Baicalein and Berberine Inhibit the Growth and Virulence of Clostridioides difficile
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Media
2.2. Tested Substances
2.3. Detection of the Minimum Inhibitory Concentration (MIC)
2.4. Checkerboard Dilution Experiment
2.5. Time–Kill Curve Assay
2.6. Scanning Electron Microscopy (SEM)
2.7. Enzyme-Linked Immunosorbnent Assay (ELISA)
2.8. Vero Cell Cytotoxicity Assay
2.9. Quantitative Reverse Transcription PCR (qRT-PCR)
2.10. Statistical Analysis
3. Results
3.1. Antibacterial Activity of Tested Substances Against C. difficile
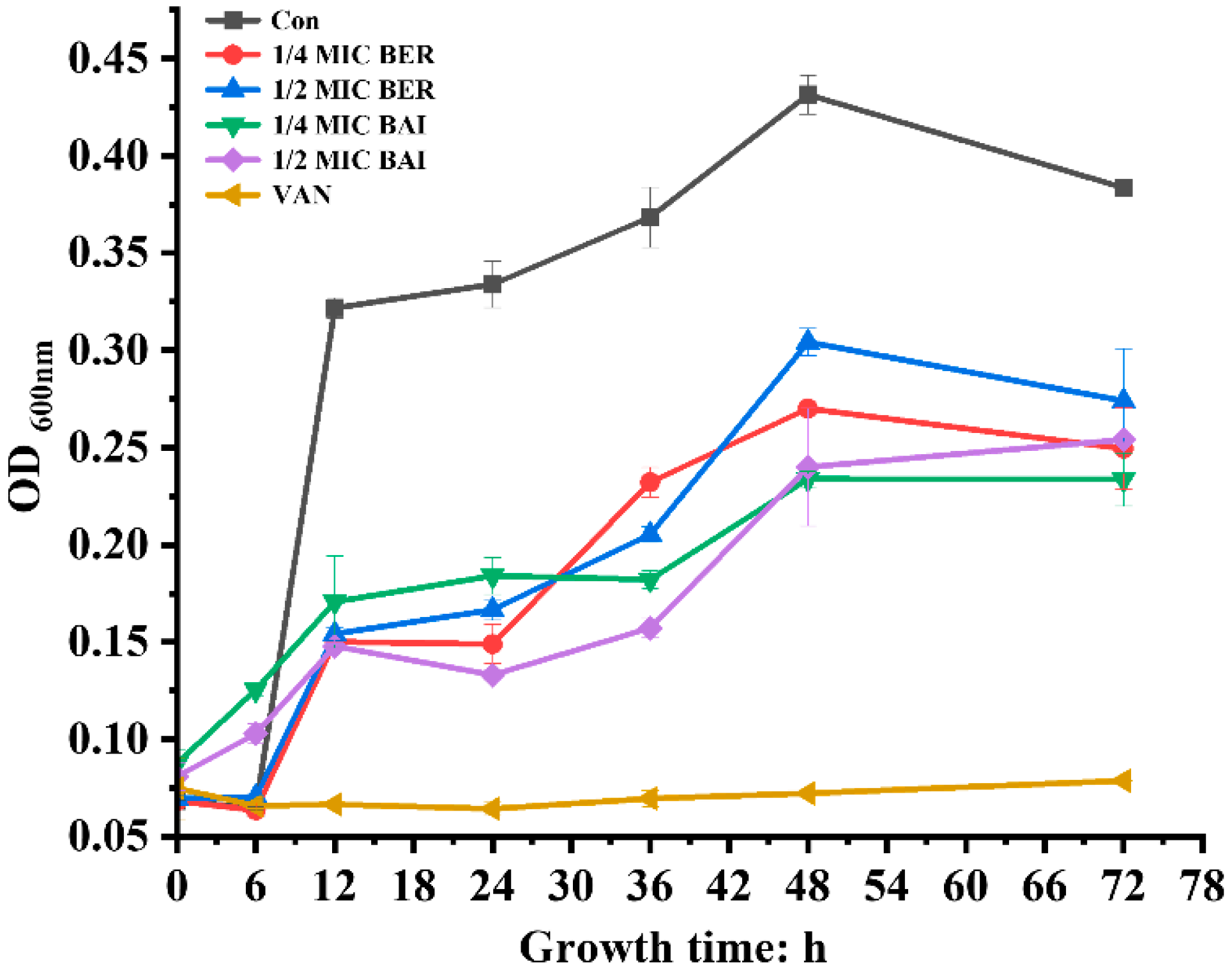
3.2. Time–Kill Curves of BER and BAI Against C. difficile
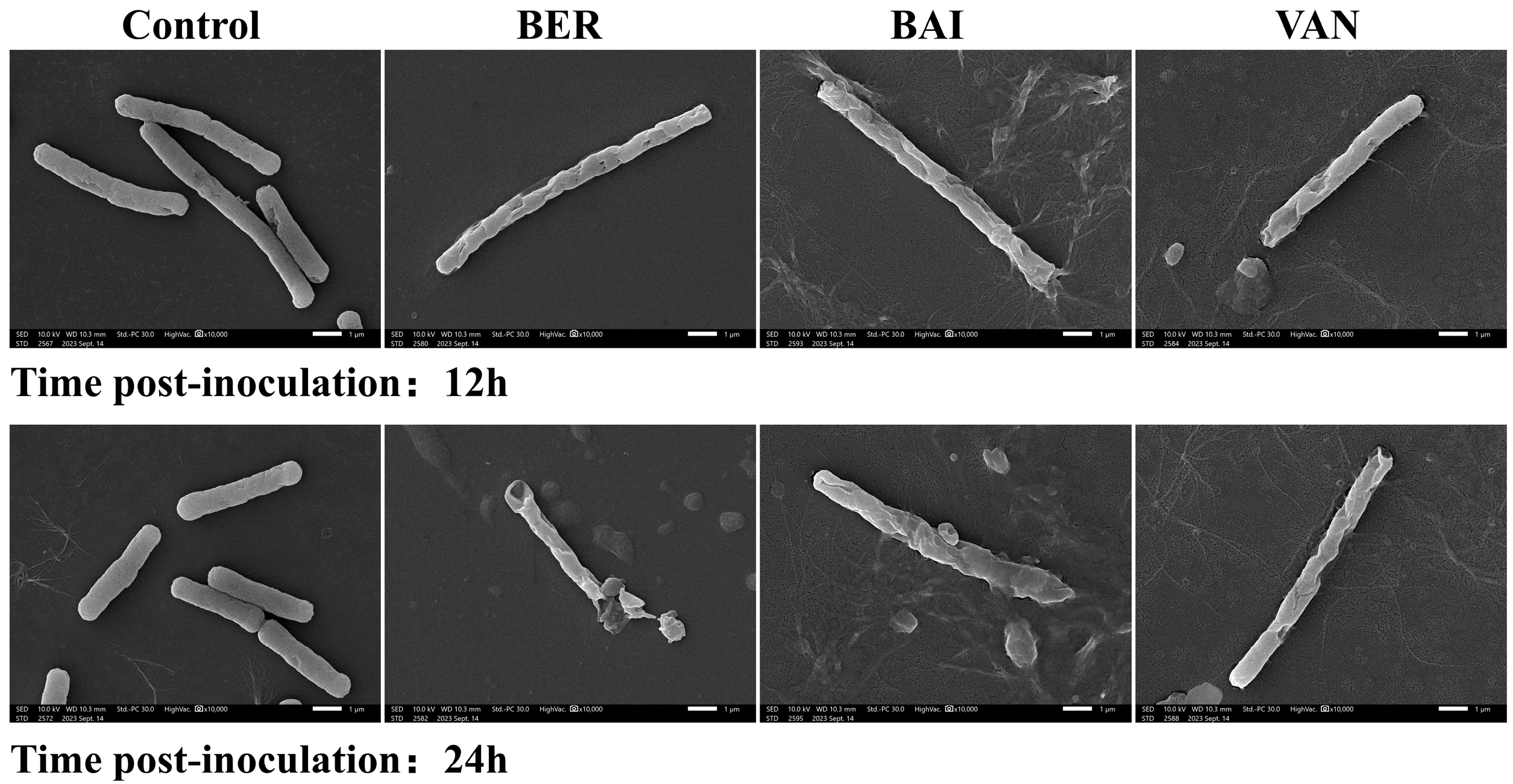
3.3. Effects of BER and BAI on the Cell Structure of C. difficile
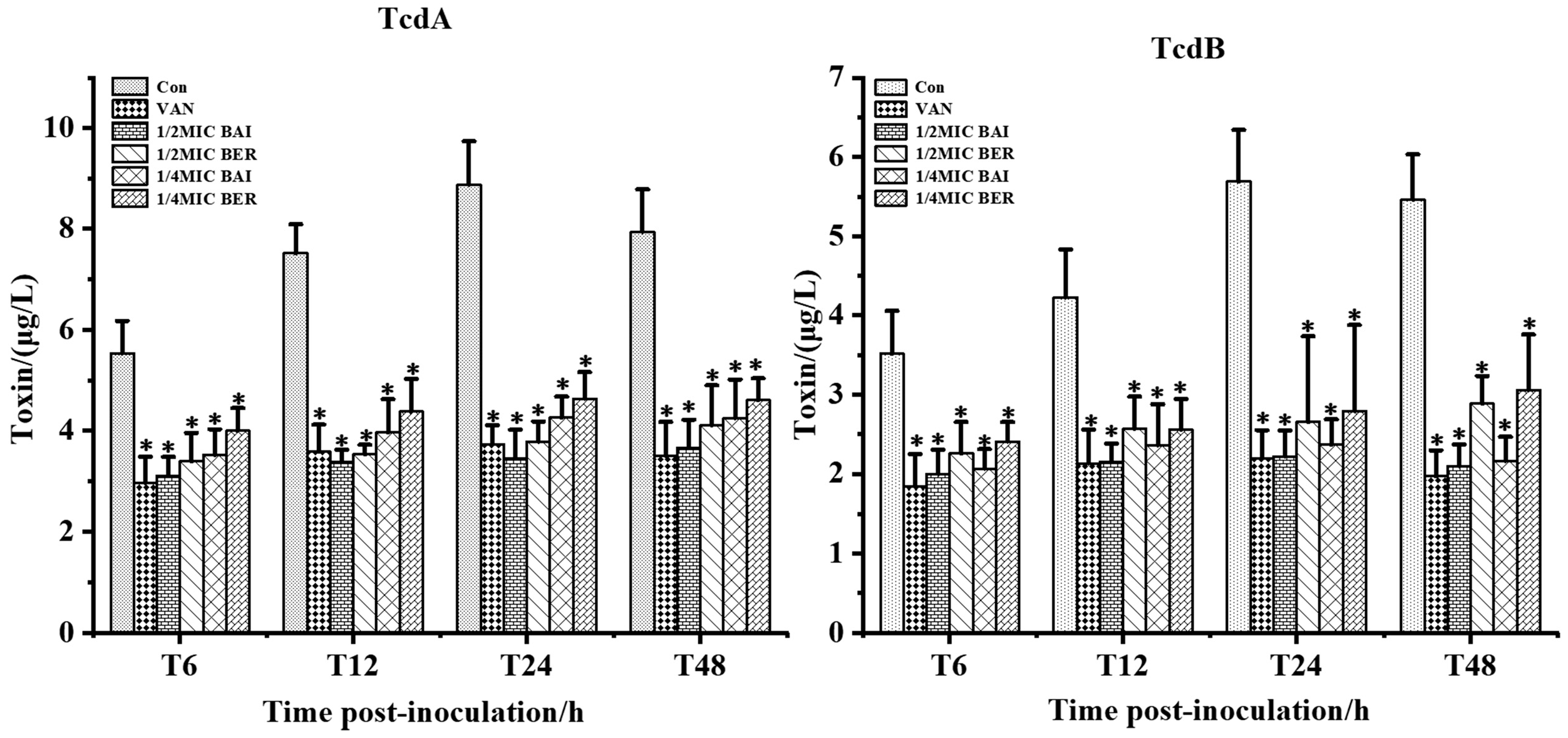
3.4. Effect of BER and BAI on C. difficile Toxin Production
3.5. Effects of BER and BAI on C. difficile-Mediated Cytotoxicity
3.6. Effects of BER and BAI on the Toxin Synthesis Genes of C. difficile
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, D.; Sorg, J.A.; Sun, X. Clostridioides difficile Biology: Sporulation, Germination, and Corresponding Therapies for C. difficile Infection. Front. Cell Infect. Microbiol. 2018, 8, 29. [Google Scholar] [CrossRef]
