Optimizing Photosensitizer Delivery for Effective Photodynamic Inactivation of Klebsiella pneumoniae Under Lung Surfactant Conditions
Abstract
1. Introduction
2. Methodology
2.1. Photosensitizer Formulation and Light Source
2.2. Strain and Growth Conditions
2.3. Antimicrobial Photodynamic Therapy
2.4. Evaluation of the Treatments
2.5. Statistical Analysis
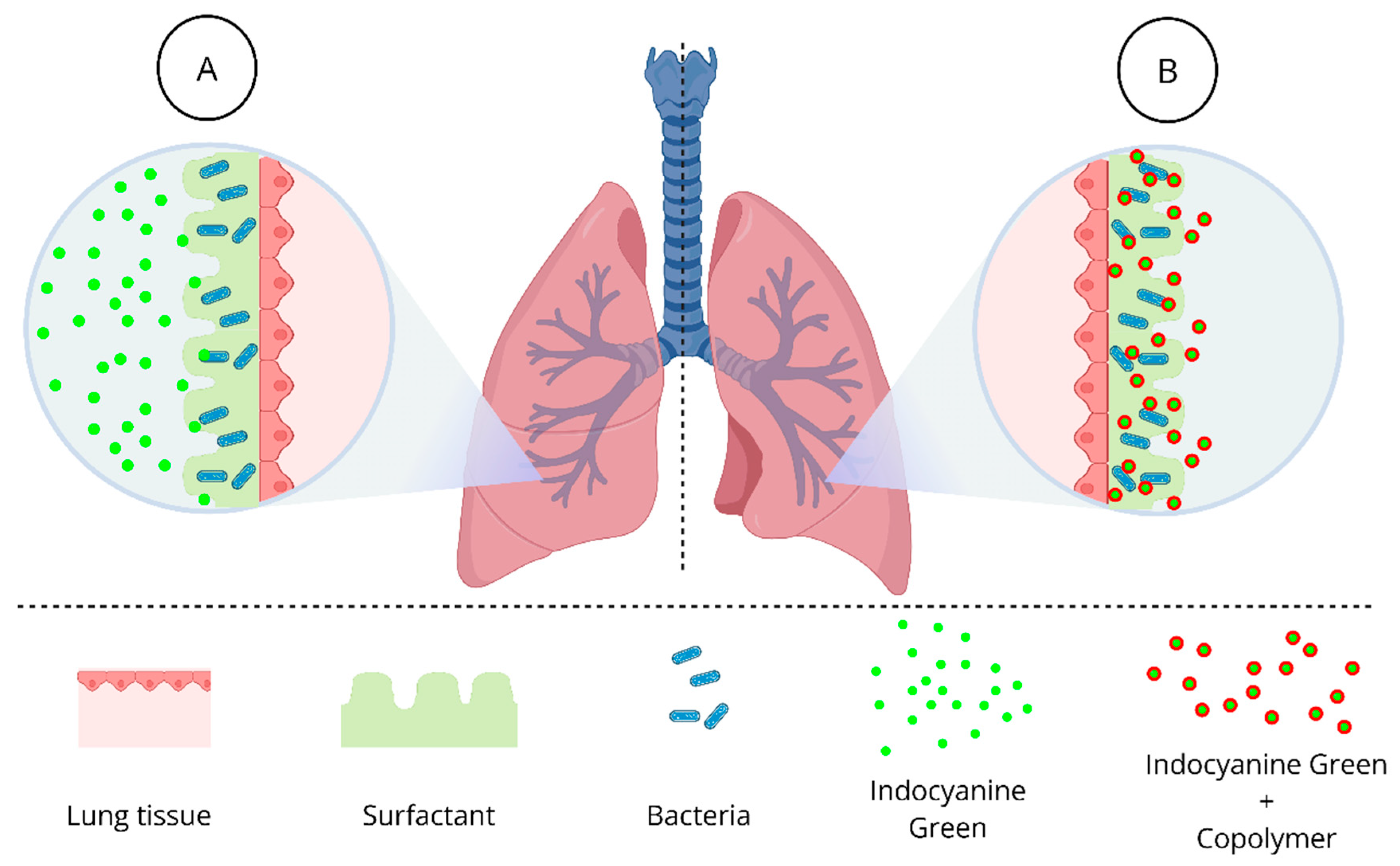
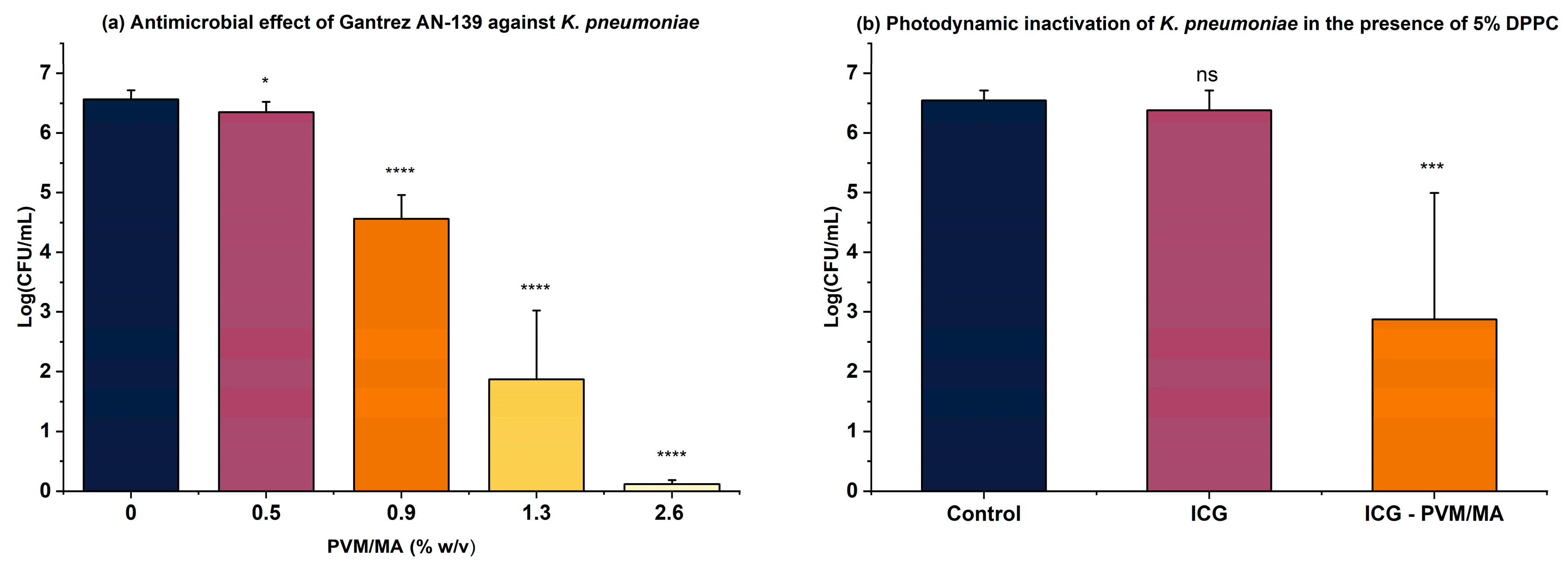
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wuerth, B.A.; Bonnewell, J.P.; Wiemken, T.L.; Arnold, F.W. Trends in Pneumonia Mortality Rates and Hospitalizations by Organism, United States, 2002–2011. Emerg. Infect. Dis. 2016, 22, 1624–1627. [Google Scholar] [CrossRef] [PubMed]
- Tufa, T.B.; Regassa, F.; Amenu, K.; Stegeman, J.A.; Hogeveen, H. Livestock producers’ knowledge, attitude, behavior (KAB) regarding antimicrobial use in Ethiopia. Front. Vet. Sci. 2023, 10, 1167847. [Google Scholar] [CrossRef]
- Núñez, C.; Palavecino, A.; González, I.A.; Dreyse, P.; Palavecino, C.E. Effective photodynamic therapy with ir(Iii) for virulent clinical isolates of extended-spectrum beta-lactamase klebsiella pneumoniae. Pharmaceutics 2021, 13, 603. [Google Scholar] [CrossRef]
- Bravo, A.R.; Fuentealba, F.A.; González, I.A.; Palavecino, C.E. Use of Antimicrobial Photodynamic Therapy to Inactivate Multidrug-Resistant Klebsiella pneumoniae: Scoping Review. Pharmaceutics 2024, 16, 1626. [Google Scholar] [CrossRef] [PubMed]
- Anas, A.; Sobhanan, J.; Sulfiya, K.M.; Jasmin, C.; Sreelakshmi, P.K.; Biju, V. Advances in photodynamic antimicrobial chemotherapy. J. Photochem. Photobiol. C Photochem. Rev. 2021, 49, 100452. [Google Scholar] [CrossRef]
- Soares, J.M.; Yakovlev, V.V.; Blanco, K.C.; Bagnato, V.S. Recovering the susceptibility of antibiotic-resistant bacteria using photooxidative damage. Proc. Natl. Acad. Sci. USA 2023, 120, e2311667120. [Google Scholar] [CrossRef]
- Willis, J.A.; Cheburkanov, V.; Chen, S.; Soares, J.M.; Kassab, G.; Blanco, K.C.; Bagnato, V.S.; de Figueiredo, P.; Yakovlev, V.V. Breaking down antibiotic resistance in methicillin-resistant Staphylococcus aureus: Combining antimicrobial photodynamic and antibiotic treatments. Proc. Natl. Acad. Sci. USA 2022, 119, e2208378119. [Google Scholar] [CrossRef] [PubMed]
