaco, IL, USA, 2015; Volume 2, pp. 144–203. [Google Scholar]
- Sloboda, M.; Kamler, M.; Bulantová, J.; Votýpka, J.; Modrý, D. Rodents as intermediate hosts of Hepatozoon ayorgbor (Apicomplexa: Adeleina: Hepatozoidae) from the African ball python Python regius. Folia Parasitol. 2008, 55, 13–16. [Google Scholar] [CrossRef]
- Ungari, L.P.; Netherlands, E.C.; Santos, A.L.Q.; Alcantara, E.P.; Emmerich, E.; Silva, R.J.; O’Dwyer, L.H. Diversity of haemogregarine parasites infecting Brazilian snakes from the Midwest and Southeast regions with a description of two new species of Hepatozoon (Apicomplexa: Adeleorina: Hepatozoidae). Parasitol. Int. 2022, 89, 102587. [Google Scholar] [CrossRef]
- Santos, F.O.; Moreira, J.C.; Gonçalves, P.R.; Lucio, C.S.; Teixeira, B.R.; D’Andrea, P.S. Necromys lasiurus (Cricetidae: Sigmodontinae) from open areas of the Atlantic Forest of Rio de Janeiro: Population structure and implications for the monitoring of hantaviroses. Zoologia 2024, 41, e23086. [Google Scholar] [CrossRef]
- Thomas, R.; Santodomingo, A.; Saboya-Acosta, L.; Quintero-Galvis, J.F.; Moreno, L.; Uribe, J.E.; Muñoz-Leal, S. Hepatozoon (Eucoccidiorida: Hepatozoidae) in wild mammals of the Americas: A systematic review. Parasite Vector 2024, 17, 1–23. [Google Scholar]
- Ferreri, M.; Qu, W.; Han, B. Phylogenetic networks: A tool to display character conflict and demographic history. Afr. J. Biothecnol. 2011, 10, 12799–12803. [Google Scholar]
- Boullianne, B.; Evans, R.C.; Smith, T.G. Phylogenetic analysis of Hepatozoon species (Apicomplexa: Adelerina) infecting frogs of Nova Scotia, Canada, determined by ITS-1 sequences. J. Parasitol. 2007, 93, 1435–1441. [Google Scholar] [CrossRef] [PubMed]
| Family | Study Areas | ||||||
|---|---|---|---|---|---|---|---|
| Cricetidae | PG | CM | LD | IG | NF | n/Total (Prevalence) | |
| Akodon cursor (Winge, 1887) | 6/29 (20.68%) | 6/29 (20.68%) | |||||
| Akodon montensis (Tomas, 1913) | 3/5 (60%) | 18/26 (69.23%) | 1/30 (3.33%) | 27/45 (60%) | 49/106 (46.23%) | ||
| Akodon paranaensis (Christoff et al., 2000) | 0/11 | 0/11 | |||||
| Delomys sublineatus (Thomas, 1903) | 0/1 | 0/1 | |||||
| Euryoryzomys russatus (Wagner, 1848) | 0/5 | 0/5 | |||||
| Juliomys ossitenuis (Costa et al., 2007) | 0/1 | 0/1 | |||||
| Necromys lasiurus (Lund, 1841) | 0/2 | 0/1 | 0/3 | ||||
| Nectomys squamipes (Brants, 1827) | 0/1 | 0/1 | |||||
| Oligoryzomys flavescens (Waterhouse, 1837) | 0/4 | 0/4 | |||||
| Oligoryzomys nigripes (Olfers, 1818) | 0/18 | 6/12 (50%) | 0/1 | 0/9 | 6/40 (15%) | ||
| Oxymycterus nasutus (Waterhouse, 1837) | 1/6 (16.66%) | 1/6 (16.66%) | |||||
| Oxymycterus quaestor (Thomas, 1903) | 3/16 (18.57%) | 3/16 (18.75%) | |||||
| Sooretamys angouya (Fischer, 1814) | 5/11 (45.45%) | 0/1 | 5/12 (38.46%) | ||||
| Thaptomys nigrita (Lichtenstein, 1829) | 0/2 | 0/2 | |||||
| Muridae | |||||||
| Mus musculus (Linnaeus, 1758) | 1/1 (100%) | 1/16 (6.25%) | 0/25 | 2/42 (4.76%) | |||
| Rattus rattus (Linnaeus, 1758) | 0/2 | 0/8 | 0/10 | ||||
| n/total (prevalence) | 4/55 (7.27%) | 30/52 (57.69%) | 2/48 (4.16%) | 0/25 | 36/109 (33%) | 72/289 (24.91%) | |