- Cymbal, M.; Chatterjee, A.; Baggott, B.; Auron, M. Management of Clostridioides difficile Infection: Diagnosis, Treatment, and Future Perspectives. Am. J. Med. 2024, 137, 571–576. [Google Scholar] [CrossRef]
- Jin, D.; Tang, Y.W.; Riley, T.V. Editorial: Clostridium difficile infection in the Asia-Pacific region. Front. Cell Infect. Microbiol. 2022, 12, 983563. [Google Scholar] [CrossRef]
- Noori, M.; Azimirad, M.; Eslami, G.; Looha, M.A.; Yadegar, A.; Ghalavand, Z.; Zali, M.R. Surface layer protein A from hypervirulent Clostridioides difficile ribotypes induce significant changes in the gene expression of tight junctions and inflammatory response in human intestinal epithelial cells. BMC Microbiol. 2022, 22, 259. [Google Scholar] [CrossRef]
- Shirley, D.A.; Tornel, W.; Warren, C.A.; Moonah, S. Clostridioides difficile Infection in Children: Recent Updates on Epidemiology, Diagnosis, Therapy. Pediatrics 2023, 152, e2023062307. [Google Scholar] [CrossRef]
- O’Grady, K.; Knight, D.R.; Riley, T.V. Antimicrobial resistance in Clostridioides difficile. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 2459–2478. [Google Scholar] [CrossRef]
- Snydman, D.R.; McDermott, L.A.; Thorpe, C.M.; Goldstein, E.J.C.; Schuetz, A.N.; Johnson, S.; Gerding, D.N.; Gluck, L.; Bourdas, D.; Carroll, K.C.; et al. A US-based national surveillance study for the susceptibility and epidemiology of Clostridioides difficile isolates with special reference to ridinilazole: 2020-2021. Antimicrob. Agents Chemother. 2023, 67, e0034923. [Google Scholar] [CrossRef]