- Klausen, M.; Ucuncu, M.; Bradley, M. Design of Photosensitizing Agents for Targeted Antimicrobial Photodynamic Therapy. Molecules 2020, 25, 5239. [Google Scholar] [CrossRef]
- Wang, X.; Peng, J.; Meng, C.; Feng, F. Recent advances for enhanced photodynamic therapy: From new mechanisms to innovative strategies. Chem. Sci. 2024, 15, 12234–12257. [Google Scholar] [CrossRef]
- Przygoda, M.; Bartusik-Aebisher, D.; Dynarowicz, K.; Cieślar, G.; Kawczyk-Krupka, A.; Aebisher, D. Cellular Mechanisms of Singlet Oxygen in Photodynamic Therapy. Int. J. Mol. Sci. 2023, 24, 16890. [Google Scholar] [CrossRef]
- Jiang, J.; Lv, X.; Cheng, H.; Yang, D.; Xu, W.; Hu, Y.; Song, Y.; Zeng, G. Type I photodynamic antimicrobial therapy: Principles, progress, future perspectives. Acta Biomater. 2024, 177, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.; Pavelka, J.M.S.; Baran, T.M. Methylene blue photodynamic therapy of bacterial species found in human abscesses: Planktonic, biofilm, 3D silicone models. Proc. SPIE Int. Soc. Opt. Eng. 2023, 12358, 1235805. [Google Scholar]
- Liu, C.; Zhou, Y.; Wang, L.; Han, L.; Lei, J.; Ishaq, H.M.; Nair, S.P.; Xu, J. Photodynamic inactivation of Klebsiella pneumoniae biofilms and planktonic cells by 5-aminolevulinic acid and 5-aminolevulinic acid methyl ester. Lasers Med. Sci. 2016, 31, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Yorozu, K.; Kaibori, M.; Kimura, S.; Ichikawa, M.; Matsui, K.; Kaneshige, S.; Kobayashi, M.; Jimbo, D.; Torikai, Y.; Fukuzawa, Y.; et al. Experience with Photodynamic Therapy Using Indocyanine Green Liposomes for Refractory Cancer. J. Pers. Med. 2022, 12, 1039. [Google Scholar] [CrossRef]
- Ara, E.S.; Noghreiyan, A.V.; Sazgarnia, A. Evaluation of photodynamic effect of Indocyanine green (ICG) on the colon and glioblastoma cancer cell lines pretreated by cold atmospheric plasma. Photodiagn. Photodyn. Ther. 2021, 35, 102408. [Google Scholar] [CrossRef]
- Wong, T.W.; Wu, E.C.; Ko, W.C.; Lee, C.C.; Hor, L.I.; Huang, I.H. Photodynamic inactivation of methicillin-resistant Staphylococcus aureus by indocyanine green and near infrared light. Dermatol. Sin. 2018, 36, 8–15. [Google Scholar] [CrossRef]
