Cathelicidins Limit Intracellular Neospora caninum-Infection in Macrophages
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Animal Models
2.2. Cell Isolation and Culture Protocols
2.3. Parasite Preparation and Infection Procedures
2.4. Quantification of Intracellular Parasite Load
2.5. Confocal Microscopy
2.6. Cytokine and Lactate Dehydrogenase Determinations
2.7. Statistical Analysis
3. Results
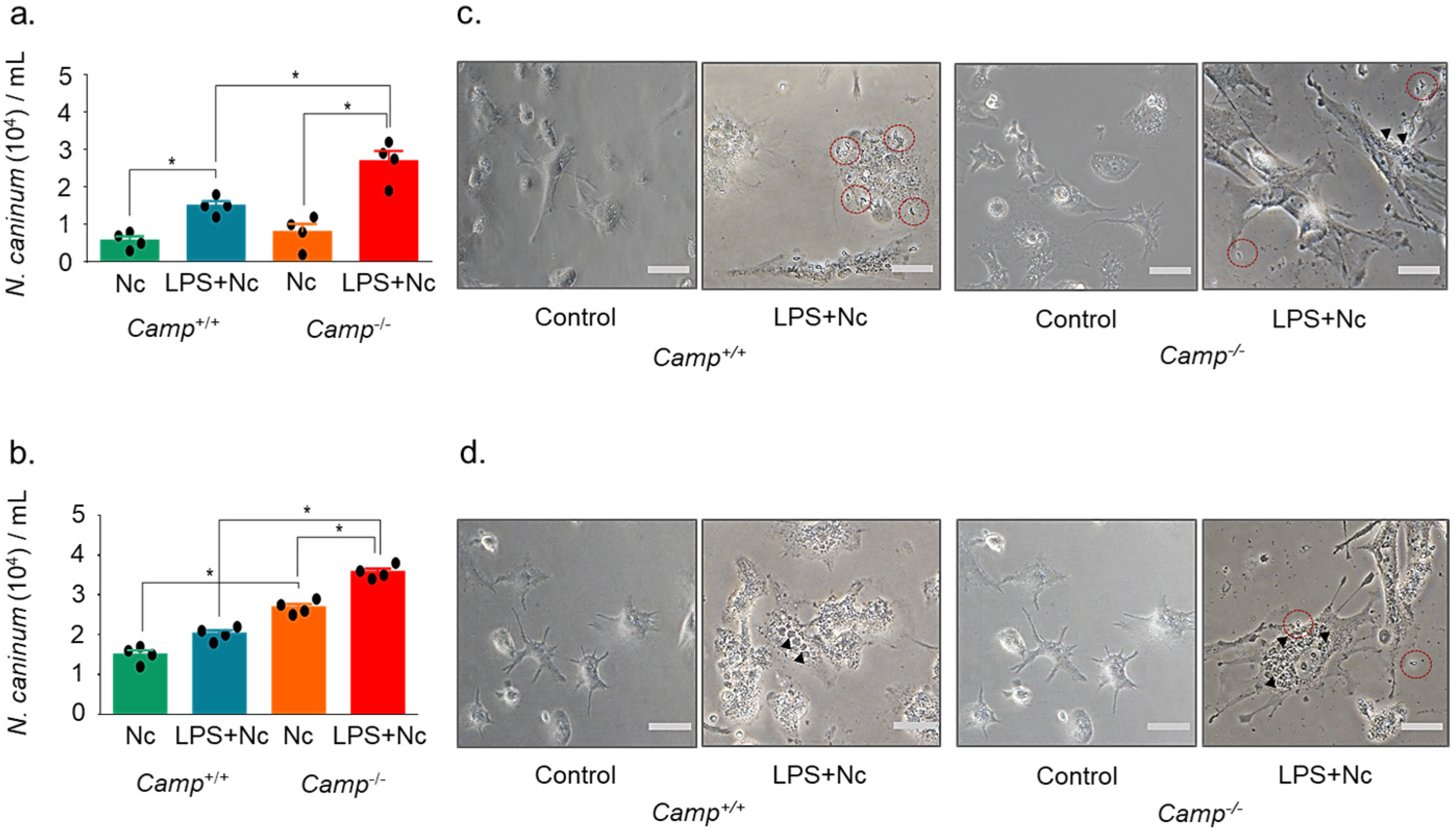
3.1. Cathelicidin Deficiency Modulates Intracellular N. caninum Burden in Macrophages
3.2. Cathelicidin Deficiency Exacerbates the Secretion of IL-1β and Cell Damage in Response to N. Caninum
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lindsay, D.S. Neosporosis, Toxoplasmosis, and Sarcocystosis in Ruminants: An Update. Vet. Clin. N. Am. Food Anim. Pract. 2020, 36, 205–222. [Google Scholar] [CrossRef] [PubMed]
- Reichel, M.P.; Ayanegui-Alcérreca, M.; Gondim, L.F.P.; Ellis, J.T. What Is the Global Economic Impact of Neospora Caninum in Cattle—The Billion Dollar Question; Australian Society for Parasitology Inc.: Smithfield, Australia, 2013; Volume 43. [Google Scholar]
- Raghupathy, R. Th1-Type Immunity Is Incompatible with Successful Pregnancy. Immunol. Today 1997, 18, 478–482. [Google Scholar] [CrossRef]
- Koga, K.; Aldo, P.B.; Mor, G. Toll-like Receptors and Pregnancy: Trophoblast as Modulators of the Immune Response. J. Obstet. Gynaecol. Res. 2009, 35, 191–202. [Google Scholar] [CrossRef]
- Fehervari, Z. NLRP3 Shapes Immunity to Leishmania. Nat. Immunol. 2015, 16, 342. [Google Scholar] [CrossRef]
- Crauwels, P.; Bank, E.; Walber, B.; Wenzel, U.A.; Agerberth, B.; Chanyalew, M.; Abebe, M.; König, R.; Ritter, U.; Reiling, N.; et al. Cathelicidin Contributes to the Restriction of Leishmania in Human Host Macrophages. Front. Immunol. 2019, 10, 469899. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.X.; Jiao, W.J.; Yang, Y.; Liu, H.L.; Wang, H.L. Role of Inflammasomes in Toxoplasma and Plasmodium Infections. Parasites Vectors 2024, 17, 466. [Google Scholar] [CrossRef]
- Wang, X.; Gong, P.; Zhang, N.; Li, L.; Chen, S.; Jia, L.; Liu, X.; Li, J.; Zhang, X. Inflammasome Activation Restrains the Intracellular Neospora Caninum Proliferation in Bovine Macrophages. Vet. Parasitol. 2019, 268, 16–20. [Google Scholar] [CrossRef]
- Wang, X.; Gong, P.; Zhang, X.; Wang, J.; Tai, L.; Wang, X.; Wei, Z.; Yang, Y.; Yang, Z.; Li, J.; et al. NLRP3 Inflammasome Activation in Murine Macrophages Caused by Neospora Caninum Infection. Parasites Vectors 2017, 10, 266. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, X.-C.; Gong, P.-T.; Zhang, N.; Zhang, X.; Li, S.; Li, X.; Liu, S.-X.; Zhang, X.-X.; Li, W.; et al. ROS-Mediated NLRP3 Inflammasome Activation Participates in the Response against Neospora Caninum Infection. Parasites Vectors 2020, 13, 449. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Gong, P.; Zhang, N.; Li, L.; Ouyang, H.; Jia, L.; Li, J.; Zhang, X. Pyroptosis Executioner Gasdermin D Contributes to Host Defense and Promotes Th 1 Immune Response during Neospora Caninum Infection. Vet. Parasitol. 2020, 286, 109254. [Google Scholar] [CrossRef]