- Bai, L.; Xu, T.; Zhang, W.; Jiang, Y.; Gu, W.; Zhao, W.; Luan, Y.; Xiong, Y.; Zou, N.; Zhang, Y.; et al. Abundant geographical divergence of Clostridioides difficile infection in China: A prospective multicenter cross-sectional study. BMC Infect. Dis. 2025, 25, 185. [Google Scholar] [CrossRef]
- Eubank, T.A.; Dureja, C.; Garey, K.W.; Hurdle, J.G.; Gonzales-Luna, A.J. Reduced Vancomycin Susceptibility in Clostridioides difficile Is Associated with Lower Rates of Initial Cure and Sustained Clinical Response. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2024, 79, 15–21. [Google Scholar] [CrossRef]
- Chandrasekaran, R.; Lacy, D.B. The role of toxins in Clostridium difficile infection. FEMS Microbiol. Rev. 2017, 41, 723–750. [Google Scholar] [CrossRef]
- Alam, M.Z.; Madan, R. Clostridioides difficile Toxins: Host Cell Interactions and Their Role in Disease Pathogenesis. Toxins 2024, 16, 241. [Google Scholar] [CrossRef]
- Li, C.; Wang, J.; Ma, R.; Li, L.; Wu, W.; Cai, D.; Lu, Q. Natural-derived alkaloids exhibit great potential in the treatment of ulcerative colitis. Pharmacol. Res. 2022, 175, 105972. [Google Scholar] [CrossRef]
