Fluazuron Baits in the Control of Amblyomma sculptum Tick: Efficacy and Pharmacokinetics Using Guinea Pigs as an Experimental Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Descriptive Pharmacokinetics (Study I)
2.2. Efficacy Against Amblyomma sculptum Larvae (Study II)
3. Results
3.1. Descriptive Pharmacokinetics (Study I)
3.2. Efficacy Against Amblyomma sculptum Larvae (Study II)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Cmax | Maximum measured concentration |
| Tmax | Time from dosing to the maximum concentration |
| AUC0-t | Area under the curve from zero to last time t |
| AUC0-∞ | Area under the curve from zero to infinity |
| SD | Standard deviation |
| CG | Control Group |
| G1 | Group 1 |
| G2 | Group 2 |
| G3 | Group 3 |
References
- Nava, S.; Beati, L.; Labruna, M.B.; Cáceres, A.G.; Mangold, A.J.; Guglielmone, A.A. Reassessment of the taxonomic status of Amblyomma cajennense (Fabricius, 1787) with the description of three new species, Amblyomma tonelliae n. sp., Amblyomma interandinum n. sp. and Amblyomma patinoi n. sp., and reinstatement of 15 Amblyomma mixtum Koch, 1844, and Amblyomma sculptum Berlese, 1888 (Ixodida: Ixodidae). Ticks Tick. Borne Dis. 2014, 5, 252–276. [Google Scholar] [CrossRef] [PubMed]
- Szabó, M.P.J.; Pinter, A.; Labruna, M.B. Ecology, biology and distribution of spotted-fever tick vectors in Brazil. Front. Cell. Infect. Microbiol. 2013, 3, 27. [Google Scholar] [CrossRef] [PubMed]
- Labruna, M.B.; Kasai, N.; Ferreira, F.; Faccini, J.L.H.; Gennari, S.M. Seasonal dynamics of ticks (Acari: Ixodidae) on horses in the state of São Paulo Brazil. Vet. Parasitol. 2002, 105, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Hernández, A.; Uchoa, F.; de Azevedo Serpa, M.C.; Binder, L.C.; Souza, C.E.; Labruna, M.B. Capybaras (Hydrochoerus hydrochaeris) as amplifying hosts of Rickettsia rickettsii to Amblyomma sculptum ticks: Evaluation during primary and subsequent exposures to R. rickettsii infection. Ticks Tick. Borne Dis. 2020, 11, 101463. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, K.M.P.; Ferraz, S.F.B.; Moreira, J.R.; Couto, H.T.Z.; Verdade, L.M. Capybara (Hydrochoerus hydrochaeris) distribution in agroecosystems: A cross-scale habitat analysis. J. Biogeogr. 2007, 34, 223–230. [Google Scholar] [CrossRef]