| Haplotype | GenBank ID | Host/Location (This Study) | Best Blast Hit ID | Host/Location (Best BLAST Hit) | Identity (%) | Query Coverage (%) | E-Value |
|---|---|---|---|---|---|---|---|
| Hap 1 | PQ807534 | O. quaestor/RJ | OM033660 e OM033663 | Akodon sp., SP | 99.81 | 100 | 0.0 |
| OM033661 e OM033662 | E. russatus, SP | ||||||
| Hap 2 | PQ807561, PQ807565 | M. musculus, O. nasutus/PR | MH111420 | Calomys callidus, MT | 99.84 | 100 | 0.0 |
| Hap 3 | PQ807530 | A. cursor/RJ | OM033663, Akodon sp., SP | Akodon sp., SP | 100 | 100 | 0.0 |
| OM033660, Akodon sp., SP | Akodon sp., SP | ||||||
| Hap 4 | PQ807539 | A. montensis/PR | OM033660 e OM033663 | Akodon sp., SP | 99.94 | 100 | 0.0 |
| OM033661 e OM033662 | E. russatus, SP |
| Haplotypes | n | Mean (µM) | SD (µM) | Limit Values | |||
|---|---|---|---|---|---|---|---|
| Minimum (µM) | Maximum (µM) | p-Value | |||||
| Gametocyte length | |||||||
| Hap 3 | 96 | 10.82 | a | 0.82 | 8.12 | 12.26 | |
| Hap 4 | 153 | 10.95 | a | 0.73 | 8.90 | 13.91 | <0.01 |
| Gametocyte width | |||||||
| Hap 3 | 96 | 4.59 | a | 0.51 | 3.33 | 5.65 | |
| Hap 4 | 153 | 4.55 | a | 0.46 | 3.29 | 5.86 | 0.560 |
| Nuclear length | |||||||
| Hap 3 | 96 | 6.05 | a | 1.14 | 3.76 | 8.72 | |
| Hap 4 | 153 | 5.94 | a | 0.92 | 3.16 | 8.98 | 0.584 |
| Nuclear width | |||||||
| Hap 3 | 96 | 3.49 | a | 0.66 | 1.56 | 5.77 | |
| Hap 4 | 153 | 3.37 | b | 0.51 | 2.19 | 4.72 | 0.109 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freitas, T.P.T.d.; Teixeira, B.R.; Machado, E.d.O.S.L.; Pinto, I.L.L.; Oliveira, L.d.S.d.; Varella, K.; Santos, H.A.; Santos, F.d.O.; Tiepolo, L.M.; Massard, C.L.; et al. Morphometric and Molecular Insights into Hepatozoon spp. in Wild and Synanthropic Rodents from Southern and Southeastern Brazil. Pathogens 2025, 14, 756. https://doi.org/10.3390/pathogens14080756
Freitas TPTd, Teixeira BR, Machado EdOSL, Pinto ILL, Oliveira LdSd, Varella K, Santos HA, Santos FdO, Tiepolo LM, Massard CL, et al. Morphometric and Molecular Insights into Hepatozoon spp. in Wild and Synanthropic Rodents from Southern and Southeastern Brazil. Pathogens. 2025; 14(8):756. https://doi.org/10.3390/pathogens14080756
Chicago/Turabian StyleFreitas, Tatiana Pádua Tavares de, Bernardo Rodrigues Teixeira, Eduarda de Oliveira Silva Lima Machado, Isaac Leandro Lira Pinto, Laís da Silva de Oliveira, Karina Varella, Huarrisson Azevedo Santos, Fernando de Oliveira Santos, Liliani Marilia Tiepolo, Carlos Luiz Massard, and et al. 2025. "Morphometric and Molecular Insights into Hepatozoon spp. in Wild and Synanthropic Rodents from Southern and Southeastern Brazil" Pathogens 14, no. 8: 756. https://doi.org/10.3390/pathogens14080756
APA StyleFreitas, T. P. T. d., Teixeira, B. R., Machado, E. d. O. S. L., Pinto, I. L. L., Oliveira, L. d. S. d., Varella, K., Santos, H. A., Santos, F. d. O., Tiepolo, L. M., Massard, C. L., & Peckle, M. (2025). Morphometric and Molecular Insights into Hepatozoon spp. in Wild and Synanthropic Rodents from Southern and Southeastern Brazil. Pathogens, 14(8), 756. https://doi.org/10.3390/pathogens14080756








