The Challenge of Lyssavirus Infections in Domestic and Other Animals: A Mix of Virological Confusion, Consternation, Chagrin, and Curiosity
Abstract
1. Introduction
2. Etiological Agents and Taxonomy
3. History of ‘Rabies-Related Viruses’
4. Host/Pathogen and Clinical Spectrum
5. Pathobiology
6. Laboratory Diagnosis of Lyssaviruses in Animals
6.1. Nucleic Acid-Based Assays
6.2. Antigen-Based Assays
6.3. Serum-Based Assays
7. Lyssavirus Occurrence and Disease Designations
8. Management
9. Selected Regional Epidemiological Highlights
9.1. Europe
9.2. Asia and the Indian Sub-Continent
9.3. Africa
9.3.1. Fragmented Control Programs
9.3.2. Challenges to Laboratory-Based Surveillance
9.3.3. Consumption of Infected Animals?
9.3.4. Veterinarians at Risk of Viral Exposure
9.4. North, Central, and South America
9.5. The Caribbean, with Special Reference to Trinidad: Cattle, Small Ruminants, and Water Buffalo
9.6. Australasian Region
10. Bats, Lyssaviruses, and Serology
11. Current and Future Concerns
12. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABC-ARV | animal birth control-antirabies vaccination program |
| ABLV | Australian bat lyssavirus |
| ACIP | Advisory Committee on Immunization Practices |
| ARAV | Aravan virus |
| BBLV | Bokeloh bat lyssavirus |
| CDC | Centers for Disease Control and Prevention |
| CNS | central nervous system |
| CSF | cerebro-spinal fluid |
| DBLV | Divaea bat lyssavirus |
| DFAT | direct fluorescent antibody test |
| DRIT | direct rapid immunohistochemical test |
| DUVV | Duvenhage virus |
| EBLV1 | European bat lyssavirus 1 |
| EBLV2 | European bat lyssavirus 2 |
| EID | emerging infectious disease |
| ELISA | enzyme-linked immunosorbent assay |
| FAO | Food and Agriculture Organization |
| G | glycoprotein |
| GBLV | Gannoruwa bat lyssavirus |
| IHC | immunohistochemistry |
| IKOV | Ikoma lyssavirus |
| IRKV | Irkut virus |
| KBLV | Kotalahti bat lyssavirus |
| KHUV | Khujand virus |
| L | ‘large’ or RNA-dependent RNA polymerase protein |
| LAMP | loop-mediated isothermal amplification |
| LBV | Lagos bat virus |
| LLEBV | Lleida bat lyssavirus |
| LMICs | lower-and-middle-income countries |
| M | matrix protein |
| MAbs | monoclonal antibodies |
| MBLV | Matlo bat lyssavirus |
| MDV | mass dog vaccination |
| MLV | modified-live virus |
| MOK | Mokola virus |
| N | nucleoprotein |
| P | phosphoprotein |
| PAHO | Pan American Health Organization |
| PBLV | Phala bat lyssavirus |
| PEP | Postexposure prophylaxis |
| PrEP | Preexposure prophylaxis |
| RIG | rabies immune globulin |
| RNP | ribonucleoprotein |
| RPA | recombinase polymerase amplification |
| SHIBV | Shimoni bat virus |
| TWBLV | Taiwan bat lyssavirus |
| TWBLV2 | Taiwan bat lyssavirus 2 |
| RABV | Rabies virus |
| VNA | virus neutralizing antibodies |
| WCBV | West Caucasian bat virus |
| WHO | World Health Organization |
| WOAH | World Organization for Animal Health |
| ZBT | ‘zero by thirty’ |
| ZRA | ZeroRabiesApp |
References
- Spellberg, B.; Taylor-Blake, B. On the exoneration of Dr. William H. Stewart: Debunking an urban legend. Infect. Dis. Poverty 2013, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Kuchipudi, S.V.; Behring, D.; Nissly, R.; Chothe, S.K.; Gontu, A.; Ravichandran, A.; Butler, T. Mitigating the Impact of Emerging Animal Infectious Disease Threats: First Emerging Animal Infectious Diseases Conference (EAIDC) Report. Viruses 2022, 14, 947. [Google Scholar] [CrossRef] [PubMed]
- Trotta, A.; Marinaro, M.; Cavalli, A.; Cordisco, M.; Piperis, A.; Buonavoglia, C.; Corrente, M. African Swine Fever-How to Unravel Fake News in Veterinary Medicine. Animals 2022, 12, 656. [Google Scholar] [CrossRef] [PubMed]
- Rudolf, I.; Kejíková, R.; Kosoy, M.; Hubálek, Z.; Mravcová, K.; Šikutová, S.; Whatmore, A.M.; Al Dahouk, S. Brucella microti and Rodent-Borne Brucellosis: A Neglected Public Health Threat. Zoonoses Public Health 2025, 72, 1–8. [Google Scholar] [CrossRef]
- Manchal, N.; Young, M.K.; Castellanos, M.E.; Leggat, P.; Adegboye, O. A systematic review and meta-analysis of ambient temperature and precipitation with infections from five food-borne bacterial pathogens. Epidemiol. Infect. 2024, 152, e98. [Google Scholar] [CrossRef]
- Pavia, G.; Branda, F.; Ciccozzi, A.; Romano, C.; Locci, C.; Azzena, I.; Pascale, N.; Marascio, N.; Quirino, A.; Gigliotti, S.; et al. The issue of climate change and the spread of tropical diseases in Europe and Italy: Vector biology, disease transmission, genome-based monitoring and public health implications. Infect. Dis. 2025, 57, 121–136. [Google Scholar] [CrossRef]
- Hagedoorn, N.N.; Maze, M.J.; Carugati, M.; Cash-Goldwasser, S.; Allan, K.J.; Chen, K.; Cossic, B.; Demeter, E.; Gallagher, S.; German, R.; et al. Global distribution of Leptospira serovar isolations and detections from animal host species: A systematic review and online database. Trop. Med. Int. Health 2024, 29, 161–172. [Google Scholar] [CrossRef]
- Teitelbaum, C.S.; Ferraz, A.; De La Cruz, S.E.W.; Gilmour, M.E.; Brosnan, I.G. The potential of remote sensing for improved infectious disease ecology research and practice. Proc. Biol. Sci. 2024, 291, 20241712. [Google Scholar] [CrossRef]
- Branda, F.; Pavia, G.; Ciccozzi, A.; Quirino, A.; Marascio, N.; Matera, G.; Romano, C.; Locci, C.; Azzena, I.; Pascale, N.; et al. Zoonotic Paramyxoviruses: Evolution, Ecology, and Public Health Strategies in a Changing World. Viruses 2024, 16, 1688. [Google Scholar] [CrossRef]
- Elrashedy, A.; Mousa, W.; Nayel, M.; Salama, A.; Zaghawa, A.; Elsify, A.; Hasan, M.E. Advances in bioinformatics and multiomics integration: Transforming viral infectious disease research in veterinary medicine. Virol. J. 2025, 22, 22. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, C.; He, J. Aquatic circoviruses: Emerging pathogens in global aquaculture—From discovery to disease management. J. Virol. 2025, 99, e0173724. [Google Scholar] [CrossRef] [PubMed]
- Salman, M.D. The role of veterinary epidemiology in combating infectious animal diseases on a global scale: The impact of training and outreach programs. Prev. Vet. Med. 2009, 92, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Reperant, L.A.; Brown, I.H.; Haenen, O.L.; de Jong, M.D.; Osterhaus, A.D.; Papa, A.; Rimstad, E.; Valarcher, J.F.; Kuiken, T. Companion Animals as a Source of Viruses for Human Beings and Food Production Animals. J. Comp. Pathol. 2016, 155 (Suppl. S1), S41–S53. [Google Scholar] [CrossRef]
- Bodjo, S.C.; Nwankpa, N.; Couacy-Hymann, E.; Tounkara, K.; Diallo, A. Rinderpest and peste des petits ruminants: A century of progress and the future. Rev. Sci. Tech. 2024, Special Edition, 36–42. [Google Scholar] [CrossRef]
- Vasconcelos, A.; King, J.D.; Nunes-Alves, C.; Anderson, R.; Argaw, D.; Basáñez, M.G.; Bilal, S.; Blok, D.J.; Blumberg, S.; Borlase, A.; et al. Accelerating Progress Towards the 2030 Neglected Tropical Diseases Targets: How Can Quantitative Modeling Support Programmatic Decisions? Clin. Infect. Dis. 2024, 78 (Suppl. S2), S83–S92. [Google Scholar] [CrossRef]
- Burgess, B.A.; Morley, P.S. Biosecurity and Infection Control for Veterinarians Caring for Livestock: A Guide for Daily Threats. Vet. Clin. N. Am. Food Anim. Pract. 2025, 41, 11–24. [Google Scholar] [CrossRef]
- Feasey, N.; Ahmad, R.; Ashley, E.; Atun, R.; Baker, K.S.; Chiari, F.; van Doorn, H.R.; Holmes, A.; Jinks, T.; Jermy, A.; et al. Insights of SEDRIC, the Surveillance and Epidemiology of Drug-Resistant Infections Consortium. Wellcome Open Res. 2025, 10, 5. [Google Scholar] [CrossRef]
- Gebeyehu, D.T.; East, L.; Wark, S.; Islam, M.S. Direct and indirect crisis of food security due to COVID-19 emergence in Addis Ababa and Amhara regions, Ethiopia: A lesson for the inevitable pandemics. BMC Public Health 2025, 25, 866. [Google Scholar] [CrossRef]
- Zerbini, F.M.; Simmonds, P.; Adriaenssens, E.M.; Lefkowitz, E.J.; Oksanen, H.M.; Alfenas-Zerbini, P.; Aylward, F.O.; Freitas-Astúa, J.; Hughes, H.R.; Łobocka, M.; et al. Virus species names have been standardized; virus names remain unchanged. mSphere 2025, 10, e0002025. [Google Scholar] [CrossRef]
- Walker, P.J.; Freitas-Astúa, J.; Bejerman, N.; Blasdell, K.R.; Breyta, R.; Dietzgen, R.G.; Fooks, A.R.; Kondo, H.; Kurath, G.; Kuzmin, I.V.; et al. ICTV Virus Taxonomy Profile: Rhabdoviridae 2022. J. Gen. Virol. 2022, 103, 001689. [Google Scholar] [CrossRef]
- Horwitz, J.A.; Jenni, S.; Harrison, S.C.; Whelan, S.P.J. Structure of a rabies virus polymerase complex from electron cryo-microscopy. Proc. Natl. Acad. Sci. USA 2020, 117, 2099–2107. [Google Scholar] [CrossRef] [PubMed]
- Gérard, F.C.A.; Bourhis, J.M.; Mas, C.; Branchard, A.; Vu, D.D.; Varhoshkova, S.; Leyrat, C.; Jamin, M. Structure and Dynamics of the Unassembled Nucleoprotein of Rabies Virus in Complex with Its Phosphoprotein Chaperone Module. Viruses 2022, 14, 2813. [Google Scholar] [CrossRef] [PubMed]
- Callaway, H.M.; Zyla, D.; Larrous, F.; de Melo, G.D.; Hastie, K.M.; Avalos, R.D.; Agarwal, A.; Corti, D.; Bourhy, H.; Saphire, E.O. Structure of the rabies virus glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Sci Adv. 2022, 8, eabp9151. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Li, M.; Zhu, J.; Li, X.; Luo, T.R.; Liang, J. Host Desmin Interacts with RABV Matrix Protein and Facilitates Virus Propagation. Viruses 2023, 15, 434. [Google Scholar] [CrossRef]
- Itakura, Y.; Tabata, K.; Saito, T.; Intaruck, K.; Kawaguchi, N.; Kishimoto, M.; Torii, S.; Kobayashi, S.; Ito, N.; Harada, M.; et al. Morphogenesis of Bullet-Shaped Rabies Virus Particles Regulated by TSG101. J. Virol. 2023, 97, e0043823. [Google Scholar] [CrossRef]
- Kleiner, V.A.; Fearns, R. How does the polymerase of non-segmented negative strand RNA viruses commit to transcription or genome replication? J. Virol. 2024, 98, e0033224. [Google Scholar] [CrossRef]
- Feige, L.; Zaeck, L.M.; Sehl-Ewert, J.; Finke, S.; Bourhy, H. Innate Immune Signaling and Role of Glial Cells in Herpes Simplex Virus- and Rabies Virus-Induced Encephalitis. Viruses 2021, 13, 2364. [Google Scholar] [CrossRef]
- Tarantola, A. Four Thousand Years of Concepts Relating to Rabies in Animals and Humans, Its Prevention and Its Cure. Trop. Med. Infect. Dis. 2017, 2, 5. [Google Scholar] [CrossRef]
- Boulger, L.R.; Porterfield, J.S. Isolation of a virus from Nigerian fruit bats. Trans. R. Soc. Trop. Med. Hyg. 1958, 52, 421–424. [Google Scholar] [CrossRef]
- Causey, O.R.; Kemp, G.E. Surveillance and study of viral infections of vertebrates in Nigeria. Nigerian J. Sci. 1968, 2, 131–135. [Google Scholar]
- Kemp, G.E.; Causey, O.R.; Moore, D.L.; Odelola, A.; Fabiyi, A. Mokola virus. Further studies on IbAn 27377, a new rabies-related etiologic agent of zoonosis in Nigeria. Am. J. Trop. Med. Hyg. 1972, 21, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Shope, R.E.; Murphy, F.A.; Harrison, A.K.; Causey, O.R.; Kemp, G.E.; Simpson, D.I.; Moore, D.L. Two African viruses serologically and morphologically related to rabies virus. J. Virol. 1970, 6, 690–692. [Google Scholar] [CrossRef] [PubMed]
- Tignor, G.H.; Shope, R.E. Vaccination and challenge of mice with viruses of the rabies serogroup. J. Infect. Dis. 1972, 125, 322–324. [Google Scholar] [CrossRef] [PubMed]
- Tignor, G.H.; Shope, R.E.; Bhatt, P.N.; Percy, D.H. Experimental infection of dogs and monkeys with two rabies serogroup viruses, Lagos bat and Mokola (IbAn 27377): Clinical, serologic, virologic, and fluorescent-antibody studies. J. Infect. Dis. 1973, 128, 471–478. [Google Scholar] [CrossRef]
- Schneider, L.G.; Dietzschold, B.; Dierks, R.E.; Matthaeus, W.; Enzmann, P.J.; Strohmaier, K. Rabies group-specific ribonucleoprotein antigen and a test system for grouping and typing of rhabdoviruses. J. Virol. 1973, 11, 748–755. [Google Scholar] [CrossRef]
- Meredith, C.D.; Rossouw, A.P.; Van Praag Koch, H. An unusual case of human rabies thought to be of Chiropteran origin. S. Afr. Med. J. 1971, 45, 767–769. [Google Scholar]
- Tignor, G.H.; Murphy, F.A.; Clark, H.F.; Shope, R.E.; Madore, P.; Bauer, S.P.; Buckley, S.M.; Meredith, C.D. Duvenhage virus: Morphological, biochemical, histopathological, and antigenic relationships to the rabies serogroup. J. Gen. Virol. 1977, 37, 595–611. [Google Scholar] [CrossRef]
- Wiktor, T.J.; Koprowski, H. Monoclonal antibodies against rabies virus produced by somatic cell hybridization: Detection of antigenic variants. Proc. Natl. Acad. Sci. USA 1978, 75, 3938–3942. [Google Scholar] [CrossRef]
- Flamand, A.; Wiktor, T.J.; Koprowski, H. Use of hybridoma monoclonal antibodies in the detection of antigenic differences between rabies and rabies-related virus proteins. I. The nucleocapsid protein. J. Gen. Virol. 1980, 48, 97–104. [Google Scholar] [CrossRef]