- Shi, J.; Gao, W.; Shao, F. Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem. Sci. 2017, 42, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.L.; Zhai, J.H.; Chai, Y.F. Recent Advances in the Molecular Mechanisms Underlying Pyroptosis in Sepsis. Mediat. Inflamm. 2018, 2018, 5823823. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Yang, L.; Pandeya, A.; Cui, J.; Zhang, Y.; Li, Z. Pyroptosis-Induced Inflammation and Tissue Damage. J. Mol. Biol. 2022, 434, 167301. [Google Scholar] [CrossRef]
- Vasudevan, S.O.; Behl, B.; Rathinam, V.A. Pyroptosis-Induced Inflammation and Tissue Damage. Semin. Immunol. 2023, 69, 101781. [Google Scholar] [CrossRef]
- Imre, G. Pyroptosis in Health and Disease. Am. J. Physiol. Cell Physiol. 2024, 326, C784–C794. [Google Scholar] [CrossRef]
- Chen, Y.; Ye, X.; Escames, G.; Lei, W.; Zhang, X.; Li, M.; Jing, T.; Yao, Y.; Qiu, Z.; Wang, Z.; et al. The NLRP3 Inflammasome: Contributions to Inflammation-Related Diseases. Cell. Mol. Biol. Lett. 2023, 28, 51. [Google Scholar] [CrossRef]
- Brown, K.L.; Hancock, R.E.W. Cationic Host Defense (Antimicrobial) Peptides. Curr. Opin. Immunol. 2006, 18, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.W.; Sahl, H.G. Antimicrobial and Host-Defense Peptides as New Anti-Infective Therapeutic Strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef]
- Hilchie, A.L.; Wuerth, K.; Hancock, R.E.W. Immune Modulation by Multifaceted Cationic Host Defense (Antimicrobial) Peptides. Nat. Chem. Biol. 2013, 9, 761–768. [Google Scholar] [CrossRef]
- Galloo, R.L.; Kim, K.J.; Bernfield, M.; Kozak, C.A.; Zanetti, M.; Merluzzi, L.; Gennaro, R. Identification of CRAMP, a Cathelin-Related Antimicrobial Peptide Expressed in the Embryonic and Adult Mouse. J. Biol. Chem. 1997, 272, 13088–13093. [Google Scholar] [CrossRef]
- Kościuczuk, E.M.; Lisowski, P.; Jarczak, J.; Strzałkowska, N.; Jóźwik, A.; Horbańczuk, J.; Krzyżewski, J.; Zwierzchowski, L.; Bagnicka, E. Cathelicidins: Family of Antimicrobial Peptides. A Rev. Mol. Biol. Rep. 2012, 39, 10957–10970. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, M. Cathelicidins, Multifunctional Peptides of the Innate Immunity. J. Leukoc. Biol. 2004, 75, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.K.K.; Wong, C.C.M.; Li, Z.J.; Zhang, L.; Ren, S.X.; Cho, C.H. Cathelicidins in Inflammation and Tissue Repair: Potential Therapeutic Applications for Gastrointestinal Disorders. Acta. Pharmacol. Sin. 2010, 31, 1118–1122. [Google Scholar] [CrossRef] [PubMed]