- Ren, Y.Y.; Zhang, X.R.; Li, T.N.; Zeng, Y.J.; Wang, J.; Huang, Q.W. Galla Chinensis, a Traditional Chinese Medicine: Comprehensive review of botany, traditional uses, chemical composition, pharmacology and toxicology. J. Ethnopharmacol. 2021, 278, 114247. [Google Scholar] [CrossRef]
- Wang, W.; Cao, J.; Yang, J.; Niu, X.; Liu, X.; Zhai, Y.; Qiang, C.; Niu, Y.; Li, Z.; Dong, N.; et al. Antimicrobial Activity of Tannic Acid In Vitro and Its Protective Effect on Mice against Clostridioides difficile. Microbiol. Spectr. 2023, 11, e0261822. [Google Scholar] [CrossRef]
- Gao, Y.; Li, H.; Yang, H.; Su, J.; Huang, L. The current novel therapeutic regimens for Clostridium difficile infection (CDI) and the potentials of Traditional Chinese Medicine in treatment of CDI. Crit. Rev. Microbiol. 2019, 45, 729–742. [Google Scholar] [CrossRef]
- Lu, J.Z.; Ye, D.; Ma, B.L. Constituents, Pharmacokinetics, and Pharmacology of Gegen-Qinlian Decoction. Front. Pharmacol. 2021, 12, 668418. [Google Scholar] [CrossRef]
- Li, Q.; Cui, Y.; Xu, B.; Wang, Y.; Lv, F.; Li, Z.; Li, H.; Chen, X.; Peng, X.; Chen, Y.; et al. Main active components of Jiawei Gegen Qinlian decoction protects against ulcerative colitis under different dietary environments in a gut microbiota-dependent manner. Pharmacol. Res. 2021, 170, 105694. [Google Scholar] [CrossRef]