- Flamand, A.; Wiktor, T.J.; Koprowski, H. Use of hybridoma monoclonal antibodies in the detection of antigenic differences between rabies and rabies-related virus proteins. II. The glycoprotein. J. Gen. Virol. 1980, 48, 105–109. [Google Scholar] [CrossRef]
- Wersching, S.; Schneider, L.G. Ein weiterer Fall von Tollwut bei einer Fledermaus in Hamburg. Berl. Munch. Tierarztl. Wochenschr. 1969, 82, 293–295. [Google Scholar] [PubMed]
- Schneider, L.G. Antigenic variants of rabies virus. Comp. Immunol. Microbiol. Infect. Dis. 1982, 5, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Lumio, J.; Hillbom, M.; Roine, R.; Ketonen, L.; Haltia, M.; Valle, M.; Neuvonen, E.; Lähdevirta, J. Human rabies of bat origin in Europe. Lancet 1986, 1, 378. [Google Scholar] [CrossRef]
- CDC. International notes bat rabies—Europe. MMWR 1986, 35, 430–432. [Google Scholar]
- Lafon, M.; Herzog, M.; Sureau, P. Human rabies vaccines induce neutralising antibodies against the European bat rabies virus (Duvenhage). Lancet 1986, 2, 515. [Google Scholar] [CrossRef]
- Roine, R.O.; Hillbom, M.; Valle, M.; Haltia, M.; Ketonen, L.; Neuvonen, E.; Lumio, J.; Lähdevirta, J. Fatal encephalitis caused by a bat-borne rabies-related virus. Clin. Find. Brain. 1988, 111, 1505–1516. [Google Scholar] [CrossRef]
- Shope, R.E. Rabies-related viruses. Yale J. Biol. Med. 1982, 55, 271–275. [Google Scholar] [PubMed]
- Wiktor, T.J.; Macfarlan, R.I.; Foggin, C.M.; Koprowski, H. Antigenic analysis of rabies and Mokola virus from Zimbabwe using monoclonal antibodies. Dev. Biol. Stand. 1984, 57, 199–211. [Google Scholar] [PubMed]
- Kuz’min, I.V.; Botvinkin, A.D.; Rybin, S.N.; Baialiev, A.B. Lissavirus s neobychnoĭ antigennoĭ strukturoĭ, vydelennoĭ ot letucheĭ myshi na iuge Kyrgyzstana. Vopr. Virusol. 1992, 37, 256–259. [Google Scholar] [PubMed]
- Botvinkin, A.D.; Kuzmin, I.V.; Rybin, S.N. The unusual bat lyssavirus Aravan from Central Asia. Myotis 1996, 34, 101–104. [Google Scholar]
- Arai, Y.T.; Kuzmin, I.V.; Kameoka, Y.; Botvinkin, A.D. New lyssavirus genotype from the Lesser Mouse-eared Bat (Myotis blythi), Kyrghyzstan. Emerg. Infect. Dis. 2003, 9, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Kuzmin, I.V.; Orciari, L.A.; Arai, Y.T.; Smith, J.S.; Hanlon, C.A.; Kameoka, Y.; Rupprecht, C.E. Bat lyssaviruses (Aravan and Khujand) from Central Asia: Phylogenetic relationships according to N, P and G gene sequences. Virus Res. 2003, 97, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Kuzmin, I.V.; Wu, X.; Tordo, N.; Rupprecht, C.E. Complete genomes of Aravan, Khujand, Irkut and West Caucasian bat viruses, with special attention to the polymerase gene and non-coding regions. Virus Res. 2008, 136, 81–90. [Google Scholar] [CrossRef]
- Fraser, G.C.; Hooper, P.T.; Lunt, R.A.; Gould, A.R.; Gleeson, L.J.; Hyatt, A.D.; Russell, G.M.; Kattenbelt, J.A. Encephalitis caused by a Lyssavirus in fruit bats in Australia. Emerg. Infect. Dis. 1996, 2, 327–331. [Google Scholar] [CrossRef]
- Annand, E.J.; Reid, P.A. Clinical review of two fatal equine cases of infection with the insectivorous bat strain of Australian bat lyssavirus. Aust. Vet. J. 2014, 92, 324–332. [Google Scholar] [CrossRef]
- Prada, D.; Boyd, V.; Baker, M.; Jackson, B.; O’Dea, M. Insights into Australian Bat Lyssavirus in Insectivorous Bats of Western Australia. Trop. Med. Infect. Dis. 2019, 4, 46. [Google Scholar] [CrossRef]
- Freuling, C.M.; Beer, M.; Conraths, F.J.; Finke, S.; Hoffmann, B.; Keller, B.; Kliemt, J.; Mettenleiter, T.C.; Mühlbach, E.; Teifke, J.P.; et al. Novel lyssavirus in Natterer’s bat, Germany. Emerg. Infect. Dis. 2011, 17, 1519–1522. [Google Scholar] [CrossRef]
- Picard-Meyer, E.; Servat, A.; Robardet, E.; Moinet, M.; Borel, C.; Cliquet, F. Isolation of Bokeloh bat lyssavirus in Myotis nattereri in France. Arch. Virol. 2013, 158, 2333–2340. [Google Scholar] [CrossRef]
- Klein, A.; Calvelage, S.; Schlottau, K.; Hoffmann, B.; Eggerbauer, E.; Müller, T.; Freuling, C.M. Retrospective Enhanced Bat Lyssavirus Surveillance in Germany between 2018–2020. Viruses 2021, 13, 1538. [Google Scholar] [CrossRef]
- Botvinkin, A.D.; Poleschuk, E.M.; Kuzmin, I.V.; Borisova, T.I.; Gazaryan, S.V.; Yager, P.; Rupprecht, C.E. Novel lyssaviruses isolated from bats in Russia. Emerg. Infect. Dis. 2003, 9, 1623–1625. [Google Scholar] [CrossRef]
- Leopardi, S.; Barneschi, E.; Manna, G.; Zecchin, B.; Priori, P.; Drzewnioková, P.; Festa, F.; Lombardo, A.; Parca, F.; Scaravelli, D.; et al. Spillover of West Caucasian Bat Lyssavirus (WCBV) in a Domestic Cat and Westward Expansion in the Palearctic Region. Viruses 2021, 13, 2064. [Google Scholar] [CrossRef] [PubMed]
- Schneider, L.G.; Barnard, B.J.H.; Schneider, H.P. Application of monoclonal antibodies for epidemiological investigations and oral vaccination studies: I. African virus. In Rabies in the Tropics; Kuwert, E., Mérieux, C., Koprowski, H., Bögel, K., Eds.; Springer: Berlin/Heidelberg, Germany, 1985; pp. 47–59. [Google Scholar]
- Paweska, J.T.; Blumberg, L.H.; Liebenberg, C.; Hewlett, R.H.; Grobbelaar, A.A.; Leman, P.A.; Croft, J.E.; Nel, L.H.; Nutt, L.; Swanepoel, R. Fatal human infection with rabies-related Duvenhage virus, South Africa. Emerg. Infect. Dis. 2006, 12, 1965–1967. [Google Scholar] [CrossRef] [PubMed]
- van Thiel, P.P.; van den Hoek, J.A.; Eftimov, F.; Tepaske, R.; Zaaijer, H.J.; Spanjaard, L.; de Boer, H.E.; van Doornum, G.J.; Schutten, M.; Osterhaus, A.; et al. Fatal case of human rabies (Duvenhage virus) from a bat in Kenya: The Netherlands, December 2007. Euro Surveill. 2008, 13, 8007. [Google Scholar] [CrossRef]
- Coertse, J.; Grobler, C.S.; Sabeta, C.T.; Seamark, E.C.J.; Kearney, T.; Paweska, J.T.; Markotter, W. Lyssaviruses in Insectivorous Bats, South Africa, 2003–2018. Emerg. Infect. Dis. 2020, 26, 3056–3060. [Google Scholar] [CrossRef]
- Hu, S.C.; Hsu, C.L.; Lee, M.S.; Tu, Y.C.; Chang, J.C.; Wu, C.H.; Lee, S.H.; Ting, L.J.; Tsai, K.R.; Cheng, M.C.; et al. Lyssavirus in Japanese Pipistrelle, Taiwan. Emerg. Infect. Dis. 2018, 24, 782–785. [Google Scholar] [CrossRef]
- Gunawardena, P.S.; Marston, D.A.; Ellis, R.J.; Wise, E.L.; Karawita, A.C.; Breed, A.C.; McElhinney, L.M.; Johnson, N.; Banyard, A.C.; Fooks, A.R. Lyssavirus in Indian Flying Foxes, Sri Lanka. Emerg. Infect. Dis. 2016, 22, 1456–1459. [Google Scholar] [CrossRef]
- Mohr, W. Die Tollwut. Med. Klin. 1957, 52, 1057–1060. [Google Scholar]
- Kappeler, A. Bat rabies surveillance in Europe. Rabies Bull. Eur. 1989, 13, 12–13. [Google Scholar]
- Selimov, M.A.; Tatarov, A.G.; Botvinkin, A.D.; Klueva, E.V.; Kulikova, L.G.; Khismatullina, N.A. Rabies-related Yuli virus; identification with a panel of monoclonal antibodies. Acta Virol. 1989, 33, 542–546. [Google Scholar] [PubMed]
- King, A.; Davies, P.; Lawrie, A. The rabies viruses of bats. Vet. Microbiol. 1990, 23, 165–174. [Google Scholar] [CrossRef]
- Bourhy, H.; Kissi, B.; Lafon, M.; Sacramento, D.; Tordo, N. Antigenic and molecular characterization of bat rabies virus in Europe. J. Clin. Microbiol. 1992, 30, 2419–2426. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.; Cox, J.; Peter, W.; Schäfer, R.; Johnson, N.; McElhinney, L.M.; Geue, J.L.; Tjørnehøj, K.; Fooks, A.R. Spill-over of European bat lyssavirus type 1 into a stone marten (Martes foina) in Germany. J. Vet. Med. B Infect. Dis. Vet. Public Health 2004, 51, 49–54. [Google Scholar] [CrossRef]
- Dacheux, L.; Larrous, F.; Mailles, A.; Boisseleau, D.; Delmas, O.; Biron, C.; Bouchier, C.; Capek, I.; Muller, M.; Ilari, F.; et al. European bat lyssavirus transmission among cats, Europe. Emerg. Infect. Dis. 2009, 15, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Kuzmin, I.V.; Botvinkin, A.D.; Poleschuk, E.M.; Orciari, L.A.; Rupprecht, C.E. Bat rabies surveillance in the former Soviet Union. Dev. Biol. 2006, 125, 273–282. [Google Scholar]
- Tjørnehøj, K.; Fooks, A.R.; Agerholm, J.S.; Rønsholt, L. Natural and experimental infection of sheep with European bat lyssavirus type-1 of Danish bat origin. J. Comp. Pathol. 2006, 134, 190–201. [Google Scholar] [CrossRef]
- Harris, S.L.; Brookes, S.M.; Jones, G.; Hutson, A.M.; Racey, P.A.; Aegerter, J.; Smith, G.C.; McElhinney, L.M.; Fooks, A.R. European bat lyssaviruses: Distribution, prevalence and implications for conservation. Biol. Conserv. 2006, 131, 193–210. [Google Scholar] [CrossRef]
- Regnault, B.; Evrard, B.; Plu, I.; Dacheux, L.; Troadec, E.; Cozette, P.; Chrétien, D.; Duchesne, M.; Vallat, J.M.; Jamet, A.; et al. First Case of Lethal Encephalitis in Western Europe Due to European Bat Lyssavirus Type 1. Clin. Infect. Dis. 2022, 74, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Eblé, P.; Dekker, A.; van den End, S.; Visser, V.; Engelsma, M.; Harders, F.; van Keulen, L.; van Weezep, E.; Holwerda, M. A case report of a cat infected with European bat lyssavirus type 1, the Netherlands, October 2024. Euro Surveill. 2025, 30, 2500154. [Google Scholar] [CrossRef]
- Nieuwenhuis, H.U. The rabies situation in The Netherlands. Parassitologia 1988, 30, 123–128. [Google Scholar] [PubMed]
- Nathwani, D.; McIntyre, P.G.; White, K.; Shearer, A.J.; Reynolds, N.; Walker, D.; Orange, G.V.; Fooks, A.R. Fatal human rabies caused by European bat Lyssavirus type 2a infection in Scotland. Clin. Infect. Dis. 2003, 37, 598–601. [Google Scholar] [CrossRef]
- Davis, P.L.; Holmes, E.C.; Larrous, F.; Van der Poel, W.H.; Tjørnehøj, K.; Alonso, W.J.; Bourhy, H. Phylogeography, population dynamics, and molecular evolution of European bat lyssaviruses. J. Virol. 2005, 79, 10487–10497. [Google Scholar] [CrossRef] [PubMed]
- Wise, E.L.; Marston, D.A.; Banyard, A.C.; Goharriz, H.; Selden, D.; Maclaren, N.; Goddard, T.; Johnson, N.; McElhinney, L.M.; Brouwer, A.; et al. Passive surveillance of United Kingdom bats for lyssaviruses (2005–2015). Epidemiol. Infect. 2017, 145, 2445–2457. [Google Scholar] [CrossRef] [PubMed]
- McElhinney, L.M.; Marston, D.A.; Wise, E.L.; Freuling, C.M.; Bourhy, H.; Zanoni, R.; Moldal, T.; Kooi, E.A.; Neubauer-Juric, A.; Nokireki, T.; et al. Molecular Epidemiology and Evolution of European Bat Lyssavirus 2. Int. J. Mol. Sci. 2018, 19, 156. [Google Scholar] [CrossRef] [PubMed]
- Horton, D.L.; Breed, A.C.; Arnold, M.E.; Smith, G.C.; Aegerter, J.N.; McElhinney, L.M.; Johnson, N.; Banyard, A.C.; Raynor, R.; Mackie, I.; et al. Between roost contact is essential for maintenance of European bat lyssavirus type-2 in Myotis daubentonii bat reservoir: ‘The Swarming Hypothesis’. Sci. Rep. 2020, 10, 1740. [Google Scholar] [CrossRef]
- Marston, D.A.; Horton, D.L.; Ngeleja, C.; Hampson, K.; McElhinney, L.M.; Banyard, A.C.; Haydon, D.; Cleaveland, S.; Rupprecht, C.E.; Bigambo, M.; et al. Ikoma lyssavirus, highly divergent novel lyssavirus in an African civet. Emerg. Infect. Dis. 2012, 18, 664–667. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, S.; Zhao, J.; Zhang, F.; Hu, R. Isolation of Irkut virus from a Murina leucogaster bat in China. PLoS Negl. Trop. Dis. 2013, 7, e2097. [Google Scholar] [CrossRef]
- Chen, T.; Miao, F.M.; Liu, Y.; Zhang, S.F.; Zhang, F.; Li, N.; Hu, R.L. Possible Transmission of Irkut Virus from Dogs to Humans. Biomed. Environ. Sci. 2018, 31, 146–148. [Google Scholar] [CrossRef]
- Nokireki, T.; Tammiranta, N.; Kokkonen, U.M.; Kantala, T.; Gadd, T. Tentative novel lyssavirus in a bat in Finland. Transbound. Emerg. Dis. 2018, 65, 593–596. [Google Scholar] [CrossRef]
- Calvelage, S.; Tammiranta, N.; Nokireki, T.; Gadd, T.; Eggerbauer, E.; Zaeck, L.M.; Potratz, M.; Wylezich, C.; Höper, D.; Müller, T.; et al. Genetic and Antigenetic Characterization of the Novel Kotalahti Bat Lyssavirus (KBLV). Viruses 2021, 13, 69. [Google Scholar] [CrossRef]
- Suu-Ire, R.D.; Fooks, A.R.; Banyard, A.C.; Selden, D.; Amponsah-Mensah, K.; Riesle, S.; Ziekah, M.Y.; Ntiamoa-Baidu, Y.; Wood, J.L.N.; Cunningham, A.A. Lagos Bat Virus Infection Dynamics in Free-Ranging Straw-Colored Fruit Bats (Eidolon helvum). Trop. Med. Infect. Dis. 2017, 2, 25. [Google Scholar] [CrossRef]
- Coertse, J.; Geldenhuys, M.; le Roux, K.; Markotter, W. Lagos Bat Virus, an Under-Reported Rabies-Related Lyssavirus. Viruses 2021, 13, 576. [Google Scholar] [CrossRef] [PubMed]
- Aréchiga Ceballos, N.; Vázquez Morón, S.; Berciano, J.M.; Nicolás, O.; Aznar López, C.; Juste, J.; Rodríguez Nevado, C.; Aguilar Setién, A.; Echevarría, J.E. Novel lyssavirus in bat, Spain. Emerg. Infect. Dis. 2013, 19, 793–795. [Google Scholar] [CrossRef] [PubMed]
- Banyard, A.C.; Selden, D.; Wu, G.; Thorne, L.; Jennings, D.; Marston, D.; Finke, S.; Freuling, C.M.; Müller, T.; Echevarría, J.E.; et al. Isolation, Antigenicity and Immunogenicity of Lleida Bat Lyssavirus. J. Gen. Virol. 2018, 99, 1590–1599. [Google Scholar] [CrossRef] [PubMed]