- Higuchi, N.; Hayashi, J.-I.; Fujita, M.; Iwamura, Y.; Sasaki, Y.; Goto, R.; Ohno, T.; Nishida, E.; Yamamoto, G.; Kikuchi, T.; et al. Photodynamic Inactivation of an Endodontic Bacteria Using Diode Laser and Indocyanine Green-Loaded Nanosphere. Int. J. Mol. Sci. 2021, 22, 8384. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Zhu, B.; Zheng, K.; He, S.; Meng, L.; Song, J.; Yang, H. Recent Progress in NIR-II Contrast Agent for Biological Imaging. Front. Bioeng. Biotechnol. 2020, 7, 487. [Google Scholar] [CrossRef]
- Chen, G.; Cao, Y.; Tang, Y.; Yang, X.; Liu, Y.; Huang, D.; Zhang, Y.; Li, C.; Wang, Q. Advanced Near-Infrared Light for Monitoring and Modulating the Spatiotemporal Dynamics of Cell Functions in Living Systems. Adv. Sci. 2020, 7, 1903783. [Google Scholar] [CrossRef]
- Kassab, G.; Cheburkanov, V.; Willis, J.; Moule, M.G.; Kurachi, C.; Yakovlev, V.; Cirillo, J.D.; Bagnato, V.S. Safety and delivery efficiency of a photodynamic treatment of the lungs using indocyanine green and extracorporeal near infrared illumination. J. Biophotonics 2020, 13, e202000176. [Google Scholar] [CrossRef]
- Tovar, J.S.D.; Kassab, G.; Buzzá, H.H.; Bagnato, V.S.; Kurachi, C. Photodynamic inactivation of Streptococcus pneumoniae with external illumination at 808 nm through the ex vivo porcine thoracic cage. J. Biophotonics 2022, 15, e202100189. [Google Scholar] [CrossRef]
- Leite, I.S.; Geralde, M.C.; Salina, A.C.; Medeiros, A.I.; Dovigo, L.N.; Bagnato, V.S.; Inada, N.M. Near–infrared photodynamic inactivation of S. pneumoniae and its interaction with RAW 264.7 macrophages. J. Biophotonics 2017, 11, e201600283. [Google Scholar] [CrossRef]
- Kassab, G.; Tovar, J.S.D.; Souza, L.M.P.; Costa, R.K.M.; Silva, R.S.; Pimentel, A.S.; Kurachi, C.; Bagnato, V.S. Lung surfactant negatively affects the photodynamic inactivation of bacteria—In vitro and molecular dynamic simulation analyses. Proc. Natl. Acad. Sci. USA 2022, 119, e2123564119. [Google Scholar] [CrossRef]
- Hidalgo, A.; Garcia-Mouton, C.; Autilio, C.; Carravilla, P.; Orellana, G.; Islam, M.N.; Bhattacharya, J.; Bhattacharya, S.; Cruz, A.; Pérez-Gil, J. Pulmonary surfactant and drug delivery: Vehiculization, release and targeting of surfactant/tacrolimus formulations. J. Control. Release 2021, 329, 205–222. [Google Scholar] [CrossRef] [PubMed]