- Lv, Z.; Peng, G.; Liu, W.; Xu, H.; Su, J. Berberine blocks the relapse of Clostridium difficile infection in C57BL/6 mice after standard vancomycin treatment. Antimicrob. Agents Chemother. 2015, 59, 3726–3735. [Google Scholar] [CrossRef]
- Vigsnaes, L.K.; Ghyselinck, J.; Van den Abbeele, P.; McConnell, B.; Moens, F.; Marzorati, M.; Bajic, D. 2’FL and LNnT Exert Antipathogenic Effects against C. difficile ATCC 9689 In Vitro, Coinciding with Increased Levels of Bifidobacteriaceae and/or Secondary Bile Acids. Pathogens 2021, 10, 927. [Google Scholar] [CrossRef]
- Wang, P.; Gui, X.; Xu, M.; Dong, F.; Li, Y.; Wang, Q.; Wang, Y.; Yao, J.; Lu, L.; Liu, R. In vivo and in vitro chemical composition and biological activity of traditional vs. dispensing granule decoctions of Coptidis Rhizoma: A comparative study. Biomed. Chromatogr. BMC 2024, 38, e5960. [Google Scholar] [CrossRef]
- Humphries, R.; Bobenchik, A.M.; Hindler, J.A.; Schuetz, A.N. Overview of changes to the clinical and laboratory standards institute performance standards for antimicrobial susceptibility testing, M100, 31st Edition. J. Clin. Microbiol. 2021, 59, e0021321. [Google Scholar] [CrossRef]
- Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria. 2018. Available online: https://webstore.ansi.org/preview-pages/CLSI/preview_CLSI+M11-Ed9.pdf?srsltid=AfmBOorsWiIp34iWYUzjIILmpzqEstOpVqwh4bx-0jrGUG15561AnWJt (accessed on 28 June 2025).