- Picard-Meyer, E.; Beven, V.; Hirchaud, E.; Guillaume, C.; Larcher, G.; Robardet, E.; Servat, A.; Blanchard, Y.; Cliquet, F. Lleida Bat Lyssavirus isolation in Miniopterus schreibersii in France. Zoonoses Public Health 2019, 66, 254–258. [Google Scholar] [CrossRef]
- Leopardi, S.; Dacheux, L.; Serra-Cobo, J.; Ábrahám, Á.; Bajić, B.; Bourhy, H.; Bücs, S.L.; Budinski, I.; Castellan, M.; Drzewniokova, P.; et al. European distribution and intramuscular pathogenicity of divergent lyssaviruses West Caucasian bat virus and Lleida bat lyssavirus. iScience 2025, 28, 111738. [Google Scholar] [CrossRef]
- Familusi, J.B.; Osunkoya, B.O.; Moore, D.L.; Kemp, G.E.; Fabiyi, A. A fatal human infection with Mokola virus. Am. J. Trop. Med. Hyg. 1972, 21, 959–963. [Google Scholar] [CrossRef]
- Foggin, C.M. Mokola virus infection in cats and a dog in Zimbabwe. Vet. Rec. 1983, 113, 115. [Google Scholar] [CrossRef]
- Swanepoel, R.; Barnard, B.J.; Meredith, C.D.; Bishop, G.C.; Brückner, G.K.; Foggin, C.M.; Hübschle, O.J. Rabies in southern Africa. Onderstepoort J. Vet. Res. 1993, 60, 325–346. [Google Scholar]
- McMahon, W.C.; Coertse, J.; Kearney, T.; Keith, M.; Swanepoel, L.H.; Markotter, W. Surveillance of the rabies-related lyssavirus, Mokola in non-volant small mammals in South Africa. Onderstepoort J. Vet. Res. 2021, 88, e1–e13. [Google Scholar] [CrossRef]
- Troupin, C.; Dacheux, L.; Tanguy, M.; Sabeta, C.; Blanc, H.; Bouchier, C.; Vignuzzi, M.; Duchene, S.; Holmes, E.C.; Bourhy, H. Large-Scale Phylogenomic Analysis Reveals the Complex Evolutionary History of Rabies Virus in Multiple Carnivore Hosts. PLoS Pathog. 2016, 12, e1006041. [Google Scholar] [CrossRef]
- Gilbert, A.T. Rabies virus vectors and reservoir species. Rev. Sci. Tech. 2018, 37, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Marston, D.A.; Banyard, A.C.; McElhinney, L.M.; Freuling, C.M.; Finke, S.; de Lamballerie, X.; Müller, T.; Fooks, A.R. The lyssavirus host-specificity conundrum-rabies virus-the exception not the rule. Curr. Opin. Virol. 2018, 28, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Kuzmin, I.V.; Mayer, A.E.; Niezgoda, M.; Markotter, W.; Agwanda, B.; Breiman, R.F.; Rupprecht, C.E. Shimoni bat virus, a new representative of the Lyssavirus genus. Virus Res. 2010, 149, 197–210. [Google Scholar] [CrossRef]
- Kuzmin, I.V.; Turmelle, A.S.; Agwanda, B.; Markotter, W.; Niezgoda, M.; Breiman, R.F.; Rupprecht, C.E. Commerson’s leaf-nosed bat (Hipposideros commersoni) is the likely reservoir of Shimoni bat virus. Vector Borne Zoonotic Dis. 2011, 11, 1465–1470. [Google Scholar] [CrossRef]
- Černe, D.; Hostnik, P.; Toplak, I.; Presetnik, P.; Maurer-Wernig, J.; Kuhar, U. Discovery of a novel bat lyssavirus in a Long-fingered bat (Myotis capaccinii) from Slovenia. PLoS Negl. Trop. Dis. 2023, 17, e0011420. [Google Scholar] [CrossRef]
- Viljoen, N.; Ismail, A.; Weyer, J.; Markotter, W. A rabies-related lyssavirus from a Nycticeinops schlieffeni bat with neurological signs, South Africa. Microbiol. Resour. Announc. 2023, 12, e0062123. [Google Scholar] [CrossRef]
- Viljoen, N.; Weyer, J.; Coertse, J.; Markotter, W. Evaluation of Taxonomic Characteristics of Matlo and Phala Bat Rabies-Related Lyssaviruses Identified in South Africa. Viruses 2023, 15, 2047. [Google Scholar] [CrossRef]
- Hu, S.C.; Hsu, C.L.; Lee, F.; Tu, Y.C.; Chen, Y.W.; Chang, J.C.; Hsu, W.C. Novel Bat Lyssaviruses Identified by Nationwide Passive Surveillance in Taiwan, 2018–2021. Viruses 2022, 14, 1562. [Google Scholar] [CrossRef]
- Relich, R.F.; Loeffelholz, M.J. Taxonomic Changes for Human Viruses, 2020 to 2022. J. Clin. Microbiol. 2023, 61, e0033722. [Google Scholar] [CrossRef]
- Murrell, K.D.; Pozio, E. Worldwide occurrence and impact of human trichinellosis, 1986–2009. Emerg. Infect. Dis. 2011, 17, 2194–2202. [Google Scholar] [CrossRef]
- Seyedmousavi, S.; Guillot, J.; Arné, P.; de Hoog, G.S.; Mouton, J.W.; Melchers, W.J.; Verweij, P.E. Aspergillus and aspergilloses in wild and domestic animals: A global health concern with parallels to human disease. Med. Mycol. 2015, 53, 765–797. [Google Scholar] [CrossRef] [PubMed]
- Ryan, U.M.; Feng, Y.; Fayer, R.; Xiao, L. Taxonomy and molecular epidemiology of Cryptosporidium and Giardia—A 50 year perspective (1971–2021). Int. J. Parasitol. 2021, 51, 1099–1119. [Google Scholar] [CrossRef] [PubMed]
- Khairullah, A.R.; Moses, I.B.; Kusala, M.K.J.; Tyasningsih, W.; Ayuti, S.R.; Rantam, F.A.; Fauziah, I.; Silaen, O.S.M.; Puspitasari, Y.; Aryaloka, S.; et al. Unveiling insights into bovine tuberculosis: A comprehensive review. Open Vet. J. 2024, 14, 1330–1344. [Google Scholar] [CrossRef] [PubMed]
- Khanna, M.; Kumar, P.; Choudhary, K.; Kumar, B.; Vijayan, V.K. Emerging influenza virus: A global threat. J. Biosci. 2008, 33, 475–482. [Google Scholar] [CrossRef]
- Baby, J.; Mani, R.S.; Abraham, S.S.; Thankappan, A.T.; Pillai, P.M.; Anand, A.M.; Madhusudana, S.N.; Ramachandran, J.; Sreekumar, S. Natural Rabies Infection in a Domestic Fowl (Gallus domesticus): A Report from India. PLoS Negl. Trop. Dis. 2015, 9, e0003942. [Google Scholar] [CrossRef]
- Rohde, R.E.; Rupprecht, C.E. Update on lyssaviruses and rabies: Will past progress play as prologue in the near term towards future elimination? Fac. Rev. 2020, 9, 9. [Google Scholar] [CrossRef]
- Fehlner-Gardiner, C.; Gongal, G.; Tenzin, T.; Sabeta, C.; De Benedictis, P.; Rocha, S.M.; Vargas, A.; Cediel-Becerra, N.; Gomez, L.C.; Maki, J.; et al. Rabies in Cats—An Emerging Public Health Issue. Viruses 2024, 16, 1635. [Google Scholar] [CrossRef]
- Zelepsky, J.; Harrison, T.M. Surveillance of rabies prevalence and bite protocols in captive mammals in 120.American zoos. J. Zoo. Wildl. Med. 2010, 41, 474–479. [Google Scholar] [CrossRef]
- CDC. Translocation of an Anteater (Tamandua tetradactyla) Infected with Rabies from Virginia to Tennessee Resulting in Multiple Human Exposures, 2021. MMWR 2022, 71, 533–537. [Google Scholar] [CrossRef]
- Hübschle, O.J. Rabies in the kudu antelope (Tragelaphus strepsiceros). Rev Infect Dis. 1988, 10 (Suppl. S4), S629–S633. [Google Scholar] [CrossRef]
- Stoltenow, C.L.; Solemsass, K.; Niezgoda, M.; Yager, P.; Rupprecht, C.E. Rabies in an American bison from North Dakota. J. Wildl. Dis. 2000, 36, 169–171. [Google Scholar] [CrossRef] [PubMed][Green Version]
- CDC. Public health response to a potentially rabid bear cub—Iowa, 1999. MMWR 1999, 48, 971–973. [Google Scholar]
- Al-Rawashdeh, O.F.; Al-Ani, F.K.; Sharrif, L.A.; Al-Qudah, K.M.; Al-Hami, Y.; Frank, N. A survey of camel (Camelus dromedarius) diseases in Jordan. J. Zoo. Wildl. Med. 2000, 31, 335–338. [Google Scholar] [CrossRef]
- CDC. Rabies in a llama—Oklahoma. MMWR 1990, 39, 203–204. [Google Scholar] [PubMed]
- Botvinkin, A.; Kosenko, M. Rabies in the European parts of Russia, Belarus and Ukraine. In Historical Perspective of Rabies in Europe and the Mediterranean Basin; OIE (World Organization for Animal Health): Paris, France, 2015; pp. 47–63. Available online: https://www.researchgate.net/publication/237149887 (accessed on 19 May 2025).
- Enurah, L.U.; Ocholi, R.A.; Adeniyi, K.O.; Ekwonu, M.C. Rabies in a civet cat (Civettictis civetta) in the Jos Zoo, Nigeria. Br. Vet. J. 1988, 144, 515–516. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, X.; Shao, X.; Ba, H.; Wang, F.; Wang, H.; Yang, Y.; Sun, N.; Ren, J.; Cheng, S.; et al. Characterization of a virulent dog-originated rabies virus affecting more than twenty fallow deer (Dama dama) in Inner Mongolia, China. Infect. Genet. Evol. 2015, 31, 127–134. [Google Scholar] [CrossRef]
- Ness, A.; Aiken, J.; McKenzie, D. Sheep scrapie and deer rabies in England prior to 1800. Prion 2023, 17, 7–15. [Google Scholar] [CrossRef]
- Petersen, B.W.; Tack, D.M.; Longenberger, A.; Simeone, A.; Moll, M.E.; Deasy, M.P.; Blanton, J.D.; Rupprecht, C.E. Rabies in captive deer, Pennsylvania, USA, 2007–2010. Emerg. Infect. Dis. 2012, 18, 138–141. [Google Scholar] [CrossRef]
- Fekadu, M. Canine rabies. Onderstepoort J. Vet. Res. 1993, 60, 421–427. [Google Scholar] [PubMed]
- Oyda, S.; Megersa, B. A review of rabies in livestock and humans in Ethiopia. Int. J. Res—Granthaalayah. 2017, 5, 561–577. [Google Scholar] [CrossRef]
- Rønsholt, L.; Sørensen, K.J.; Bruschke, C.J.; Wellenberg, G.J.; van Oirschot, J.T.; Johnstone, P.; Whitby, J.E.; Bourhy, H. Clinically silent rabies infection in (zoo) bats. Vet. Rec. 1998, 142, 519–520. [Google Scholar] [CrossRef] [PubMed]
- Nanayakkara, S.; Smith, J.S.; Rupprecht, C.E. Rabies in Sri Lanka: Splendid isolation. Emerg. Infect. Dis. 2003, 9, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Langlois, I. Viral diseases of ferrets. Vet. Clin. N. Am. Exot. Anim. Pract. 2005, 8, 139–160. [Google Scholar] [CrossRef] [PubMed]
- Pushpakumara, D.P.N.; Ashoka, D.; Ranjani, H.; Preeni, A.; Craig, S. Surveillance opportunities and the need for intersectoral collaboration on rabies in Sri Lanka. J. Vet. Med. 2019, 2019, 7808517. [Google Scholar] [CrossRef][Green Version]
- Eidson, M.; Matthews, S.D.; Willsey, A.L.; Cherry, B.; Rudd, R.J.; Trimarchi, C.V. Rabies virus infection in a pet guinea pig and seven pet rabbits. J. Am. Vet. Med. Assoc. 2005, 227, 932–935. [Google Scholar] [CrossRef]
- Singh, B.; Singh, N.; Chandra, M.; Joshi, D.V. Causes of mortality of some zoo animals. Zentralbl. Vet. B. 1981, 28, 596–602. [Google Scholar] [CrossRef]
- Krebs, J.W.; Williams, S.M.; Smith, J.S.; Rupprecht, C.E.; Childs, J.E. Rabies among infrequently reported mammalian carnivores in the United States, 1960–2000. J. Wildl. Dis. 2003, 39, 253–261. [Google Scholar] [CrossRef]
- Winkler, M.P.; Parker, S. Rabies in seals: Visitors to Cape Town marine areas urged to be alert. J. Travel Med. 2024, 31, taae106. [Google Scholar] [CrossRef]
- Gautret, P.; Blanton, J.; Dacheux, L.; Ribadeau-Dumas, F.; Brouqui, P.; Parola, P.; Esposito, D.H.; Bourhy, H. Rabies in nonhuman primates and potential for transmission to humans: A literature review and examination of selected French national data. PLoS Negl. Trop. Dis. 2014, 8, e2863. [Google Scholar] [CrossRef]
- Karp, B.E.; Ball, N.E.; Scott, C.R.; Walcoff, J.B. Rabies in two privately owned domestic rabbits. J. Am. Vet. Med. Assoc. 1999, 215, 1806, 1824–1827. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yu, X.; Wang, L.; Lu, Z.; Liu, H.; Xuan, H.; Hu, Z.; Tu, C. An outbreak of pig rabies in Hunan province, China. Epidemiol. Infect. 2008, 136, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Chandel, B.S.; Patel, A.C.; Dadawala, A.I.; Chauhan, H.C.; Parsani, H.R.; Shrimali, M.D.; Raval, S.H. Confirmation of Rabies in Buffalo from Organized Farm of North Gujarat. Buffalo Bull. 2016, 35, 661–666. [Google Scholar]
- Devleesschauwer, B.; Aryal, A.; Sharma, B.K.; Ale, A.; Declercq, A.; Depraz, S.; Gaire, T.N.; Gongal, G.; Karki, S.; Pandey, B.D.; et al. Epidemiology, Impact and Control of Rabies in Nepal: A Systematic Review. PLoS Negl. Trop. Dis. 2016, 10, e0004461. [Google Scholar] [CrossRef] [PubMed]
- Obonyo, M.; Arvelo, W.; Kadivane, S.; Orundu, M.; Lankau, E.; Gakuya, F.; Munyua, P.; Githinji, J.; Marano, N.; Njenga, K.; et al. Investigation to determine staff exposure and describe animal bite surveillance after detection of a rabid zebra in a safari lodge in Kenya, 2011. Pan Afr. Med. J. 2014, 19, 10. [Google Scholar] [CrossRef]
- Ma, X.; Blanton, J.D.; Millien, M.F.; Medley, A.M.; Etheart, M.D.; Fénelon, N.; Wallace, R.M. Quantifying the risk of rabies in biting dogs in Haiti. Sci. Rep. 2020, 10, 1062. [Google Scholar] [CrossRef]
- Coertse, J.; Markotter, W.; le Roux, K.; Stewart, D.; Sabeta, C.T.; Nel, L.H. New isolations of the rabies-related Mokola virus from South Africa. BMC Vet. Res. 2017, 13, 37. [Google Scholar] [CrossRef]
- Zakia, L.S.; Albertino, L.G.; Andrade, D.G.A.; Amorim, R.M.; Takahira, R.R.; Oliveira-Filho, J.P.; Borges, A.S. Cerebrospinal fluid analysis in horses, cattle, and sheep diagnosed with rabies: A retrospective study of 62 cases. Can. Vet. J. 2022, 63, 1242–1246. [Google Scholar] [PubMed]
- Laothamatas, J.; Wacharapluesadee, S.; Lumlertdacha, B.; Ampawong, S.; Tepsumethanon, V.; Shuangshoti, S.; Phumesin, P.; Asavaphatiboon, S.; Worapruekjaru, L.; Avihingsanon, Y.; et al. Furious and paralytic rabies of canine origin: Neuroimaging with virological and cytokine studies. J. Neurovirol. 2008, 14, 119–129. [Google Scholar] [CrossRef]
- CDC. Animal rabies—South Dakota, 1995. MMWR 1996, 45, 164–166. [Google Scholar] [PubMed]
- Rupprecht, C.E. Rabies in Animals. Merck Veterinary Manual; Merck & Co., Inc.: Rahway, NJ, USA, 2025; Available online: https://www.merckvetmanual.com/nervous-system/rabies/rabies-in-animals (accessed on 19 May 2025).