- Confalonieri, M.; Salton, F.; Ruaro, B.; Confalonieri, P.; Volpe, M.C. Alveolar Epithelial Type II Cells. In Encyclopedia of Respiratory Medicine, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 1, pp. 10–17. [Google Scholar] [CrossRef]
- de Lima, I.A.; Fiuza, L.G.; Tovar, J.S.D.; Bejar, D.S.L.; Tomé, A.J.B.; Requena, M.B.; Pires, L.; Zheng, G.; Inada, N.M.; Kurachi, C.; et al. Strategies for overcoming the lung surfactant barrier and achieving success in antimicrobial photodynamic therapy. J. Photochem. Photobiol. 2024, 24, 100252. [Google Scholar] [CrossRef]
- Rajagopalan, R.; Uetrecht, P.; Bugaj, J.E.; Achilefu, S.A.; Dorshow, R.B. Stabilization of the Optical Tracer Agent Indocyanine Green Using Noncovalent Interactions. J. Photochem. Photobiol. 2000, 71, 347–350. [Google Scholar] [CrossRef]
- Caffarel-Salvador, E.; Kearney, M.-C.; Mairs, R.; Gallo, L.; Stewart, S.A.; Brady, A.J.; Donnelly, R.F. Methylene blue-loaded dissolving microneedles: Potential use in photodynamic antimicrobial chemotherapy of infected wounds. Pharmaceutics 2015, 7, 397–412. [Google Scholar] [CrossRef]
- Rapacka-Zdończyk, A.; Woźniak, A.; Michalska, K.; Pierański, M.; Ogonowska, P.; Grinholc, M.; Nakonieczna, J. Factors Determining the Susceptibility of Bacteria to Antibacterial Photodynamic Inactivation. Front. Med. 2021, 8, 642609. [Google Scholar] [CrossRef]
- Reik, W.; Dean, W. Antimicrobial Photodynamic Therapy to Kill Gram-negative Bacteria. Recent. Pat. Antiinfect. Drug Discov. 2013, 8, 108–120. [Google Scholar] [CrossRef]
- Huang, L.; Szewczyk, G.; Sarna, T.; Hamblin, M.R. Potassium Iodide Potentiates Broad-Spectrum Antimicrobial Photodynamic Inactivation Using Photofrin. ACS Infect. Dis. 2018, 3, 320–328. [Google Scholar] [CrossRef]
- Li, Q.; Huang, L.; Xuan, W.; Yin, X.; Zan, Y. Study of photodynamic effect of Photofrin plus potassium iodide against Gram-negative bacteria. J. Guangxi Med. Univ. 2023, 40, 1972–1977. [Google Scholar] [CrossRef]