- Guerra, M.E.S.; Vieira, B.; Calazans, A.P.C.T.; Destro, G.V.; Melo, K.; Rodrigues, E.; Waz, N.T.; Girardello, R.; Darrieux, M.; Converso, T.R. Recent Advances in the Therapeutic Potential of Cathelicidins. Front. Microbiol. 2024, 15, 1405760. [Google Scholar] [CrossRef]
- Hu, Z.; Murakami, T.; Suzuki, K.; Tamura, H.; Reich, J.; Kuwahara-Arai, K.; Iba, T.; Nagaoka, I. Antimicrobial Cathelicidin Peptide LL-37 Inhibits the Pyroptosis of Macrophages and Improves the Survival of Polybacterial Septic Mice. Int. Immunol. 2016, 28, 245–253. [Google Scholar] [CrossRef]
- Boucher, E.; Marine, M.; Holani, R.; Young-Speirs, M.; Moore, D.M.; Cobo, E.R. Characteristic Pro-Inflammatory Cytokines and Host Defence Cathelicidin Peptide Produced by Human Monocyte-Derived Macrophages Infected with Neospora Caninum. Parasitology 2018, 145, 871–884. [Google Scholar] [CrossRef]
- Cirone, K.M.; Lahiri, P.; Holani, R.; Tan, Y.L.; Arrazuria, R.; De Buck, J.; Barkema, H.W.; Cobo, E.R. Synthetic Cathelicidin LL-37 Reduces Mycobacterium Avium Subsp. Paratuberculosis Internalization and pro-Inflammatory Cytokines in Macrophages. Cell Tissue Res. 2020, 379, 207–217. [Google Scholar] [CrossRef]
- Toda, G.; Yamauchi, T.; Kadowaki, T.; Ueki, K. Preparation and Culture of Bone Marrow-Derived Macrophages from Mice for Functional Analysis. STAR Protoc. 2021, 2, 100246. [Google Scholar] [CrossRef]
- Xi, H.; Zhang, Y.; Xu, Y.; Yang, W.Y.; Jiang, X.; Sha, X.; Cheng, X.; Wang, J.; Qin, X.; Yu, J.; et al. Caspase-1 Inflammasome Activation Mediates Homocysteine-Induced Pyrop-Apoptosis in Endothelial Cells. Circ. Res. 2016, 118, 1525–1539. [Google Scholar] [CrossRef]
- Rivera-Fernández, N.; Anacleto-Santos, J.; Casarrubias-Tabarez, B.; López-Pérez, T.d.J.; Rojas-Lemus, M.; López-Valdez, N.; Fortoul, T.I. Bioactive Peptides against Human Apicomplexan Parasites. Antibiotics 2022, 11, 1658. [Google Scholar] [CrossRef]
- Kulkarni, M.M.; Barbi, J.; Mcmaster, W.R.; Gallo, R.L.; Satoskar, A.R.; Mcgwire, B.S. Mammalian Antimicrobial Peptide Influences Control of Cutaneous Leishmania Infection. Cell. Microbiol. 2011, 13, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Cauchard, S.; Van Reet, N.; Büscher, P.; Goux, D.; Grötzinger, J.; Leippe, M.; Cattoir, V.; Laugier, C.; Cauchard, J. Killing of Trypanozoon Parasites by the Equine Cathelicidin ECATH1. Antimicrob. Agents Chemother. 2016, 60, 2610–2619. [Google Scholar] [CrossRef]
- Klaffenbach, D.; Friedrich, D.; Strick, R.; Strissel, P.L.; Beckmann, M.W.; Rascher, W.; Gessner, A.; Dötsch, J.; Meißner, U.; Schnare, M. Contribution of Different Placental Cells to the Expression and Stimulation of Antimicrobial Proteins (AMPs). Placenta 2011, 32, 830–837. [Google Scholar] [CrossRef]