- Hudson, L.C.; Weinstock, D.; Jordan, T.; Bold-Fletcher, N.O. Clinical presentation of experimentally induced rabies in horses. Zentralbl. Vet. B. 1996, 43, 277–285. [Google Scholar] [CrossRef]
- Siepker, C.L.; Dalton, M.F.; McHale, B.J.; Sakamoto, K.; Rissi, D.R. Neuropathology and diagnostic features of rabies in a litter of piglets, with a brief review of the literature. J. Vet. Diagn. Investig. 2020, 32, 166–168. [Google Scholar] [CrossRef] [PubMed]
- White, J.; Taylor, S.M.; Wolfram, K.L.; O’Conner, B.P. Rabies in a 10-week-old puppy. Can. Vet. J. 2007, 48, 931–934. [Google Scholar] [PubMed] [PubMed Central]
- Thiptara, A.; Atwill, E.R.; Kongkaew, W.; Chomel, B.B. Epidemiologic trends of rabies in domestic animals in southern Thailand, 1994–2008. Am. J. Trop. Med. Hyg. 2011, 85, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Hudson, L.C.; Weinstock, D.; Jordan, T.; Bold-Fletcher, N.O. Clinical features of experimentally induced rabies in cattle and sheep. Zentralbl Vet. B. 1996, 43, 85–95. [Google Scholar] [CrossRef]
- Meyer, E.E.; Morris, P.G.; Elcock, L.H.; Weil, J. Hindlimb hyperesthesia associated with rabies in two horses. J. Am. Vet. Med. Assoc. 1986, 188, 629–632. [Google Scholar] [CrossRef] [PubMed]
- da Silva, L.H.; Bissoto, C.E.; Delbem, A.C.; Ferrari, C.I.; Perri, S.H.; Nunes, C.M. Canine rabies epidemiology in Araçatuba and neighborhood, Northwestern São Paulo State-Brazil. Rev. Soc. Bras. Med. Trop. 2004, 37, 139–142. [Google Scholar] [CrossRef]
- Tepsumethanon, V.; Wilde, H.; Meslin, F.X. Six criteria for rabies diagnosis in living dogs. J. Med. Assoc. Thai. 2005, 88, 419–422. [Google Scholar] [PubMed]
- Martínez-Burnes, J.; Barrios-García, H.; Carvajal-de la Fuente, V.; Corona-González, B.; Obregón Alvarez, D.; Romero-Salas, D. Viral Diseases in Water Buffalo (Bubalus bubalis): New Insights and Perspectives. Animals 2024, 14, 845. [Google Scholar] [CrossRef]
- Den, K.; Sato, T.; Schneebeli, M.; Morita, Y. Acoustic characteristics of voiceless bellowing typical of bovine rabies. Am. J. Trop. Med. Hyg. 2012, 86, 528–530. [Google Scholar] [CrossRef][Green Version]
- Bloch, N.; Diallo, I. A probable outbreak of rabies in a group of camels in Niger. Vet. Microbiol. 1995, 46, 281–283. [Google Scholar] [CrossRef]
- Henderson, H.; Carpenter, L.R.; Dunn, J.R. Rabies risk and use of post-exposure prophylaxis associated with dog bites in Tennessee. Zoonoses Public Health 2018, 65, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Niezgoda, M.; Briggs, D.J.; Shaddock, J.; Dreesen, D.W.; Rupprecht, C.E. Pathogenesis of experimentally induced rabies in domestic ferrets. Am. J. Vet. Res. 1997, 58, 1327–1331. [Google Scholar] [CrossRef] [PubMed]
- Weyna, A.A.W.; Ruder, M.G.; Dalton, M.F.; Bahnson, C.; Keel, M.K.; Fenton, H.; Ballard, J.R.; Nemeth, N.M. Distinctive Gross Presentation in Free-Ranging White-Tailed Deer (Odocoileus virginianus) with Rabies. J. Wildl. Dis. 2022, 58, 664–669. [Google Scholar] [CrossRef]
- Barnes, H.L.; Chrisman, C.L.; Farina, L.; Detrisac, C.J. Clinical evaluation of rabies virus meningoencephalomyelitis in a dog. J. Am. Anim. Hosp. Assoc. 2003, 39, 547–550. [Google Scholar] [CrossRef]
- Green, S.L.; Smith, L.L.; Vernau, W.; Beacock, S.M. Rabies in horses: 21 cases (1970–1990). J. Am. Vet. Med. Assoc. 1992, 200, 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- Sabeta, C.T.; Markotter, W.; Mohale, D.K.; Shumba, W.; Wandeler, A.I.; Nel, L.H. Mokola virus in domestic mammals, South Africa. Emerg. Infect. Dis. 2007, 13, 1371–1373. [Google Scholar] [CrossRef]
- Millar, K. Another litter of pups positive for rabies. News Views 2005, 227, 1912–1925. [Google Scholar]
- Green, S.L. Rabies. Vet. Clin. N. Am. Equine Pract. 1997, 13, 65. [Google Scholar] [CrossRef]
- Medley, A.M.; Millien, M.F.; Blanton, J.D.; Ma, X.; Augustin, P.; Crowdis, K.; Wallace, R.M. Retrospective Cohort Study to Assess the Risk of Rabies in Biting Dogs, 2013–2015, Republic of Haiti. Trop. Med. Infect. Dis. 2017, 2, 14. [Google Scholar] [CrossRef]
- Charlton, K.M. The pathogenesis of rabies and other lyssaviral infections: Recent studies. Curr. Top. Microbiol. Immunol. 1994, 187, 95–119. [Google Scholar] [CrossRef]
- Mshelbwala, P.P.; Audu, S.W.; Ogunkoya, A.B.; Okaiyeto, S.O.; James, A.A.; Kumbish, P.R.; Abdullahi, S.U.; Ibrahim, S.; Abubakar, U.B. A case study of rabies in a six month old calf in Zaria, Nigeria. J. Exp. Biol. Agric. Sci. 2013, 1, 217–222. [Google Scholar]
- Khalafalla, A.I.; Ali, Y.H. Rabies virus infection in livestock. In Rabies Virus in the Beginning of the 21st Century; Tkachev, S., Ed.; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Brookes, S.M.; Klopfleisch, R.; Müller, T.; Healy, D.M.; Teifke, J.P.; Lange, E.; Kliemt, J.; Johnson, N.; Johnson, L.; Kaden, V.; et al. Susceptibility of sheep to European bat lyssavirus type-1 and -2 infection: A clinical pathogenesis study. Vet. Microbiol. 2007, 125, 210–223. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Expert Consultation on Rabies; WHO Technical Report Series; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Manning, S.E.; Rupprecht, C.E.; Fishbein, D.; Hanlon, C.A.; Lumlertdacha, B.; Guerra, M.; Meltzer, M.I.; Dhankhar, P.; Vaidya, S.A.; Jenkins, S.R.; et al. Human rabies prevention—United States, 2008: Recommendations of the Advisory Committee on Immunization Practices. MMWR 2008, 57, 1–28. [Google Scholar] [PubMed]
- Fooks, A.R.; Cliquet, F.; Finke, S.; Freuling, C.; Hemachudha, T.; Mani, R.S.; Müller, T.; Nadin-Davis, S.; Picard-Meyer, E.; Wilde, H.; et al. Rabies. Nat. Rev. Dis. Primers 2017, 3, 17091. [Google Scholar] [CrossRef]
- Shuangshoti, S.; Thorner, P.S.; Teerapakpinyo, C.; Thepa, N.; Phukpattaranont, P.; Intarut, N.; Lumlertdacha, B.; Tepsumethanon, V.; Hemachudha, T. Intracellular Spread of Rabies Virus Is Reduced in the Paralytic Form of Canine Rabies Compared to the Furious Form. PLoS Negl. Trop. Dis. 2016, 10, e0004748. [Google Scholar] [CrossRef]
- Jackson, A.C. Rabies: A medical perspective. Rev. Sci. Tech. 2018, 37, 569–580. [Google Scholar] [CrossRef]
- Vaughn, J.B., Jr.; Gerhardt, P.; Newell, K.W. Excretion of street rabies virus in the saliva of dogs. JAMA 1965, 193, 363–368. [Google Scholar] [CrossRef]
- Vaughn, J.B., Jr.; Gerhardt, P.; Paterson, J.C. Excretion of street rabies virus in saliva of cats. JAMA 1963, 184, 705–708. [Google Scholar] [CrossRef]
- Fleming, G. Rabies and Hydrophobia: Their History, Nature, Causes, Symptoms and Prevention; Chapman and Hall: London, UK, 1872; 405p. [Google Scholar]
- Negri, A. Contributo allo studio dell’eziologia della rabbia. Boll. Della Soc. Med.-Chir. Pavia 1904, 2, 88–115. [Google Scholar]
- Williams, A.W.; Lowden, M.M. The etiology and diagnosis of hydrophobia. J. Inf. Dis. 1906, 3, 452–483. [Google Scholar] [CrossRef]
- Niezgoda, M.; Subbian Satheshkumar, P. Immunohistochemistry Test for the Lyssavirus Antigen Detection from Formalin-Fixed Tissues. J. Vis. Exp. 2021, 176, e60138. [Google Scholar] [CrossRef]
- Klein, A.; Eggerbauer, E.; Potratz, M.; Zaeck, L.M.; Calvelage, S.; Finke, S.; Müller, T.; Freuling, C.M. Comparative pathogenesis of different phylogroup I bat lyssaviruses in a standardized mouse model. PLoS Negl. Trop. Dis. 2022, 16, e0009845. [Google Scholar] [CrossRef] [PubMed]
- Castellan, M.; Zamperin, G.; Foiani, G.; Zorzan, M.; Priore, M.F.; Drzewnioková, P.; Melchiotti, E.; Vascellari, M.; Monne, I.; Crovella, S.; et al. Immunological findings of West Caucasian bat virus in an accidental host. J. Virol. 2025, 99, e0191424. [Google Scholar] [CrossRef] [PubMed]
- Charlton, K.M. Rabies: Spongiform lesions in the brain. Acta Neuropathol. 1984, 63, 198–202. [Google Scholar] [CrossRef]
- Claassen, D.D.; Odendaal, L.; Sabeta, C.T.; Fosgate, G.T.; Mohale, D.K.; Williams, J.H.; Clift, S.J. Diagnostic sensitivity and specificity of immunohistochemistry for the detection of rabies virus in domestic and wild animals in South Africa. J. Vet. Diagn. Investig. 2023, 35, 236–245. [Google Scholar] [CrossRef]
- Markbordee, B.; Cabic, A.G.B.; Iamohbhars, N.; Shiwa-Sudo, N.; Kimitsuki, K.; Espino, M.J.M.; Nacion, L.B.; Manalo, D.L.; Inoue, S.; Park, C.H. Histopathological and immunohistochemical examination of the brains of rabid dogs in the Philippines. J. Vet. Med. Sci. 2024, 86, 1243–1251. [Google Scholar] [CrossRef]
- Rupprecht, C.E.; Fooks, A.R.; Abela-Ridder, B. Laboratory Techniques in Rabies, 5th ed.; World Health Organization: Geneva, Switzerland, 2018; Volumes 1 and 2. [Google Scholar]
- World Organization for Animal Health (WOAH). Rabies (Infection with Rabies Virus and Other Lyssaviruses). In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals; WOAH: Paris, France, 2024. [Google Scholar]
- Ermine, A.; Larzul, D.; Ceccaldi, P.E.; Guesdon, J.-L.; Tsiang, H. Polymerase Chain Reaction Amplification of Rabies Virus Nucleic Acids from Total Mouse Brain RNA. Mol. Cell. Probes 1990, 4, 189–191. [Google Scholar] [CrossRef]
- Sacramento, D.; Bourhy, H.; Tordo, N. PCR Technique as an Alternative Method for Diagnosis and Molecular Epidemiology of Rabies Virus. Mol. Cell. Probes 1991, 5, 229–240. [Google Scholar] [CrossRef]
- Heaton, P.R.; Johnstone, P.; McElhinney, L.M.; Cowley, R.; O’Sullivan, E.; Whitby, J.E. Heminested PCR Assay for Detection of Six Genotypes of Rabies and Rabies-Related Viruses. J. Clin. Microbiol. 1997, 35, 2762–2766. [Google Scholar] [CrossRef]
- Gigante, C.M.; Wicker, V.; Wilkins, K.; Seiders, M.; Zhao, H.; Patel, P.; Orciari, L.; Condori, R.E.; Dettinger, L.; Yager, P.; et al. Optimization of Pan-Lyssavirus LN34 Assay for Streamlined Rabies Diagnostics by Real-Time RT-PCR. J. Virol. Methods 2025, 333, 115070. [Google Scholar] [CrossRef]
- Wadhwa, A.; Wilkins, K.; Gao, J.; Condori Condori, R.E.; Gigante, C.M.; Zhao, H.; Ma, X.; Ellison, J.A.; Greenberg, L.; Velasco-Villa, A.; et al. A Pan-Lyssavirus Taqman Real-Time RT-PCR Assay for the Detection of Highly Variable Rabies Virus and Other Lyssaviruses. PLoS Negl. Trop. Dis. 2017, 11, e0005258. [Google Scholar] [CrossRef] [PubMed]
- Wakeley, P.R.; Johnson, N.; McElhinney, L.M.; Marston, D.; Sawyer, J.; Fooks, A.R. Development of a Real-Time, TaqMan Reverse Transcription-PCR Assay for Detection and Differentiation of Lyssavirus Genotypes 1, 5, and 6. J. Clin. Microbiol. 2005, 43, 2786–2792. [Google Scholar] [CrossRef]
- Hayman, D.T.S.; Banyard, A.C.; Wakeley, P.R.; Harkess, G.; Marston, D.; Wood, J.L.N.; Cunningham, A.A.; Fooks, A.R. A Universal Real-Time Assay for the Detection of Lyssaviruses. J. Virol. Methods 2011, 177, 87–93. [Google Scholar] [CrossRef] [PubMed]
- CDC. Protocol for Postmortem Diagnosis of Rabies in Animals by Direct Fluorescent Antibody Testing: A Minimum Standard for Rabies Diagnosis in the United States; CDC: Atlanta, GA, USA, 2004; Available online: https://books.google.com/books/about/Protocol_for_Postmortem_Diagnosis_of_Rab.html?id=b9XqjwEACAAJ (accessed on 19 May 2025).