- Wultańska, D.; Piotrowski, M.; Pituch, H. The effect of berberine chloride and/or its combination with vancomycin on the growth, biofilm formation, and motility of Clostridioides difficile. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1391–1399. [Google Scholar] [CrossRef]
- Hurdle, J.G.; Heathcott, A.E.; Yang, L.; Yan, B.; Lee, R.E. Reutericyclin and related analogues kill stationary phase Clostridium difficile at achievable colonic concentrations. J. Antimicrob. Chemother. 2011, 66, 1773–1776. [Google Scholar] [CrossRef]
- Harnvoravongchai, P.; Chankhamhaengdecha, S.; Ounjai, P.; Singhakaew, S.; Boonthaworn, K.; Janvilisri, T. Antimicrobial Effect of Asiatic Acid Against Clostridium difficile Is Associated with Disruption of Membrane Permeability. Front. Microbiol. 2018, 9, 2125. [Google Scholar] [CrossRef]
- Endres, B.T.; Bassères, E.; Khaleduzzaman, M.; Alam, M.J.; Chesnel, L.; Garey, K.W. Evaluating the Effects of Surotomycin Treatment on Clostridium difficile Toxin A and B Production, Immune Response, and Morphological Changes. Antimicrob. Agents Chemother. 2016, 60, 3519–3523. [Google Scholar] [CrossRef]
- Mooyottu, S.; Kollanoor-Johny, A.; Flock, G.; Bouillaut, L.; Upadhyay, A.; Sonenshein, A.L.; Venkitanarayanan, K. Carvacrol and trans-cinnamaldehyde reduce Clostridium difficile toxin production and cytotoxicity in vitro. Int. J. Mol. Sci. 2014, 15, 4415–4430. [Google Scholar] [CrossRef]
- IBM Corp. IBM SPSS Statistics for Windows, version 26.0; IBM Corp: Armonk, NY, USA, 2019. [Google Scholar]
- Kvasničková, E.; Paulíček, V.; Paldrychová, M.; Ježdík, R.; Maťátková, O.; Masák, J. Aspergillus fumigatus DBM 4057 biofilm formation is inhibited by chitosan, in contrast to baicalein and rhamnolipid. World J. Microbiol. Biotechnol. 2016, 32, 187. [Google Scholar] [CrossRef]
- Lim, S.C.; Knight, D.R.; Riley, T.V. Clostridium difficile and One Health. Clinical microbiology and infection: The official publication of the European Society of Clinical Microbiology and Infectious Diseases. Clin. Microbiol. Infect. 2020, 26, 857–863. [Google Scholar] [CrossRef]
- Boyanova, L.; Dimitrov, G.; Gergova, R.; Hadzhiyski, P.; Markovska, R. Clostridioides difficile resistance to antibiotics, including post-COVID-19 data. Expert. Rev. Clin. Pharmacol. 2023, 16, 925–938. [Google Scholar] [CrossRef]
- Lan, S.; Li, Z.; Su, A.; Peng, Y.; Liao, Y.; Liu, X.; Tan, Q. Study on the mechanisms of the cross-resistance to TET, PIP, and GEN in Staphylococcus aureus mediated by the Rhizoma Coptidis extracts. J. Antibiot. 2021, 74, 330–336. [Google Scholar] [CrossRef]
- Bao, M.; Bu, Q.; Pan, M.; Xu, R.; Chen, Y.; Yang, Y.; Wang, C.; Wang, T. Coptidis rhizoma extract alleviates oropharyngeal candidiasis by gC1qR-EGFR/ERK/c-fos axis-induced endocytosis of oral epithelial cells. J. Ethnopharmacol. 2024, 331, 118305. [Google Scholar] [CrossRef]
- Tseng, C.Y.; Sun, M.F.; Kao, T.C.; Li, T.C.; Lin, C.T. Role of Coptis chinensis in antibiotic susceptibility of carbapenem-resistant Klebsiella pneumoniae. J. Microbiol. Immunol. Infect. 2022, 55, 946–955. [Google Scholar] [CrossRef]