- Davis, R.M. Use of orally administered chitin inhibitor (lufenuron) to control flea vectors of plague on ground squirrels in California. J. Med. Entomol. 1999, 36, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Slowik, T.J.; Lane, R.S.; Davis, R.M. Field trial of systemically delivered arthropod development-inhibitor (fluazuron) used to control woodrat fleas (Siphonaptera: Ceratophyllidae) and ticks (Acari: Ixodidae). J. Med. Entomol. 2001, 38, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.M.; Cleugh, E.; Smith, R.T.; Fritz, C.L. Use of a chitin synthesis inhibitor to control fleas on wild rodents important in the maintenance of plague, Yersinia pestis, in California. J. Vector Ecol. 2008, 33, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Borges, D.A.; Cid, Y.P.; Magalhães, V.D.S.; Alves, M.C.C.; Ferreira, T.P.; Bonfim, I.V.; Lima, E.A.S.; Freitas, J.P.; Scott, F.B. Fluazuron orally administered to guinea pigs: Pharmacokinetic and efficacy against Amblyomma sculptum. Parasit. Vectors 2022, 15, 198. [Google Scholar] [CrossRef] [PubMed]
- Ayres, M.; Ayres, J.; Ayres, D.L.; Santos, A.S. BioEstat 5.3: Aplicações Estatísticas nas Áreas das Ciências Biológicas e Médicas; Sociedade Civil Mamirauá: Belém, Brazil, 2011. [Google Scholar]
- Oliveira, P.R.; Calligaris, I.B.; Roma, G.C.; Bechara, G.H.; Pizano, M.A.; Mathias, M.I.C. Potential of the insect growth regulator, fluazuron, in the control of Rhipicephalus sanguineus nymphs (Latreille, 1806) (Acari: Ixodidae): Determination of the LD95 and LD50. Exp. Parasitol. 2012, 131, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.R.; Borges, L.M.F.; Lopes, C.M.L.; Leite, R.C. Population dynamics of the free living stages of Amblyomma cajennense (Fabricius, 1787) (Acari: Ixodidae) on pastures of Pedro Leopoldo, Minas Gerais State, Brazil. Vet. Parasitol. 2000, 92, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Graf, J.F. The role of insect growth regulators in arthropod control. Parasitol. Today 1993, 9, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Junquera, P.; Hosking, B.; Gameiro, M.; Macdolnal, A. Benzoylphenyl ureas as veterinary antiparasitics. An overview and outlook with emphasis on efficacy, usage and resistance. Parasite 2019, 26, 26. [Google Scholar] [CrossRef] [PubMed]
- Zapa, D.M.B.; Couto, L.F.M.; Heller, L.M.; De Assis Cavalcante, A.S.; Nicaretta, J.E.; Cruvinel, L.B.; Melo Júnior, R.D.; Ferreira, L.L.; Bastos, T.S.A.; Soares, V.E.; et al. Do rainfall and tick burden affect the efficacy of pour-on formulations against Rhipicephalus (Boophilus) microplus? Prev. Vet. Med. 2020, 177, 104950. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, D.W. Dwindling resources and the social behaviour of capybaras, (Hydrochoerus hydrochaeris) (Mammalia). J. Zool. 1981, 194, 371–391. [Google Scholar] [CrossRef]