- Goldwasser, R.A.; Kissling, R.E. Fluorescent Antibody Staining of Street and Fixed Rabies Virus Antigens. Exp. Biol. Med. 1958, 98, 219–223. [Google Scholar] [CrossRef]
- Rudd, R.J.; Smith, J.S.; Yager, P.A.; Orciari, L.A.; Trimarchi, C.V. A Need for Standardized Rabies-Virus Diagnostic Procedures: Effect of Cover-Glass Mountant on the Reliability of Antigen Detection by the Fluorescent Antibody Test. Virus Res. 2005, 111, 83–88. [Google Scholar] [CrossRef]
- Smith, J.S. Rabies Virus Epitopic Variation: Use in Ecologic Studies. In Advances in Virus Research; Elsevier: Amsterdam, The Netherlands, 1989; Volume 36, pp. 215–253. ISBN 978-0-12-039836-2. [Google Scholar]
- Lembo, T.; Niezgoda, M.; Velasco-Villa, A.; Cleaveland, S.; Ernest, E.; Rupprecht, C.E. Evaluation of a Direct, Rapid Immunohistochemical Test for Rabies Diagnosis. Emerg. Infect. Dis. 2012, 12, 310–313. [Google Scholar] [CrossRef]
- Certoma, A.; Lunt, R.A.; Vosloo, W.; Smith, I.; Colling, A.; Williams, D.T.; Tran, T.; Blacksell, S.D. Assessment of a Rabies Virus Rapid Diagnostic Test for the Detection of Australian Bat Lyssavirus. Trop. Med. Infect. Dis. 2018, 3, 109. [Google Scholar] [CrossRef]
- Eggerbauer, E.; De Benedictis, P.; Hoffmann, B.; Mettenleiter, T.C.; Schlottau, K.; Ngoepe, E.C.; Sabeta, C.T.; Freuling, C.M.; Müller, T. Evaluation of Six Commercially Available Rapid Immunochromatographic Tests for the Diagnosis of Rabies in Brain Material. PLoS Negl. Trop. Dis. 2016, 10, e0004776. [Google Scholar] [CrossRef]
- Klein, A.; Fahrion, A.; Finke, S.; Eyngor, M.; Novak, S.; Yakobson, B.; Ngoepe, E.; Phahladira, B.; Sabeta, C.; De Benedictis, P.; et al. Further Evidence of Inadequate Quality in Lateral Flow Devices Commercially Offered for the Diagnosis of Rabies. Trop. Med. Infect. Dis. 2020, 5, 13. [Google Scholar] [CrossRef]
- Servat, A.; Picard-Meyer, E.; Robardet, E.; Muzniece, Z.; Must, K.; Cliquet, F. Evaluation of a Rapid Immunochromatographic Diagnostic Test for the Detection of Rabies from Brain Material of European Mammals. Biologicals 2012, 40, 61–66. [Google Scholar] [CrossRef]
- Servat, A.; Robardet, E.; Cliquet, F. An Inter-Laboratory Comparison to Evaluate the Technical Performance of Rabies Diagnosis Lateral Flow Assays. J. Virol. Methods 2019, 272, 113702. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.S.; Yager, P.A.; Baer, G.M. A Rapid Reproducible Test for Determining Rabies Neutralizing Antibody. Bull. World Health Organ. 1973, 48, 535–541. [Google Scholar] [PubMed]
- Cliquet, F.; Aubert, M.; Sagné, L. Development of a fluorescent antibody virus neutralisation test (FAVN test) for the quantitation of rabies-neutralising antibody. J. Immunol. Methods 1998, 212, 79–87. [Google Scholar] [CrossRef]
- Muhamuda, K.; Madhusudana, S.N.; Ravi, V.; Desai, A. Presence of Rabies Specific Immune Complexes in Cerebro-Spinal Fluid Can Help in Ante-Mortem Diagnosis of Human Paralytic Rabies. J. Clin. Virol. 2006, 37, 162–167. [Google Scholar] [CrossRef]
- Xu, G.; Weber, P.; Hu, Q.; Xue, H.; Audry, L.; Li, C.; Wu, J.; Bourhy, H. A Simple Sandwich ELISA (WELYSSA) for the Detection of Lyssavirus Nucleocapsid in Rabies Suspected Specimens Using Mouse Monoclonal Antibodies. Biologicals 2007, 35, 297–302. [Google Scholar] [CrossRef]
- Bingham, J.; Merwe, M. van der Distribution of Rabies Antigen in Infected Brain Material: Determining the Reliability of Different Regions of the Brain for the Rabies Fluorescent Antibody Test. J. Virol. Methods 2002, 101, 85–94. [Google Scholar] [CrossRef]
- McElhinney, L.M.; Marston, D.A.; Brookes, S.M.; Fooks, A.R. Effects of Carcass Decomposition on Rabies Virus Infectivity and Detection. J. Virol. Methods 2014, 207, 110–113. [Google Scholar] [CrossRef]
- Hughes, G.J.; Smith, J.S.; Hanlon, C.A.; Rupprecht, C.E. Evaluation of a TaqMan PCR Assay To Detect Rabies Virus RNA: Influence of Sequence Variation and Application to Quantification of Viral Loads. J. Clin. Microbiol. 2004, 42, 299–306. [Google Scholar] [CrossRef]
- Vázquez-Morón, S.; Avellón, A.; Echevarría, J.E. RT-PCR for Detection of All Seven Genotypes of Lyssavirus Genus. J. Virol. Methods 2006, 135, 281–287. [Google Scholar] [CrossRef]
- Condori, R.E.; Niezgoda, M.; Lopez, G.; Matos, C.A.; Mateo, E.D.; Gigante, C.; Hartloge, C.; Filpo, A.P.; Haim, J.; Satheshkumar, P.S.; et al. Using the LN34 Pan-Lyssavirus Real-Time RT-PCR Assay for Rabies Diagnosis and Rapid Genetic Typing from Formalin-Fixed Human Brain Tissue. Viruses 2020, 12, 120. [Google Scholar] [CrossRef]
- Léchenne, M.; Naïssengar, K.; Lepelletier, A.; Alfaroukh, I.O.; Bourhy, H.; Zinsstag, J.; Dacheux, L. Validation of a Rapid Rabies Diagnostic Tool for Field Surveillance in Developing Countries. PLoS Negl. Trop. Dis. 2016, 10, e0005010. [Google Scholar] [CrossRef] [PubMed]
- Mauhay, J.D.; Saito, N.; Kimitsuki, K.; Mananggit, M.R.; Cruz, J.L.; Lagayan, M.G.; Garcia, A.M.; Lacanilao, P.M.; Yamada, K.; Saito-Obata, M.; et al. Molecular Analysis of Rabies Virus Using RNA Extracted from Used Lateral Flow Devices. J. Clin. Microbiol. 2023, 61, e01543-22. [Google Scholar] [CrossRef] [PubMed]
- Ukamaka, E.U.; Coetzer, A.; Scott, T.P.; Anene, B.M.; Ezeokonkwo, R.C.; Nwosuh, C.I.; Nel, L.H.; Sabeta, C.T. Economic and Feasibility Comparison of the dRIT and DFA for Decentralized Rabies Diagnosis in Resource-Limited Settings: The Use of Nigerian Dog Meat Markets as a Case Study. PLoS Negl. Trop. Dis. 2020, 14, e0008088. [Google Scholar] [CrossRef]
- Appler, K.; Brunt, S.; Jarvis, J.A.; Davis, A.D. Clarifying Indeterminate Results on the Rabies Direct Fluorescent Antibody Test Using Real-Time Reverse Transcriptase Polymerase Chain Reaction. Public Health Rep. 2019, 134, 57–62. [Google Scholar] [CrossRef]
- Gigante, C.M.; Dettinger, L.; Powell, J.W.; Seiders, M.; Condori, R.E.C.; Griesser, R.; Okogi, K.; Carlos, M.; Pesko, K.; Breckenridge, M.; et al. Multi-Site Evaluation of the LN34 Pan-Lyssavirus Real-Time RT-PCR Assay for Post-Mortem Rabies Diagnostics. PLoS ONE 2018, 13, e0197074. [Google Scholar] [CrossRef]
- Robardet, E.; Servat, A.; Rieder, J.; Picard-Meyer, E.; Cliquet, F. Multi-Annual Performance Evaluation of Laboratories in Post-Mortem Diagnosis of Animal Rabies: Which Techniques Lead to the Most Reliable Results in Practice? PLoS Negl. Trop. Dis. 2021, 15, e0009111. [Google Scholar] [CrossRef]
- Robardet, E.; Picard-Meyer, E.; Andrieu, S.; Servat, A.; Cliquet, F. International Interlaboratory Trials on Rabies Diagnosis: An Overview of Results and Variation in Reference Diagnosis Techniques (Fluorescent Antibody Test, Rabies Tissue Culture Infection Test, Mouse Inoculation Test) and Molecular Biology Techniques. J. Virol. Methods 2011, 177, 15–25. [Google Scholar] [CrossRef]
- Robardet, E.; Andrieu, S.; Rasmussen, T.B.; Dobrostana, M.; Horton, D.L.; Hostnik, P.; Jaceviciene, I.; Juhasz, T.; Müller, T.; Mutinelli, F.; et al. Comparative Assay of Fluorescent Antibody Test Results among Twelve European National Reference Laboratories Using Various Anti-Rabies Conjugates. J. Virol. Methods 2013, 191, 88–94. [Google Scholar] [CrossRef]
- Da Silva, G.H.; Santos Da Silva, J.H.; Iamamoto, K.; De Arruda, T.S.; Katz, I.S.S.; Fernandes, E.R.; Guedes, F.; Rodrigues Da Silva, A.D.C.; Silva, S.R. Performance Evaluation of the Polyclonal Anti-Rabies Virus Ribonucleoprotein IgG Antibodies Produced in-House for Use in Direct Fluorescent Antibody Test. J. Virol. Methods 2020, 280, 113879. [Google Scholar] [CrossRef]
- Coetzer, A.; Sabeta, C.T.; Markotter, W.; Rupprecht, C.E.; Nel, L.H. Comparison of Biotinylated Monoclonal and Polyclonal Antibodies in an Evaluation of a Direct Rapid Immunohistochemical Test for the Routine Diagnosis of Rabies in Southern Africa. PLoS Negl. Trop. Dis. 2014, 8, e3189. [Google Scholar] [CrossRef]
- Castro, B.S.; Guedes, F.; Fernandes, E.R.; Koike, G.; Katz, I.S.S.; Chaves, L.B.; Silva, S.R. Development of Biotinylated Polyclonal Anti-Ribonucleoprotein IgG for Detection of Rabies Virus Antigen by Direct Rapid Immunohistochemical Test. Biologicals 2020, 68, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.-Y.; Niezgoda, M.; Du, J.-L.; Li, H.; Wang, X.-G.; Huang, Y.; Jiao, Y.; Cao, L.; Tang, Q.; Liang, G.-D. [The primary application of direct rapid immunohistochemical test to rabies diagnosis in China]. Zhonghua Shi Yan He Lin Chuang Bing Xue Za Zhi Zhonghua Shiyan He Linchuang Bingduxue Zazhi Chin. J. Exp. Clin. Virol. 2008, 22, 168–170. [Google Scholar]
- Coetzer, A.; Anahory, I.; Dias, P.T.; Sabeta, C.T.; Scott, T.P.; Markotter, W.; Nel, L.H. Enhanced Diagnosis of Rabies and Molecular Evidence for the Transboundary Spread of the Disease in Mozambique. J. S. Afr. Vet. Assoc. 2017, 88, e1–e9. [Google Scholar] [CrossRef]
- World Health Organization for Animal Health (WOAH) Rabies Chapter. Page 9, Section 1.3.3. Available online: https://www.woah.org/fileadmin/Home/eng/Health_standards/tahm/3.01.18_RABIES.pdf (accessed on 19 May 2025).