- Mekseepralard, C.; Kamkaen, N.; Wilkinson, J.M. Antimicrobial and antioxidant activities of traditional Thai herbal remedies for aphthous ulcers. Phytother. Res. 2010, 24, 1514–1519. [Google Scholar] [CrossRef]
- Li, C.; Wang, J.; Li, H.; Wang, Y.; Wu, H.; Wei, W.; Wu, D.; Shao, J.; Wang, T.; Wang, C. Suppressing the virulence factors of Candida auris with baicalein through multifaceted mechanisms. Arch. Microbiol. 2024, 206, 349. [Google Scholar] [CrossRef]
- Güran, M.; Çakıral, K.; Teralı, K.; Kandemir, T.; Şanlıtürk, G.; Öcal, M.M.; Nagiyev, T.; Köksal, F. Meropenem in combination with baicalein exhibits synergism against extensively drug resistant and pan-drug-resistant Acinetobacter baumannii clinical isolates in vitro. Pathog. Dis. 2023, 81, ftad007. [Google Scholar] [CrossRef]
- Augostine, C.R.; Avery, S.V. Discovery of Natural Products with Antifungal Potential Through Combinatorial Synergy. Front. Microbiol. 2022, 13, 866840. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, W.; Cai, L.; Yang, T. Potentiation and Mechanism of Berberine as an Antibiotic Adjuvant Against Multidrug-Resistant Bacteria. Infect. Drug Resist. 2023, 16, 7313–7326. [Google Scholar] [CrossRef]
- Ding, J.; Yan, Z.; Peng, L.; Li, J.; Yang, F.; Zheng, D. Inhibitory effects of berberine on fungal growth, biofilm formation, virulence, and drug resistance as an antifungal drug and adjuvant with prospects for future applications. World J. Microbiol. Biotechnol. 2024, 41, 5. [Google Scholar] [CrossRef]
- Gonzales, M.; Pepin, J.; Frost, E.H.; Carrier, J.C.; Sirard, S.; Fortier, L.C.; Valiquette, L. Faecal pharmacokinetics of orally administered vancomycin in patients with suspected Clostridium difficile infection. BMC Infect. Dis. 2010, 10, 363. [Google Scholar] [CrossRef]
- Gargis, A.S.; Karlsson, M.; Paulick, A.L.; Anderson, K.F.; Adamczyk, M.; Vlachos, N.; Kent, A.G.; McAllister, G.; McKay, S.L.; Halpin, A.L.; et al. Reference Susceptibility Testing and Genomic Surveillance of Clostridioides difficile, United States, 2012–2017. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2023, 76, 890–896. [Google Scholar] [CrossRef]
- Li, Y.; Yin, Y.M.; Wang, X.Y.; Wu, H.; Ge, X.Z. Evaluation of berberine as a natural fungicide: Biodegradation and antimicrobial mechanism. J. Asian Nat. Prod. Res. 2018, 20, 148–162. [Google Scholar] [CrossRef]
- Zhou, F.F.; Gu, X.M.; Wang, L.; Lin, M. The mechanism of berberine on Methicillin resistant Staphylococcus aureus in vitro. Zhonghua Yu Fang Yi Xue Za Zhi [Chin. J. Prev. Med.] 2023, 57, 1217–1221. [Google Scholar]
- Teng, X.; Wu, B.; Liang, Z.; Zhang, L.; Yang, M.; Liu, Z.; Liang, Q.; Wang, C. Three bioactive compounds from Huangqin decoction ameliorate Irinotecan-induced diarrhea via dual-targeting of Escherichia coli and bacterial β-glucuronidase. Cell Biol. Toxicol. 2024, 40, 88. [Google Scholar] [CrossRef]
- Wilcox, M.H.; Gerding, D.N.; Poxton, I.R.; Kelly, C.; Nathan, R.; Birch, T.; Cornely, O.A.; Rahav, G.; Bouza, E.; Lee, C.; et al. Bezlotoxumab for Prevention of Recurrent Clostridium difficile Infection. N. Engl. J. Med. 2017, 376, 305–317. [Google Scholar] [CrossRef]
- Pellissery, A.J.; Vinayamohan, P.G.; Venkitanarayanan, K. In vitro antivirulence activity of baicalin against Clostridioides difficile. J. Med. Microbiol. 2020, 69, 631–639. [Google Scholar] [CrossRef]