- Gudmundsson, G.H.; Agerberth, B.; Odeberg, J.; Bergman, T.; Olsson, B.; Salcedo, R. The Human Gene FALL39 and Processing of the Cathelin Precursor to the Antibacterial Peptide LL-37 in Granulocytes. Eur. J. Biochem. 1996, 238, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Holani, R.; Babbar, A.; Blyth, G.A.D.; Lopes, F.; Jijon, H.; McKay, D.M.; Hollenberg, M.D.; Cobo, E.R. Cathelicidin-Mediated Lipopolysaccharide Signaling via Intracellular TLR4 in Colonic Epithelial Cells Evokes CXCL8 Production. Gut Microbes 2020, 12, 1785802. [Google Scholar] [CrossRef]
- Sørensen, O.E.; Follin, P.; Johnsen, A.H.; Calafat, J.; Sandra Tjabringa, G.; Hiemstra, P.S.; Borregaard, N. Human Cathelicidin, HCAP-18, Is Processed to the Antimicrobial Peptide LL-37 by Extracellular Cleavage with Proteinase 3. Blood 2001, 97, 3951–3959. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Buxton, D.; Wouda, W. Pathogenesis of bovine neosporosis. J. Comp. Pathol. 2006, 134, 267–289. [Google Scholar] [CrossRef]
- Buxton, D.; McAllister, M.M.; Dubey, J.P. The comparative pathogenesis of neosporosis. Trends Parasitol. 2002, 18, 546–552. [Google Scholar] [CrossRef]
- Khan, I.A.; Schwartzman, J.D.; Fonseka, S.; Kasper, L.H. Neospora Caninum: Role for Immune Cytokines in Host Immunity. Exp. Parasitol. 1997, 85, 24–34. [Google Scholar] [CrossRef]
- Innes, E.A.; Andrianarivo, A.G.; Björkman, C.; Williams, D.J.L.; Conrad, P.A. Immune Responses to Neospora Caninum and Prospects for Vaccination. Trends Parasitol. 2002, 18, 497–504. [Google Scholar] [CrossRef]
- Quinn, H.E.; Ellis, J.T.; Smith, N.C. Neospora caninum: A cause of immune-mediated failure of pregnancy? Trends Parasitol. 2002, 18, 391–394. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiorani, F.; Lahiri, P.; Puentes, R.; Bradley, P.J.; Moore, D.P.; Cobo, E.R. Cathelicidins Limit Intracellular Neospora caninum-Infection in Macrophages. Pathogens 2025, 14, 663. https://doi.org/10.3390/pathogens14070663
Fiorani F, Lahiri P, Puentes R, Bradley PJ, Moore DP, Cobo ER. Cathelicidins Limit Intracellular Neospora caninum-Infection in Macrophages. Pathogens. 2025; 14(7):663. https://doi.org/10.3390/pathogens14070663
Chicago/Turabian StyleFiorani, Franco, Priyoshi Lahiri, Rodrigo Puentes, Peter John Bradley, Dadin Prando Moore, and Eduardo Ruben Cobo. 2025. "Cathelicidins Limit Intracellular Neospora caninum-Infection in Macrophages" Pathogens 14, no. 7: 663. https://doi.org/10.3390/pathogens14070663
APA StyleFiorani, F., Lahiri, P., Puentes, R., Bradley, P. J., Moore, D. P., & Cobo, E. R. (2025). Cathelicidins Limit Intracellular Neospora caninum-Infection in Macrophages. Pathogens, 14(7), 663. https://doi.org/10.3390/pathogens14070663