- Topaloglu, N.; Guney, M.; Aysan, N.; Gulsoy, M.; Yuksel, S. The role of reactive oxygen species in the antibacterial photodynamic treatment: Photoinactivation vs proliferation. Lett. Appl. Microbiol. 2016, 62, 230–236. [Google Scholar] [CrossRef]
- Afrasiabi, S.; Benedicenti, S.; Signore, A.; Arshad, M.; Chiniforush, N. Simultaneous Dual-Wavelength Laser Irradiation against Implant-Adherent Biofilms of Staphylococcus aureus, Escherichia coli, Candida albicans for Improved Antimicrobial Photodynamic Therapy. Bioengineering 2024, 11, 48. [Google Scholar] [CrossRef] [PubMed]
- Gnanasekar, S.; Kasi, G.; He, X.; Zhang, K.; Xu, L.; Kang, E.T. Recent advances in engineered polymeric materials for efficient photodynamic inactivation of bacterial pathogens. Bioact. Mater. 2023, 21, 157–174. [Google Scholar] [CrossRef] [PubMed]
- Requena, M.B.; Permana, A.D.; Vollet-Filho, J.D.; González-Vázquez, P.; Garcia, M.R.; De Faria, C.M.G.; Pratavieira, S.; Donnelly, R.F.; Bagnato, V.S. Dissolving microneedles containing aminolevulinic acid improves protoporphyrin IX distribution. J. Biophotonics 2021, 14, e202000128. [Google Scholar] [CrossRef]
- Patel, M.D.; Date, P.V.; Gaikwad, R.V.; Samad, A.; Malshe, V.C.; Devarajan, P.V. Comparative evaluation of polymeric nanoparticles of rifampicin comprising gantrez and poly(ethylene sebacate) on pharmacokinetics, biodistribution and lung uptake following oral administration. J. Biomed. Nanotechnol. 2014, 10, 687–694. [Google Scholar] [CrossRef]
- Lai, S.K.; Wang, Y.Y.; Hanes, J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv. Drug Deliv. Rev. 2009, 61, 158–171. [Google Scholar] [CrossRef]
- McChlery, S.; Ramage, G.; Bagg, J. Respiratory tract infections and pneumonia. Periodontology 2000 2009, 49, 151–165. [Google Scholar] [CrossRef]
- Thakur, A.K.; Kaundle, B.; Singh, I. Mucoadhesive drug delivery systems in respiratory diseases. In Targeting Chronic Inflammatory Lung Diseases Using Advanced Drug Delivery Systems; Academic Press: Cambridge, MA, USA, 2020; pp. 475–491. [Google Scholar] [CrossRef]
- Makhlof, A.; Werle, M.; Takeuchi, H. Mucoadhesive drug carriers and polymers for effective drug delivery. J. Drug Deliv. Sci. Technol. 2008, 18, 375–386. [Google Scholar] [CrossRef]