- Lewis, B.B.; Carter, R.A.; Ling, L.; Leiner, I.; Taur, Y.; Kamboj, M.; Dubberke, E.R.; Xavier, J.; Pamer, E.G. Pathogenicity Locus, Core Genome, and Accessory Gene Contributions to Clostridium difficile Virulence. mBio 2017, 8, e00885-17. [Google Scholar] [CrossRef]
- Jabbari, S.; Cartman, S.T.; King, J.R. Mathematical modelling reveals properties of TcdC required for it to be a negative regulator of toxin production in Clostridium difficile. J. Math. Biol. 2015, 70, 773–804. [Google Scholar] [CrossRef]
- Dyer, C.; Hutt, L.P.; Burky, R.; Joshi, L.T. Biocide Resistance and Transmission of Clostridium difficile Spores Spiked onto Clinical Surfaces from an American Health Care Facility. Appl. Environ. Microbiol. 2019, 85, e01090-19. [Google Scholar] [CrossRef]


| Oligo Name | Sequence (5′ to 3′) | GC (%) | TM (°C) | Product Size (bp) |
|---|---|---|---|---|
| 16S RNA-F | AGCGGTGAAATGCGTAGATAT | 42.86 | 57.61 | 72 |
| 16S RNA-R | CAGCGTCAGTTACAGTCCAGA | 52.38 | 59.73 | |
| SPO0A-F | TTTTAGCAGATGACAATAAGG | 33.33 | 51.54 | 674 |
| SPO0A-R | GTCAACTTTTCCTCTACTCCA | 42.86 | 54.89 | |
| tcdA-F | GCTTTCGCTTTAGGCAGTGT | 50.00 | 59.12 | 126 |
| tcdA-R | GGCTGGGTTAAGGTGTTGGT | 55.00 | 60.18 | |
| tcdB-F | ATGGAAGGTGGTTCAGGTCA | 50.00 | 58.56 | 204 |
| tcdB-R | ACCTGGTGTCCATCCTGTTTC | 52.38 | 59.93 | |
| tcdC-F | ACCATGGTTCAGCATCAGACA | 47.62 | 59.65 | 171 |
| tcdC-R | AGGGTATTGCTCTACTGGCA | 50.00 | 58.12 | |
| tcdE-F | ACCTAGGAGGCGTTATGAATATGAC | 44.00 | 60.05 | 170 |
| tcdE-R | TGCTACTTTTCTGATTCCTCCA | 40.91 | 57.09 | |
| tcdR-F | AACTCAGTAGATGATTTGCAAGAA | 33.33 | 56.62 | 100 |
| tcdR-R | TTAAATCTGTTTCTCCCTCTTCA | 34.78 | 55.25 |
| Category | Substance | MIC (μg/mL) |
|---|---|---|
| Decoction-Free Chinese Herbal Granules | Galla chinensis | 64 |
| Coptis chinensis | 1024 | |
| Glycyrrhiza uralensis | >40,960 | |
| Scutellaria baicalensis | >40,960 | |
| Pueraria lobata | >40,960 | |
| Herbs | Berberine | 256 |
| Baicalein | 320 | |
| Baicalin | 512 | |
| Tannic acid | 2560 | |
| Antibiotics | Vancomycin | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Zheng, D.; Yong, J.; Li, Y.; Sun, Y.; Zhao, F.; Tang, D.; Xie, Y.; Bi, D. Baicalein and Berberine Inhibit the Growth and Virulence of Clostridioides difficile. Pathogens 2025, 14, 662. https://doi.org/10.3390/pathogens14070662
Yang X, Zheng D, Yong J, Li Y, Sun Y, Zhao F, Tang D, Xie Y, Bi D. Baicalein and Berberine Inhibit the Growth and Virulence of Clostridioides difficile. Pathogens. 2025; 14(7):662. https://doi.org/10.3390/pathogens14070662
Chicago/Turabian StyleYang, Xue, Dongming Zheng, Jiangyan Yong, Yuchen Li, Yunzhi Sun, Fei Zhao, Daiyan Tang, Yi Xie, and Dongming Bi. 2025. "Baicalein and Berberine Inhibit the Growth and Virulence of Clostridioides difficile" Pathogens 14, no. 7: 662. https://doi.org/10.3390/pathogens14070662
APA StyleYang, X., Zheng, D., Yong, J., Li, Y., Sun, Y., Zhao, F., Tang, D., Xie, Y., & Bi, D. (2025). Baicalein and Berberine Inhibit the Growth and Virulence of Clostridioides difficile. Pathogens, 14(7), 662. https://doi.org/10.3390/pathogens14070662