- Pasay, C.; Rothwell, J.; Mounsey, K.; Kelly, A.; Hutchinson, B.; Miezler, A.; McCarthy, J. An exploratory study to assess the activity of the acarine growth inhibitor, fluazuron, against Sarcoptes scabei infestation in pigs. Parasit. Vectors 2012, 5, 40. [Google Scholar] [CrossRef] [PubMed]
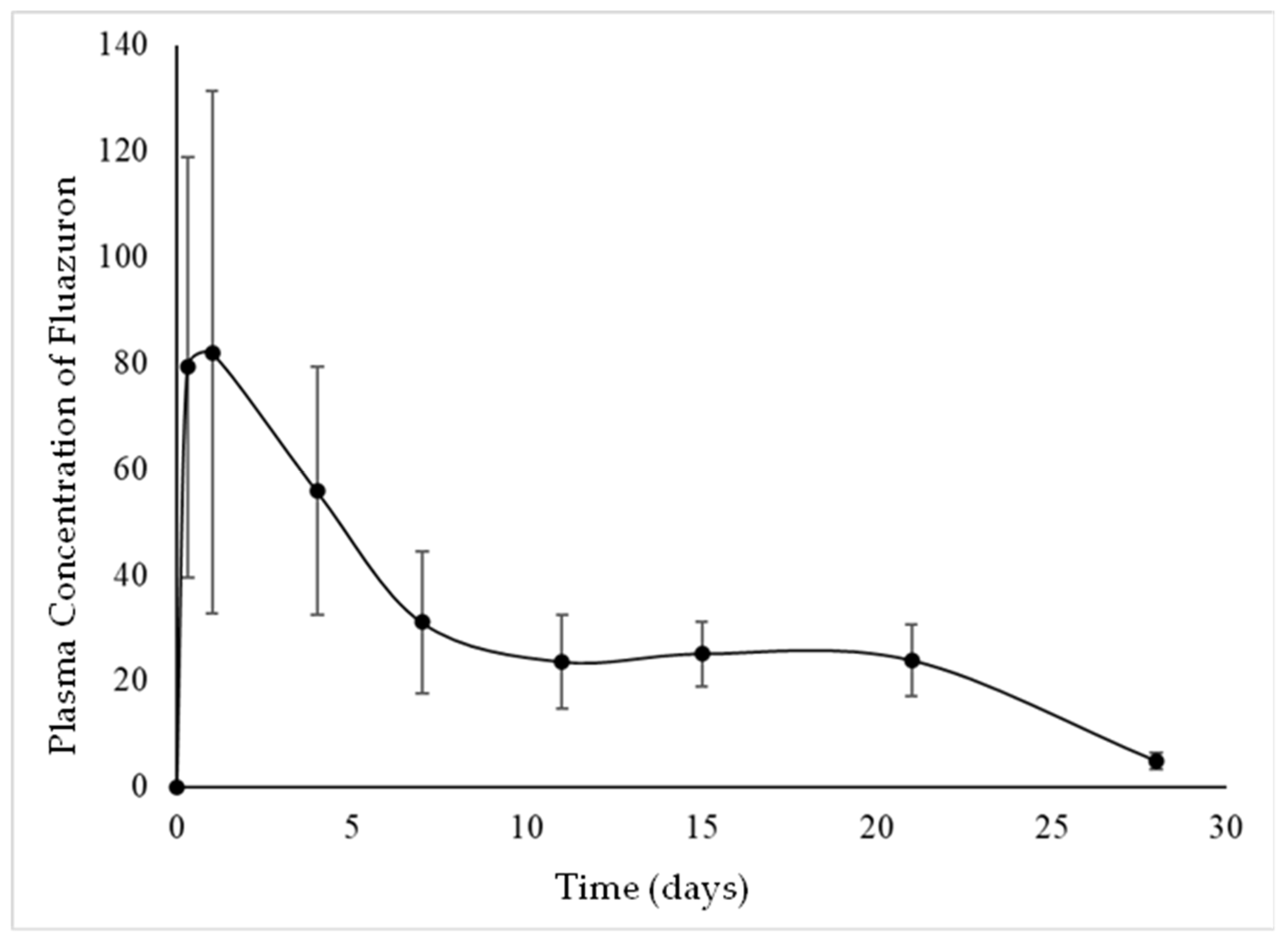
| Experimental Day | D−7 | D0+8 h | D+1 | D+4 | D+7 | D+11 | D+15 | D+21 | D+28 |
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | 0 | 79.24 ± 39.73 a | 81.99 ± 49.30 a | 55.86 ± 23.51 a | 31.08 ± 13.41 b | 23.66 ± 8.81 b | 25.15 ± 6.09 b | 23.95 ± 6.76 b | 4.97 ± 1.58 c |
| Pharmacokinetic Parameters | Arithmetic Mean ± SD |
|---|---|
| Cmax (ng/mL) | 107.23 ± 46.69 |
| Tmax (d) | 0.90 ± 1.04 |
| AUC0-t (ng.d/mL) | 862.99 ± 182.05 |
| AUC0-∞ (ng.d/mL) | 911.65 ± 181.09 |
| T1/2 (d) | 6.63 ± 2.02 |
| Groups | Average of Engorged Larvae Detached | Larvicidal Efficacy (%) | Average of Molted Nymphs | Molted Nymphs (%) | Molting Process Inhibition (%) |
|---|---|---|---|---|---|
| GC | 546.6 ± 379.6 a | --- | 380.0 ± 239.9 | 70.8 ± 6.6 a | --- |
| G1 | 790.9 ± 570.8 a | 0 | 254.5 ± 292.3 | 24.8 ± 13.2 b | 64.99 |
| G2 | 573.1 ± 278.7 a | 0 | 192.9 ± 180.4 | 27.0 ± 18.7 b | 61.88 |
| G3 | 626.4 ± 297.6 a | 0 | 200.3 ± 113.6 | 28.8 ± 12.3 b | 59.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borges, D.A.; Bonfim, I.V.; Dutra, C.R.; Jesus, I.L.R.d.; Monteiro, R.; Assis, R.C.P.d.; Miranda, F.R.; Cid, Y.P.; Scott, F.B. Fluazuron Baits in the Control of Amblyomma sculptum Tick: Efficacy and Pharmacokinetics Using Guinea Pigs as an Experimental Model. Pathogens 2025, 14, 854. https://doi.org/10.3390/pathogens14090854
Borges DA, Bonfim IV, Dutra CR, Jesus ILRd, Monteiro R, Assis RCPd, Miranda FR, Cid YP, Scott FB. Fluazuron Baits in the Control of Amblyomma sculptum Tick: Efficacy and Pharmacokinetics Using Guinea Pigs as an Experimental Model. Pathogens. 2025; 14(9):854. https://doi.org/10.3390/pathogens14090854
Chicago/Turabian StyleBorges, Debora Azevedo, Isabelle Vilela Bonfim, Clara Rodrigues Dutra, Ingrid Lins Raquel de Jesus, Rayane Monteiro, Rayane Christine Pereira de Assis, Fernando Rocha Miranda, Yara Peluso Cid, and Fabio Barbour Scott. 2025. "Fluazuron Baits in the Control of Amblyomma sculptum Tick: Efficacy and Pharmacokinetics Using Guinea Pigs as an Experimental Model" Pathogens 14, no. 9: 854. https://doi.org/10.3390/pathogens14090854
APA StyleBorges, D. A., Bonfim, I. V., Dutra, C. R., Jesus, I. L. R. d., Monteiro, R., Assis, R. C. P. d., Miranda, F. R., Cid, Y. P., & Scott, F. B. (2025). Fluazuron Baits in the Control of Amblyomma sculptum Tick: Efficacy and Pharmacokinetics Using Guinea Pigs as an Experimental Model. Pathogens, 14(9), 854. https://doi.org/10.3390/pathogens14090854