- Kimitsuki, K.; Saito, N.; Yamada, K.; Park, C.-H.; Inoue, S.; Suzuki, M.; Saito-Obata, M.; Kamiya, Y.; Manalo, D.L.; Demetria, C.S.; et al. Evaluation of the Diagnostic Accuracy of Lateral Flow Devices as a Tool to Diagnose Rabies in Post-Mortem Animals. PLoS Negl. Trop. Dis. 2020, 14, e0008844. [Google Scholar] [CrossRef]
- Djegui, F.; Gourlaouen, M.; Coetzer, A.; Adjin, R.; Tohozin, R.; Leopardi, S.; Mauti, S.; Akpo, Y.; Gnanvi, C.; Nel, L.H.; et al. Capacity Building Efforts for Rabies Diagnosis in Resource-Limited Countries in Sub-Saharan Africa: A Case Report of the Central Veterinary Laboratory in Benin (Parakou). Front. Vet. Sci. 2022, 8, 769114. [Google Scholar] [CrossRef]
- Cruz, J.L.; Garcia, A.M.; Saito, N.; Lagayan, M.G.O.; Dela Peña, R.C.; Usana, M.S.; Agustin, S.P.; Tattao, J.Z.; Mamauag, C.V.; Ducayag, O.P.; et al. Evaluation of Lateral Flow Devices for Postmortem Rabies Diagnosis in Animals in the Philippines: A Multicenter Study. J. Clin. Microbiol. 2023, 61, e00842-23. [Google Scholar] [CrossRef]
- Echevarría, J.E.; Banyard, A.C.; McElhinney, L.M.; Fooks, A.R. Current Rabies Vaccines Do Not Confer Protective Immunity against Divergent Lyssaviruses Circulating in Europe. Viruses 2019, 11, 892. [Google Scholar] [CrossRef]
- Moh’d, A.Z.; Coetzer, A.; Malan, A.J.; Scott, T.P.; Ramadhan, R.J.; Wright, N.; Nel, L.H. Investigating the Impact That Diagnostic Screening with Lateral Flow Devices Had on the Rabies Surveillance Program in Zanzibar, Tanzania. Microorganisms 2024, 12, 1314. [Google Scholar] [CrossRef]
- Mani, R.S.; Madhusudana, S.N.; Mahadevan, A.; Reddy, V.; Belludi, A.Y.; Shankar, S.K. Utility of Real-Time Taqman PCR for Antemortem and Postmortem Diagnosis of Human Rabies: Real-Time PCR for Diagnosis of Human Rabies. J. Med. Virol. 2014, 86, 1804–1812. [Google Scholar] [CrossRef]
- Swedberg, C.; Bote, K.; Gamble, L.; Fénelon, N.; King, A.; Wallace, R.M. Eliminating invisible deaths: The woeful state of global rabies data and its impact on progress towards 2030 sustainable development goals for neglected tropical diseases. Front. Trop. Dis. 2024, 5, 1303359. [Google Scholar] [CrossRef]
- Ma, X.; Boutelle, C.; Bonaparte, S.; Orciari, L.A.; Condori, R.E.; Kirby, J.D.; Chipman, R.B.; Fehlner-Gardiner, C.; Thang, C.; Cedillo, V.G.; et al. Rabies surveillance in the United States during 2022. J. Am. Vet. Med. Assoc. 2024, 262, 1518–1525. [Google Scholar] [CrossRef] [PubMed]
- Vos, A.; Un, H.; Hampson, K.; De Balogh, K.; Aylan, O.; Freuling, C.M.; Müller, T.; Fooks, A.R.; Johnson, N. Bovine rabies in Turkey: Patterns of infection and implications for costs and control. Epidemiol. Infect. 2014, 142, 1925–1933. [Google Scholar] [CrossRef] [PubMed]
- Jibat, T.; Mourits, M.C.; Hogeveen, H. Incidence and economic impact of rabies in the cattle population of Ethiopia. Prev. Vet. Med. 2016, 130, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Jemberu, W.T.; Molla, W.; Almaw, G.; Alemu, S. Incidence of rabies in humans and domestic animals and people’s awareness in North Gondar Zone, Ethiopia. PLoS Negl. Trop. Dis. 2013, 7, e2216. [Google Scholar] [CrossRef]
- Dinbiso, T.D.; Mekonnen, A.T.; Olana, T.N.; Ayana, G.M.; Kelbesa, K.A.; Deso, H.D.; Gudina, S.B.; Biru, M.K.; Hordofa, M.A.; Berecha, M.S. Community knowledge, attitude, and practice, incidence of suspected cases, and epidemiological distribution of rabies in humans and animals in Southwest Shewa zone, Oromia, Ethiopia. Front. Vet. Sci. 2025, 12, 1448448. [Google Scholar] [CrossRef]
- Feng, Y.; Shi, Y.; Yu, M.; Xu, W.; Gong, W.; Tu, Z.; Ding, L.; He, B.; Guo, H.; Tu, C. Livestock rabies outbreaks in Shanxi province, China. Arch. Virol. 2016, 161, 2851–2854. [Google Scholar] [CrossRef]
- El-Neweshy, M.S.; Al Mayahi, N.; Al Mamari, W.; Al Rashdi, Z.; Al Mawly, J.H. Animal rabies situation in Sultanate of Oman (2017–2019). Trop. Anim. Health Prod. 2020, 52, 3069–3076. [Google Scholar] [CrossRef]
- Ali, Y.H.; Mohieddeen, T.A.G.; Abdellatif, M.M.; Ahmed, B.M.; Saeed, I.K.; Attaalfadeel, H.M.; Ali, A.A. Rabies in equids in Sudan. Onderstepoort J. Vet. Res. 2024, 91, e1–e10. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Johnson, N.; Montano Hirose, J.A. The impact of paralytic bovine rabies transmitted by vampire bats in Latin America and the Caribbean. Rev. Sci. Tech. 2018, 37, 451–459. [Google Scholar] [CrossRef]
- Soler-Tovar, D.; Escobar, L.E. Rabies transmitted from vampires to cattle: An overview. PLoS ONE 2025, 20, e0317214. [Google Scholar] [CrossRef]
- Rodrigues da Silva, A.C.; Caporale, G.M.; Gonçalves, C.A.; Targueta, M.C.; Comin, F.; Zanetti, C.R.; Kotait, I. Antibody response in cattle after vaccination with inactivated and attenuated rabies vaccines. Rev. Inst. Med. Trop. Sao Paulo 2000, 42, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Ventura, M.C.D.S.; Neves, J.M.M.; Pinheiro, R.D.S.; Santos, M.V.C.; de Lemos, E.R.S.; Horta, M.A.P. The Silent Threat: Unraveling the Impact of Rabies in Herbivores in Brazil. Animals 2024, 14, 2305. [Google Scholar] [CrossRef] [PubMed]
- Bonilla-Aldana, D.K.; Jimenez-Diaz, S.D.; Barboza, J.J.; Rodriguez-Morales, A.J. Mapping the Spatiotemporal Distribution of Bovine Rabies in Colombia, 2005–2019. Trop. Med. Infect. Dis. 2022, 7, 406. [Google Scholar] [CrossRef] [PubMed]
- Van de Vuurst, P.; Gohlke, J.M.; Escobar, L.E. Future climate change and the distributional shift of the common vampire bat, Desmodus rotundus. Sci. Rep. 2025, 15, 5989. [Google Scholar] [CrossRef]
- Crozet, G.; Rivière, J.; Canini, L.; Cliquet, F.; Robardet, E.; Dufour, B. Evaluation of the Worldwide Occurrence of Rabies in Dogs and Cats Using a Simple and Homogenous Framework for Quantitative Risk Assessments of Rabies Reintroduction in Disease-Free Areas through Pet Movements. Vet. Sci. 2020, 7, 207. [Google Scholar] [CrossRef]
- Cliquet, F.; Wasniewski, M. Maintenance of rabies-free areas. Rev. Sci. Tech. 2018, 37, 691–702. (In English) [Google Scholar] [CrossRef] [PubMed]
- Rupprecht, C.E.; Barrett, J.; Briggs, D.; Cliquet, F.; Fooks, A.R.; Lumlertdacha, B.; Meslin, F.X.; Müler, T.; Nel, L.H.; Schneider, C.; et al. Can rabies be eradicated? Dev. Biol. 2008, 131, 95–121. [Google Scholar] [PubMed]
- Greene, C.E.; Schultz, R.D.; Ford, R.B. Canine vaccination. Vet. Clin. N. Am. Small Anim. Pract. 2001, 31, 473–492, v–vi. [Google Scholar] [CrossRef]
- National Association of State Public Health Veterinarians (NASPHV). Compendium of measures to prevent disease associated with animals in public settings 2013. J. Am. Vet. Med. Assoc. 2013, 243, 1270–1288. [Google Scholar] [CrossRef]
- Gilbert, A.; Greenberg, L.; Moran, D.; Alvarez, D.; Alvarado, M.; Garcia, D.L.; Peruski, L. Antibody response of cattle to vaccination with commercial modified live rabies vaccines in Guatemala. Prev. Vet. Med. 2015, 118, 36–44. [Google Scholar] [CrossRef]
- National Association of State Public Health Veterinarians (NASPHV). Compendium of Animal Rabies Prevention Control 2016. J. Am. Vet. Med. Assoc. 2016, 248, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Cleaveland, S.; Thumbi, S.M.; Sambo, M.; Lugelo, A.; Lushasi, K.; Hampson, K.; Lankester, F. Proof of concept of mass dog vaccination for thecontrol and elimination of canine rabies. Rev. Sci. Tech. 2018, 37, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Stuchin, M.; Machalaba, C.M.; Olival, K.J.; Artois, M.; Bengis, R.G.; Caceres, P.; Diaz, F.; Erlacher-Vindel, E.; Forcella, S.; Leighton, F.A.; et al. Rabies as a threat to wildlife. Rev. Sci. Tech. 2018, 37, 341–357. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.M. Rabies: Current Preventive Strategies. Vet. Clin. N. Am. Small Anim. Pract. 2019, 49, 629–641. [Google Scholar] [CrossRef]
- Robardet, E.; Bosnjak, D.; Englund, L.; Demetriou, P.; Martín, P.R.; Cliquet, F. Zero Endemic Cases of Wildlife Rabies (Classical Rabies Virus, RABV) in the European Union by 2020: An Achievable Goal. Trop. Med. Infect. Dis. 2019, 4, 124. [Google Scholar] [CrossRef]
- Yin, C. Commentary: Progress in the development of animal rabies vaccines in China. China CDC Wkly. 2021, 3, 825–830. [Google Scholar] [CrossRef]
- Nadal, D.; Bote, K.; Abela, B. Is there hope to reach the Zero by 30 target for dog-mediated human rabies? Lancet Glob. Health 2023, 11, e1682–e1683. [Google Scholar] [CrossRef]
- Barber, S.; Hathaway, M.J. Rabies in China: The role of rabies ecologies and pet activism. In One Health for Dog-Mediated Rabies Elimination in Asia: A Collection of Local Experiences; CABI: Egham, UK, 2023; pp. 199–206. [Google Scholar] [CrossRef]
- Shen, T.; Welburn, S.C.; Sun, L.; Yang, G.J. Progress towards dog-mediated rabies elimination in PR China: A scoping review. Infect. Dis. Poverty 2023, 12, 30. [Google Scholar] [CrossRef]
- Rupprecht, C.E.; Buchanan, T.; Cliquet, F.; King, R.; Müller, T.; Yakobson, B.; Yang, D.K. A Global Perspective on Oral Vaccination of Wildlife against Rabies. J. Wildl. Dis. 2024, 60, 241–284. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Vaccinations Immunization—Rabies; WHO: Geneva, Switzerland; Available online: https://www.who.int/teams/control-of-neglected-tropical-diseases/rabies/vaccinations-and-immunization (accessed on 12 March 2025).
- Weyl Feinstein, S.; Novak, S.; Eyngor, M.; Lavon, Y.; Yakobson, B. Rabies Vaccination for Sheep and Goats: Influence of Booster on Persistence of Antibody Response. Vet. Sci. 2024, 11, 502. [Google Scholar] [CrossRef]
- World Organisation for Animal Health (WOAH). Rabies; WOAH: Paris, France, 2025; Available online: https://www.woah.org/en/disease/rabies/ (accessed on 19 May 2025).
- Tirosh-Levy, S.; Shaiman Barom, L.; Novak, S.; Eyngor, M.; Schvartz, G.; Yakobson, B.; Steinman, A. Persistence of Anti-Rabies Antibody Response in Horses Following Vaccination. Pathogens 2024, 13, 125. [Google Scholar] [CrossRef] [PubMed]
- Arbeitskreis Pferde der Ständigen Impfkommission Veterinärmedizin (StIKo Vet) Leitlinie zur Impfung von Pferden—5 Auflage Tierarztl Prax Ausg, G. Grosstiere Nutztiere 2025, 53, 33–38. [CrossRef]
- U.S. Department of Agriculture. Animal and Plant Health Inspection Service, Product Summaries, March 17, 2025. Available online: https://www.aphis.usda.gov/veterinary-biologics/product-summaries (accessed on 19 May 2025).
- Colombi, D.; Poletto, C.; Nakouné, E.; Bourhy, H.; Colizza, V. Long-range movements coupled with heterogeneous incubation period sustain dog rabies at the national scale in Africa. PLoS Negl. Trop. Dis. 2020, 14, e0008317. [Google Scholar] [CrossRef]
- Kaneda, Y.; Sakeshima, K.; Takahashi, K.; Ozaki, A.; Tanimoto, T. Public health risks for relaxing quarantine for pet dogs entering with Ukrainian refugees. QJM 2022, 115, 495–496. [Google Scholar] [CrossRef]
- Pieracci, E.G.; Maskery, B.; Stauffer, K.; Gertz, A.; Brown, C. Risk factors for death and illness in dogs imported into the United States, 2010–2018. Transbound. Emerg. Dis. 2022, 69, e1749–e1757. [Google Scholar] [CrossRef]
- Rysava, K.; Tildesley, M.J. Identification of dynamical changes of rabies transmission under quarantine: Community-based measures towards rabies elimination. PLoS Comput. Biol. 2023, 19, e1011187. [Google Scholar] [CrossRef]
- Lushasi, K.; Brunker, K.; Rajeev, M.; Ferguson, E.A.; Jaswant, G.; Baker, L.L.; Biek, R.; Changalucha, J.; Cleaveland, S.; Czupryna, A.; et al. Integrating contact tracing and whole-genome sequencing to track the elimination of dog-mediated rabies: An observational and genomic study. eLife 2023, 12, e85262. [Google Scholar] [CrossRef]
- Ross, Y.B.; Vo, C.D.; Bonaparte, S.; Phan, M.Q.; Nguyen, D.T.; Nguyen, T.X.; Nguyen, T.T.; Orciari, L.; Nguyen, T.D.; Nguyen, O.K.T.; et al. Measuring the impact of an integrated bite case management program on the detection of canine rabies cases in Vietnam. Front. Public Health 2023, 11, 1150228. [Google Scholar] [CrossRef]
- Mohanty, P.; Boro, P.K.; Heydtmann, S.; Durr, S.; Tiwari, H.K. Rabies in rural northeast India: A case report emphasising the urgency of the One Health approach. One Health 2024, 19, 100850. [Google Scholar] [CrossRef]
- Carpenter, A.; Price, E.R.; Stein, S.R.; Beron, A.J.; Divis, A.; Mix, S.; Hess, A.R.; Nelson, K.M.; Wetzel, C.T.; Fredrick, J.; et al. Identification of raccoon rabies virus variant in a stray kitten: The role of veterinary practitioners in detection and reporting of a non-native zoonotic pathogen-Nebraska, 2023. J. Am. Vet. Med. Assoc. 2024, 263, 149–152. [Google Scholar] [CrossRef]
- Kasempimolporn, S.; Mitmoonpitak, C.; Chaiyabutr, N.; Supakorn, K.; Brahmasa, R.; Sitprija, V. Maternal antibodies against rabies in Thai puppies. A preliminary study. J. Med. Assoc. Thai 1996, 79, 36–39. [Google Scholar] [PubMed]
- Seghaier, C.; Cliquet, F.; Hammami, S.; Aouina, T.; Tlatli, A.; Aubert, M. Rabies mass vaccination campaigns in Tunisia: Are vaccinated dogs correctly immunized? Am. J. Trop. Med. Hyg. 1999, 61, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Berndtsson, L.T.; Nyman, A.K.; Rivera, E.; Klingeborn, B. Factors associated with the success of rabies vaccination of dogs in Sweden. Acta Vet. Scand. 2011, 53, 22. [Google Scholar] [CrossRef]
- Morters, M.K.; McNabb, S.; Horton, D.L.; Fooks, A.R.; Schoeman, J.P.; Whay, H.R.; Wood, J.L.; Cleaveland, S. Effective vaccination against rabies in puppies in rabies endemic regions. Vet. Rec. 2015, 177, 150. [Google Scholar] [CrossRef]
- Rota Nodari, E.; Alonso, S.; Mancin, M.; De Nardi, M.; Hudson-Cooke, S.; Veggiato, C.; Cattoli, G.; De Benedictis, P. Rabies Vaccination: Higher Failure Rates in Imported Dogs than in those Vaccinated in Italy. Zoonoses Public Health 2017, 64, 146–155. [Google Scholar] [CrossRef]
- Wallace, R.M.; Pees, A.; Blanton, J.B.; Moore, S.M. Risk factors for inadequate antibody response to primary rabies vaccination in dogs under one year of age. PLoS Negl. Trop. Dis. 2017, 11, e0005761. [Google Scholar] [CrossRef]