- Mira, A.; Mateo, C.R.; Mallavia, R.; Falco, A. Poly(methyl vinyl ether-alt-maleic acid) and ethyl monoester as building polymers for drug-loadable electrospun nanofibers. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Gardner, C.M.; Burke, N.A.D.; Chu, T.; Shen, F.; Potter, M.A.; Stöver, H.D.H. Poly(methyl vinyl ether-alt-maleic acid) polymers for cell encapsulation. J. Biomater. Sci. Polym. Ed. 2011, 22, 2127–2145. [Google Scholar] [CrossRef] [PubMed]
- Meijer, D.K.F.; Weert, B.; Vermeer, G.A. Pharmacokinetics of biliary excretion in man. VI. Indocyanine green. Eur. J. Clin. Pharmacol. 1988, 35, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; McCrudden, M.T.C.; Alkilani, A.Z.; Larrañeta, E.; McAlister, E.; Courtenay, A.J.; Kearney, M.-C.; Singh, T.R.R.; McCarthy, H.O.; Kett, V.L.; et al. Hydrogel-Forming Microneedles Prepared from ‘Super Swelling’ Polymers Combined with Lyophilised Wafers for Transdermal Drug Delivery. PLoS ONE 2014, 9, e111547. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, F.; de Lima, I.A.; Fiuza, L.G.; A. Arnaut, Z.; Inada, N.M.; Bagnato, V.S. Optimizing Photosensitizer Delivery for Effective Photodynamic Inactivation of Klebsiella pneumoniae Under Lung Surfactant Conditions. Pathogens 2025, 14, 618. https://doi.org/10.3390/pathogens14070618
Alves F, de Lima IA, Fiuza LG, A. Arnaut Z, Inada NM, Bagnato VS. Optimizing Photosensitizer Delivery for Effective Photodynamic Inactivation of Klebsiella pneumoniae Under Lung Surfactant Conditions. Pathogens. 2025; 14(7):618. https://doi.org/10.3390/pathogens14070618
Chicago/Turabian StyleAlves, Fernanda, Isabelle Almeida de Lima, Lorraine Gabriele Fiuza, Zoe A. Arnaut, Natalia Mayumi Inada, and Vanderlei Salvador Bagnato. 2025. "Optimizing Photosensitizer Delivery for Effective Photodynamic Inactivation of Klebsiella pneumoniae Under Lung Surfactant Conditions" Pathogens 14, no. 7: 618. https://doi.org/10.3390/pathogens14070618
APA StyleAlves, F., de Lima, I. A., Fiuza, L. G., A. Arnaut, Z., Inada, N. M., & Bagnato, V. S. (2025). Optimizing Photosensitizer Delivery for Effective Photodynamic Inactivation of Klebsiella pneumoniae Under Lung Surfactant Conditions. Pathogens, 14(7), 618. https://doi.org/10.3390/pathogens14070618