- Kaila, M.; Marjoniemi, J.; Nokireki, T. Comparative study of rabies antibody titers of dogs vaccinated in Finland and imported street dogs vaccinated abroad. Acta Vet. Scand. 2019, 61, 15. [Google Scholar] [CrossRef]
- Arega, S.; Conan, A.; Sabeta, C.T.; Crafford, J.E.; Wentzel, J.; Reininghaus, B.; Biggs, L.; Leisewitz, A.L.; Quan, M.; Toka, F.; et al. Rabies Vaccination of 6-Week-Old Puppies Born to Immunized Mothers: A Randomized Controlled Trial in a High-Mortality Population of Owned, Free-Roaming Dogs. Trop. Med. Infect. Dis. 2020, 5, 45. [Google Scholar] [CrossRef]
- Dodds, W.J.; Larson, L.J.; Christine, K.L.; Schultz, R.D. Duration of immunity after rabies vaccination in dogs: The Rabies Challenge Fund research study. Can. J. Vet. Res. 2020, 84, 153–158. [Google Scholar]
- Manzano, M.D.; Cereza, J.; García, J.; Yus, L.J.; Badiola, J.J.; Echevarria, J.E.; Monzón, M. Factors Involved in the Immunological Protection against Rabies Virus in Dogs in Spain. Vaccines 2024, 12, 293. [Google Scholar] [CrossRef]
- Mansfield, K.L.; Burr, P.D.; Snodgrass, D.R.; Sayers, R.; Fooks, A.R. Factors affecting the serological response of dogs and cats to rabies vaccination. Vet. Rec. 2004, 154, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Kappus, K.D. Canine rabies in the United States, 1971-1973: Study of reported cases with reference to vaccination history. Am. J. Epidemiol. 1976, 103, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Okoh, A.E. Canine rabies in Nigeria, 1970–1980 reported cases in vaccinated dogs. Int. J. Zoonoses 1982, 9, 118–125. [Google Scholar] [PubMed]
- Eng, T.R.; Fishbein, D.B. Epidemiologic factors, clinical findings, and vaccination status of rabies in cats and dogs in the United States in 1988. National Study Group on Rabies. J. Am. Vet. Med. Assoc. 1990, 197, 201–209. [Google Scholar] [CrossRef]
- Murray, K.O.; Holmes, K.C.; Hanlon, C.A. Rabies in vaccinated dogs and cats in the United States, 1997–2001. J. Am. Vet. Med. Assoc. 2009, 235, 691–695. [Google Scholar] [CrossRef]
- Malerczyk, C.; Freuling, C.; Gniel, D.; Giesen, A.; Selhorst, T.; Müller, T. Cross-neutralization of antibodies induced by vaccination with Purified Chick Embryo Cell Vaccine (PCECV) against different Lyssavirus species. Hum. Vaccin. Immunother. 2014, 10, 2799–2804. [Google Scholar] [CrossRef]
- Fooks, A.R.; Shipley, R.; Markotter, W.; Tordo, N.; Freuling, C.M.; Müller, T.; McElhinney, L.M.; Banyard, A.C.; Rupprecht, C.E. Renewed Public Health Threat from Emerging Lyssaviruses. Viruses 2021, 13, 1769. [Google Scholar] [CrossRef]
- Inoue, Y.; Kaku, Y.; Harada, M.; Ishijima, K.; Kuroda, Y.; Tatemoto, K.; Virhuez-Mendoza, M.; Nishino, A.; Yamamoto, T.; Inoue, S.; et al. Cross-Neutralization Activities of Antibodies against 18 Lyssavirus Glycoproteins. Jpn. J. Infect. Dis. 2024, 77, 169–173. [Google Scholar] [CrossRef]
- Tsie, K.; Ngoepe, E.; Phahladira, B.; Khumalo, N.; Sabeta, C. Molecular Characterization of Lyssaviruses Originating from Domestic and Wild Cats Provides an Insight on the Diversity of Lyssaviruses and a Risk of Rabies Transmission to Other Susceptible Mammals and Humans in South Africa. Pathogens 2023, 12, 1212. [Google Scholar] [CrossRef]
- Kgaladi, J.; Faber, M.; Dietzschold, B.; Nel, L.H.; Markotter, W. Pathogenicity and Immunogenicity of Recombinant Rabies Viruses Expressing the Lagos Bat Virus Matrix and Glycoprotein: Perspectives for a Pan-Lyssavirus Vaccine. Trop. Med. Infect. Dis. 2017, 2, 37. [Google Scholar] [CrossRef]
- Evans, J.S.; Wu, G.; Selden, D.; Buczkowski, H.; Thorne, L.; Fooks, A.R.; Banyard, A.C. Utilisation of Chimeric Lyssaviruses to Assess Vaccine Protection against Highly Divergent Lyssaviruses. Viruses 2018, 10, 130. [Google Scholar] [CrossRef] [PubMed]
- Ben Hamed, S.; Myers, J.F.; Chandwani, A.; Wirblich, C.; Kurup, D.; Paran, N.; Schnell, M.J. Toward the Development of a Pan-Lyssavirus Vaccine. Viruses 2024, 16, 1107. [Google Scholar] [CrossRef]
- Miao, F.; Li, N.; Yang, J.; Chen, T.; Liu, Y.; Zhang, S.; Hu, R. Neglected challenges in the control of animal rabies in China. One Health 2021, 12, 100212. [Google Scholar] [CrossRef]
- Taylor, E.; Banyard, A.C.; Bourhy, H.; Cliquet, F.; Ertl, H.; Fehlner-Gardiner, C.; Horton, D.L.; Mani, R.S.; Müller, T.; Rupprecht, C.E.; et al. Avoiding preventable deaths: The scourge of counterfeit rabies vaccines. Vaccine 2019, 37, 2285–2287. [Google Scholar] [CrossRef]
- Henson, K.E.R.; Santiago, A.A.C.; Namqui, S.S. Counterfeit Rabies Vaccines: The Philippine Experience. Open Forum Infect. Dis. 2020, 7, ofaa313. [Google Scholar] [CrossRef]
- Kogan, L.R.; Rishniw, M. Canine and feline core vaccinations: US veterinarians’ concerns and perceived impact of COVID-19 antivaccination views on veterinary medicine. J. Am. Vet. Med. Assoc. 2022, 260, 1482–1488. [Google Scholar] [CrossRef]
- Motta, M.; Motta, G.; Stecula, D. Sick as a dog? The prevalence, politicization, and health policy consequences of canine vaccine hesitancy (CVH). Vaccine 2023, 41, 5946–5950. [Google Scholar] [CrossRef]
- Haeder, S.F. Assessing vaccine hesitancy and support for vaccination requirements for pets and potential Spillovers from humans. Vaccine 2023, 41, 7322–7332. [Google Scholar] [CrossRef]
- Mshelbwala, P.P.; Rupprecht, C.E.; Osinubi, M.O.; Njoga, E.O.; Orum, T.G.; Weese, J.S.; Clark, N.J. Factors influencing canine rabies vaccination among dog-owning households in Nigeria. One Health 2024, 18, 100751. [Google Scholar] [CrossRef]
- Mshelbwala, P.P.; Wangdi, K.; Bunting-Graden, J.A.; Bamayange, S.; Adamu, A.M.; Gupta, S.D.; Suluku, R.; Adamu, C.S.; Weese, J.S.; Rupprecht, C.E.; et al. Insights into canine rabies vaccination disparities in Sierra Leone: A cross-sectional household study. PLoS Negl. Trop. Dis. 2024, 18, e0012332. [Google Scholar] [CrossRef]
- Basheer, A.M.; Ramakrishna, J.; Manickam, R. Evaluation of post-exposure vaccination against rabies in cattle. New Microbiol. 1997, 20, 289–294. [Google Scholar] [PubMed]
- Wilson, P.J.; Hunt, P.R.; Fonken, E.P. Rabies postexposure prophylaxis protocol option for unvaccinated domestic animals, Texas: 2010–2019. J. Am. Vet. Med. Assoc. 2024, 262, 627–634. [Google Scholar] [CrossRef] [PubMed]
- King, A.A.; Fooks, A.R.; Aubert, M.; Wandeler, A.I. (Eds.) Historical Perspective of Rabies in Europe and the Mediterranean Basin; World Organization for Animal Health (OIE): Paris, France, 2004; 384p. [Google Scholar]
- Steck, F.; Wandeler, A.; Bichsel, P.; Capt, S.; Häfliger, U.; Schneider, L. Oral immunization of foxes against rabies. Laboratory and field studies. Comp. Immunol. Microbiol. Infect. Dis. 1982, 5, 165–171. [Google Scholar] [CrossRef]
- Cliquet, F.; Picard-Meyer, E.; Robardet, E. Rabies in Europe: What are the risks? Expert Rev. Anti Infect. Ther. 2014, 12, 905–908. [Google Scholar] [CrossRef]
- Ribadeau-Dumas, F.; Cliquet, F.; Gautret, P.; Robardet, E.; Le Pen, C.; Bourhy, H. Travel-Associated Rabies in Pets and Residual Rabies Risk, Western Europe. Emerg. Infect. Dis. 2016, 22, 1268–1271. [Google Scholar] [CrossRef]
- Crozet, G.; Rivière, J.; Rapenne, E.; Cliquet, F.; Robardet, E.; Dufour, B. Quantitative risk assessment of rabies being introduced into mainland France through worldwide noncommercial dog and cat movements. Risk Anal. 2023, 43, 896–916. [Google Scholar] [CrossRef]
- Hochedez, P.; Jidar, K.; Taieb, F.; Itani, O.; Benabdelmoumen, G.; Parize, P.; Bourhy, H.; Consigny, P.H.; Poujol, P. Rabies exposure in international travellers: Experience from a single travel clinic in Paris, France, 2018–2022. Travel. Med. Infect. Dis. 2025, 64, 102821. [Google Scholar] [CrossRef]
- Jelesicj, Z.; Nikolic, M. Isolation of rabies virus from insectivorous bats in Yugoslavia. Bull. World Health Organ. 1956, 14, 801–804. [Google Scholar] [PubMed]
- Dundarova, H.; Ivanova-Aleksandrova, N.; Bednarikova, S.; Georgieva, I.; Kirov, K.; Miteva, K.; Neov, B.; Ostoich, P.; Pikula, J.; Zukal, J.; et al. Phylogeographic Aspects of Bat Lyssaviruses in Europe: A Review. Pathogens 2023, 12, 1089. [Google Scholar] [CrossRef]
- van Thiel, P.P.; de Bie, R.M.; Eftimov, F.; Tepaske, R.; Zaaijer, H.L.; van Doornum, G.J.; Schutten, M.; Osterhaus, A.D.; Majoie, C.B.; Aronica, E.; et al. Fatal human rabies due to Duvenhage virus from a bat in Kenya: Failure of treatment with coma-induction, ketamine, and antiviral drugs. PLoS Negl. Trop. Dis. 2009, 3, e428. [Google Scholar] [CrossRef]
- Kannan, A.; Chen, R.; Akhtar, Z.; Sutton, B.; Quigley, A.; Morris, M.J.; MacIntyre, C.R. Use of Open-Source Epidemic Intelligence for Infectious Disease Outbreaks, Ukraine, 2022. Emerg. Infect. Dis. 2024, 30, 1865–1871. [Google Scholar] [CrossRef] [PubMed]
- Cobby, T.R.; Eisler, M.C. Risk of rabies reintroduction into the European Union as a result of the Russo-Ukrainian war: A quantitative disease risk analysis. Zoonoses Public Health 2024, 71, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Ploin, D.; Alexandre, M.; Ventelou, B.; Che, D.; Coignard, B.; Boulanger, N.; Burucoa, C.; Caron, F.; Gallian, P.; Hansmann, Y.; et al. Prioritisation of infectious diseases from a public health perspective: A multi-criteria decision analysis study, France, 2024. Euro Surveill. 2024, 29, 2400074. [Google Scholar] [CrossRef]
- Anagnostopoulos, A.; Niederer-Loher, A.; Hüssy, D.; Staehelin, C.; Fehr, J. Tollwut-Update 2025: Das Wichtigste in Kürze. Praxis 2024, 113, 306–312. [Google Scholar] [CrossRef]
- Overduin, L.A.; Koopman, J.P.R.; Prins, C.; Verbeek-Menken, P.H.; de Pijper, C.A.; Heerink, F.; van Genderen, P.J.J.; Grobusch, M.P.; Visser, L.G. Rabies knowledge gaps and risk behaviour in Dutch travellers: An observational cohort study. Travel. Med. Infect. Dis. 2024, 60, 102739. [Google Scholar] [CrossRef]
- Begeman, L.; Geschiere, M.J.M.; de Boer, W.F.; van den Brand, J.M.A.; Eblé, P.L.; van der Kerkhof, J.H.T.C.; Keur, I.; Lina, P.H.C.; Reusken, C.B.E.M.; de Rosa, M.; et al. Human-bat contacts in the Netherlands, and potential risks for virus exchange. One Health Outlook 2025, 7, 7. [Google Scholar] [CrossRef]
- Hampson, K.; Coudeville, L.; Lembo, T.; Sambo, M.; Kieffer, A.; Attlan, M.; Barrat, J.; Blanton, J.D.; Briggs, D.J.; Cleaveland, S.; et al. Estimating the global burden of endemic canine rabies. PLoS Negl. Trop. Dis. 2015, 9, e0003709. [Google Scholar] [CrossRef]
- Leonova, G.N.; Belikov, S.I.; Kondratov, I.G.; Krylova, N.V.; Pavlenko, E.V.; Romanova, E.V.; Chentsova, I.V.; Petukhova, S.A. A fatal case of bat lyssavirus infection in Primorye Territory of the Russian Far East. WHO Rabies Bull Eur. 2009, 33, 5–8. [Google Scholar]
- Zhou, H.; Vong, S.; Liu, K.; Li, Y.; Mu, D.; Wang, L.; Yin, W.; Yu, H. Human Rabies in China, 1960–2014: A Descriptive Epidemiological Study. PLoS Negl. Trop. Dis. 2016, 10, e0004874. [Google Scholar] [CrossRef]
- Yue, Y.; Chen, Q.; Mu, D.; Li, Y.; Yin, W. A Descriptive Analysis of Human Rabies in Mainland China, 2005–2020. Int. J. Environ. Res. Public Health 2022, 20, 380. [Google Scholar] [CrossRef]
- World Health Organization. Western Pacific Health Data Platform. 2024. Available online: https://data.wpro.who.int/rabies-western-pacific-region (accessed on 1 May 2025).
- Smith, C.; Ortal, A.; Girasol, M.; Suzuki, S.; Coughlan, C. Rabies in the Philippines: A call to action. Lancet Reg. Health West Pac. 2024, 49, 101156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cruz, J.; Tian, Y.; Wang, Y.; Jiang, J.; Gonzales, R.M.; Azul, R.R.; Peña, R.C.D.; Sun, S.; Liu, Y.; et al. Phylogeography Analysis Reveals Rabies Epidemiology, Evolution, and Transmission in the Philippines. Mol. Biol. Evol. 2025, 42, msaf007. [Google Scholar] [CrossRef] [PubMed]
- Thangaraj, J.W.V.; Krishna, N.S.; Devika, S.; Egambaram, S.; Dhanapal, S.R.; Khan, S.A.; Srivastava, A.K.; Mishra, A.; Shrinivasa, B.; Gour, D.; et al. Estimates of the burden of human rabies deaths and animal bites in India, 2022–2023: A community-based cross-sectional survey and probability decision-tree modelling study. Lancet Infect. Dis. 2025, 25, 126–134. [Google Scholar] [CrossRef]
- Ashwini, M.A.; Pattanaik, A.; Mani, R.S. Recent updates on laboratory diagnosis of rabies. Indian. J. Med. Res. 2024, 159, 48–61. [Google Scholar] [CrossRef]
- Pal, S.R.; Arora, B.; Chhuttani, P.N.; Broor, S.; Choudhury, S.; Joshi, R.M.; Ray, S.D. Rabies virus infection of a flying fox bat, Pteropus policephalus in Chandigarh, Northern India. Trop. Geogr. Med. 1980, 32, 265–267. [Google Scholar] [PubMed]
- Mani, R.S.; Dovih, D.P.; Ashwini, M.A.; Chattopadhyay, B.; Harsha, P.K.; Garg, K.M.; Sudarshan, S.; Puttaswamaiah, R.; Ramakrishnan, U.; Madhusudana, S.N. Serological Evidence of Lyssavirus Infection among Bats in Nagaland, a North-Eastern State in India. Epidemiol. Infect. 2017, 145, 1635–1641. [Google Scholar] [CrossRef]
- Das, T.; Dutta, J.B.; Boro, P.K.; Isloor, S.; Arif, S.A.; Boro, A.R.; Saikia, U. A Preliminary Study on Bat Lyssavirus in Assam, India. Ind. J. Anim. Res. 2024, 58, 1543–1549. [Google Scholar] [CrossRef]
- Sudarshan, M.K.; Madhusudana, S.N.; Mahendra, B.J.; Rao, N.S.; Ashwath Narayana, D.H.; Abdul Rahman, S.; Meslin, F.-; Lobo, D.; Ravikumar, K. Gangaboraiah Assessing the burden of human rabies in India: Results of a national multi-center epidemiological survey. Int. J. Infect. Dis. 2007, 11, 29–35. [Google Scholar] [CrossRef]
- Gongal, G.; Wright, A.E. Human Rabies in the WHO Southeast Asia Region: Forward Steps for Elimination. Adv. Prev. Med. 2011, 2011, 383870. [Google Scholar] [CrossRef]
- Warrell, M. Rabies and African bat lyssavirus encephalitis and its prevention. Int. J. Antimicrob. Agents 2010, 36 (Suppl. S1), S47–S52. [Google Scholar] [CrossRef]
- Gompper, M.E. (Ed.) Free-Ranging Dogs and Wildlife Conservation, 1st ed.; Oxford University Press: Oxford, UK, 2014; Volume 1. [Google Scholar]
- Mars Petcare. State of Pet Homelessness Index. 2023. Available online: https://stateofpethomelessness.com/ (accessed on 26 March 2025).
- Corfmat, J.; Gibson, A.; Mellanby, R.; Watson, W.; Appupillai, M.; Yale, G.; Gamble, L.; Mazeri, S. Community attitudes and perceptions towards free-roaming dogs in Goa, India. J. Appl. Anim. Welf. Sci. 2021, 26, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Belotto, A.; Leanes, L.F.; Schneider, M.C.; Tamayo, H.; Correa, E. Overview of rabies in the Americas. Virus Res. 2005, 111, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Cleaveland, S.; Kaare, M.; Knobel, D.; Laurenson, M.K. Canine vaccination—Providing broader benefits for disease control. Vet. Microbiol. 2006, 117, 43–50. [Google Scholar] [CrossRef]
- Freire de Carvalho, M.; Vigilato, M.A.N.; Pompei, J.A.; Rocha, F.; Vokaty, A.; Molina-Flores, B.; Cosivi, O.; Del Rio Vilas, V.J. Rabies in the Americas: 1998–2014. PLoS. Negl. Trop. Dis. 2018, 12, e0006271. [Google Scholar] [CrossRef]
- Rupprecht, C.E.; Mani, R.S.; Mshelbwala, P.P.; Recuenco, S.E.; Ward, M.P. Rabies in the Tropics. Curr. Trop. Med. Rep. 2022, 9, 28–39. [Google Scholar] [CrossRef]
- Hampson, K.; Dushoff, J.; Cleaveland, S.; Haydon, D.T.; Kaare, M.; Packer, C.; Dobson, A. Transmission dynamics and prospects for the elimination of canine rabies. PLoS Biol. 2009, 7, e53. [Google Scholar] [CrossRef]
- World Health Organization. Committee on Rabies, Eighth Report; WHO Technical Report Series, 824; WHO: Geneva, Switzerland, 1992. [Google Scholar]
- Animal Welfare Board of India. Animal Birth Control (Dogs) Rules; Animal Welfare Board of India: Chennai, India, 2001; p. 7. [Google Scholar]
- World Health Organization. WHO Expert Consultation on Rabies, First Report; WHO Technical Report Series; WHO: Geneva, Switzerland, 2004; Volume 931. [Google Scholar]
- Belsare, A.; Vanak, A.T. Modelling the challenges of managing free-ranging dog populations. Sci. Rep. 2020, 10, 18874. [Google Scholar] [CrossRef]
- Boro, P.K. Epidemiology of Rabies in Assam. Doctoral Dissertation, College of Veterinary Science, Assam Agricultural University, Khanapara Campus, Guwahati, India, 2022. [Google Scholar]
- Brookes, V.J.; Gill, G.S.; Singh, B.B.; Sandhu, B.S.; Dhand, N.K.; Aulakh, R.S.; Ward, M.P. Challenges to human rabies elimination highlighted following a rabies outbreak in bovines and a human in Punjab, India. Zoonoses Public Health 2019, 66, 325–336. [Google Scholar] [CrossRef]
- Knobel, D.L.; Cleaveland, S.; Coleman, P.G.; Fèvre, E.M.; Meltzer, M.I.; Miranda, M.E.; Shaw, A.; Zinsstag, J.; Meslin, F.X. Re-evaluating the burden of rabies in Africa and Asia. Bull. World Health Organ. 2005, 83, 360–368. [Google Scholar] [PubMed]
- CDC. Mass treatment of humans who drank unpasteurized milk from rabid cows—Massachusetts, 1996–1998. MMWR 1999, 48, 228–229. [Google Scholar]
- ProMED-mail. Archive Number: 20250323.8723089. Available online: www.promedmail.org (accessed on 4 November 2024).
- Sreenivasan, N.; Li, A.; Shiferaw, M.; Tran, C.H.; Wallace, R.; Blanton, J.; Knopf, L.; Abela-Ridder, B.; Hyde, T.; Working Group on Rabies PEP logistics. Overview of rabies post-exposure prophylaxis access, procurement and distribution in selected countries in Asia and Africa, 2017–2018. Vaccine 2019, 37 (Suppl. S1), A6–A13. [Google Scholar] [CrossRef] [PubMed]
- Yasmeen, A. Patients Hit as Government Hospitals Run Out of Antirabies Vaccine. The Hindu. 2019. Available online: https://www.thehindu.com/news/national/karnataka/patients-hit-as-government-hospitals-run-out-of-anti-rabies-vaccine/article28119449.ece (accessed on 15 February 2024).
- Kole, A.K.; Roy, R.; Kole, D.C. Human rabies in India: A problem needing more attention. Bull. World Health Organ. 2014, 92, 230. [Google Scholar] [CrossRef] [PubMed]
- Belsare, A.V.; Briggs, D.J.; Gongal, G.; Mani, R.S.; Rupprecht, C.E. Enhancing human post-exposure rabies prophylaxis in canine rabies endemic regions with an interactive web solution. Publ. Health Chall. 2025, 4, e70030. [Google Scholar] [CrossRef]
- Sabeta, C.; Ngoepe, E.C. Controlling dog rabies in Africa: Successes, failures and prospects for the future. Rev. Sci. Tech. 2018, 37, 439–449. [Google Scholar] [CrossRef]
- Sambo, M.; Ferguson, E.A.; Abela-Ridder, B.; Changalucha, J.; Cleaveland, S.; Lushasi, K.; Mchau, G.J.; Nanai, A.; Nonga, H.; Steenson, R.; et al. Scaling-up the delivery of dog vaccination campaigns against rabies in Tanzania. PLoS Negl. Trop. Dis. 2022, 16, e0010124. [Google Scholar] [CrossRef]
- Lembo, T.; Attlan, M.; Bourhy, H.; Cleaveland, S.; Costa, P.; de Balogh, K.; Dodet, B.; Fooks, A.R.; Hiby, E.; Leanes, F.; et al. Renewed global partnerships and redesigned roadmaps for rabies prevention and control. Vet. Med. Int. 2011, 2011, 923149. [Google Scholar] [CrossRef]
- LeRoux, K.; Stewart, D.; Perrett, K.D.; Nel, L.H.; Kessels, J.A.; Abela-Ridder, B. Rabies control in KwaZulu-Natal, South Africa. Bull. World Health Organ. 2018, 96, 360–365. [Google Scholar] [CrossRef]
- Mbilo, C.; Coetzer, A.; Bonfoh, B.; Angot, A.; Bebay, C.; Cassamá, B.; De Benedictis, P.; Ebou, M.H.; Gnanvi, C.; Kallo, V.; et al. Dog rabies control in West and Central Africa: A review. Acta Trop. 2021, 224, 105459. [Google Scholar] [CrossRef]
- Minghui, R.; Stone, M.; Semedo, M.H.; Nel, L. New global strategic plan to eliminate dog-mediated rabies by 2030. Lancet Glob. Health 2018, 6, e828–e829. [Google Scholar] [CrossRef]
- Mshelbwala, P.P.; Weese, J.S.; Sanni-Adeniyi, O.A.; Chakma, S.; Okeme, S.S.; Mamun, A.A.; Rupprecht, C.E.; Magalhaes, R.J.S. Rabies epidemiology, prevention and control in Nigeria: Scoping progress towards elimination. PLoS Negl. Trop. Dis. 2021, 15, e0009617. [Google Scholar] [CrossRef]
- Yang, H.; Chen, S.; Dai, Z.; Luo, L.; Sun, Q.; Zhao, S.; Zeng, G.; Liu, Z.; Hu, S. Integrated Rabies Surveillance—Hunan Province, China, 2020. China CDC Wkly. 2022, 4, 986–989. [Google Scholar] [CrossRef] [PubMed]
- Broban, A.; Tejiokem, M.C.; Tiembré, I.; Druelles, S.; L’Azou, M. Bolstering human rabies surveillance in Africa is crucial to eliminating canine-mediated rabies. PLoS Negl. Trop. Dis. 2018, 12, e0006367. [Google Scholar] [CrossRef] [PubMed]
- Mashura, G.; Maburutse, B.; Chidoti, V.; Zinyakasa, T.R.; Porovha, E.; Nhara, R.B.; Mwandiringana, E.; Gori, E. Bat Rhabdoviruses: Occurrence, detection and challenges in Africa. Trop. Anim. Health Prod. 2025, 57, 108. [Google Scholar] [CrossRef] [PubMed]
- Dreyfus, A.; Volasoa, M.H.; Guis, H.; Razafindraibe, N.P.; Razafindramparany, M.H.; Arivony, N.L.; Rakotondrabe, N.; Andriamananjara, M.A.; Dussart, P.; Kassie, D.; et al. Challenges of rabies surveillance in Madagascar based on a mixed method survey amongst veterinary health officers. Front. Vet. Sci. 2024, 11, 1270547. [Google Scholar] [CrossRef]
- Viljoen, N.; Sabeta, C.; Markotter, W.; Weyer, J. Temporal and Spatial Analysis of Rabies Virus Lineages in South Africa. Viruses 2025, 17, 340. [Google Scholar] [CrossRef]
- Owiny, M.O.; Ngare, B.K.; Mugo, B.C.; Rotich, J.; Mutembei, A.; Chepkorir, K.; Sitawa, R.; Obonyo, M.; Onono, J.O. Assessment of community perceptions and risk to common zoonotic diseases among communities living at the human-livestock-wildlife interface in Nakuru West, Kenya: A participatory epidemiology approach. PLoS Negl. Trop. Dis. 2023, 17, e0011086. [Google Scholar] [CrossRef]
- Njoga, E.O.; Ilo, S.U.; Nwobi, O.C.; Onwumere-Idolor, O.S.; Ajibo, F.E.; Okoli, C.E.; Jaja, I.F.; Oguttu, J.W. Pre-slaughter, slaughter and post-slaughter practices of slaughterhouse workers in Southeast, Nigeria: Animal welfare, meat quality, food safety and public health implications. PLoS ONE 2023, 18, e0282418. [Google Scholar] [CrossRef]
- Qekwana, N.D.; Oguttu, J.W. Assessment of food safety risks associated with preslaughter activities during the traditional slaughter of goats in Gauteng, South Africa. J. Food Prot. 2014, 77, 1031–1037. [Google Scholar] [CrossRef]
- Gadaga, B.M.; Etter, E.M.; Mukamuri, B.; Makwangudze, K.J.; Pfukenyi, D.M.; Matope, G. Living at the edge of an interface area in Zimbabwe: Cattle owners, commodity chain and health workers’ awareness, perceptions and practices on zoonoses. BMC Public Health. 2016, 16, 84. [Google Scholar] [CrossRef]
- Abrahim, A.; Bekele, B.; Tahir, M.; Ahmed, S.; Ahmedin, L. Associations of community knowledge, perceptions, and practices related to zoonotic disease with sociodemographic factors in and around Chiro Town, Eastern Ethiopia: A cross-sectional study. One Health Outlook 2024, 6, 10. [Google Scholar] [CrossRef]
- Gurman, T.A.; Diallo, K.; Larson, E.; Sugg, K.; Tibbels, N. Balancing the uncertain and unpredictable nature of possible zoonotic disease transmission with the value placed on animals: Findings from a qualitative study in Guinea. PLoS Glob. Public Health 2024, 4, e0001174. [Google Scholar] [CrossRef] [PubMed]
- Singano, N.; Kainga, H.; Chatanga, E.; Nkhoma, J.; Njunga, G.; Chulu, J.; Tembo, R.; Sawa, H.; Muleya, W. One Health Lens on Rabies: Human-Bat Interactions and Genomic Insights of Rabies Virus in Rural Lilongwe, Malawi. Trop. Med. Infect. Dis. 2025, 10, 95. [Google Scholar] [CrossRef] [PubMed]
- Adetayo, O.A.; Adetayo, O.C.; Koleosho, S.A.; Adeyemo, D.O.; Obafemi, O.M.; Olakojo, T.A. Prevalence of Rabies Pre-exposure Vaccination Risk Perception among Veterinary Students at the University of Ibadan—A Cross Sectional Survey. Int. J. Med. Public Health 2021, 11, 108–112. [Google Scholar] [CrossRef]
- O’Brien, K.L.; Nolan, T. The WHO position on rabies immunization—2018 updates. Vaccine 2019, 37 (Suppl. S1), A85–A87. [Google Scholar] [CrossRef]
- Rao, A.K.; Briggs, D.; Moore, S.M.; Whitehill, F.; Campos-Outcalt, D.; Morgan, R.L.; Wallace, R.M.; Romero, J.R.; Bahta, L.; Frey, S.E.; et al. Use of a Modified Preexposure Prophylaxis Vaccination Schedule to Prevent Human Rabies: Recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR 2022, 71, 619–627. [Google Scholar] [CrossRef]
- Davis, P.; Montroy, J.; Warshawsky, B.; Abrams, E.M.; Coward, L.; Killikelly, A. Immunogenicity of pre-exposure rabies vaccination comparing number of doses and routes of administration: A systematic review and meta-analyses. Vaccine 2025, 53, 126878. [Google Scholar] [CrossRef]
- Holmes, E.C.; Harvey, E.H. The diversity, evolution and emergence of rabies virus in the Americas. In History of Rabies in the Americas: From the Pre-Columbian to the Present, Volume I, Insights into Specific Cross-Cutting Aspects of the Disease in the Americas; Rupprecht, C.E., Ed.; Fascinating Life Sciences; Springer: Cham, Switzerland, 2023; pp. 43–59. [Google Scholar]
- Fehlner-Gardiner, C. Rabies control in North America—Past, present and future. Rev. Sci. Tech. 2018, 37, 421–437. (In English) [Google Scholar] [CrossRef]
- World Health Organization. Mexico Is Free from Human Rabies Transmitted by Dogs. 2019. Available online: https://www.paho.org/en/news/21-12-2019-mexico-free-human-rabies-transmitted-dogs (accessed on 19 May 2025).
- Vigilato, M.A.N.; Molina-Flores, B.; Del Rio Vilas, V.J.; Pompei, J.C.; Cosivi, O. Canine rabies elimination: Governance principles. Rev. Sci. Tech. 2018, 37, 703–709. (In English) [Google Scholar] [CrossRef]
- Pan American Health Organization. Canine Vaccination: Advances and Challenges to Rabies Elimination in Bolivia. 2024. Available online: https://www.paho.org/en/stories/canine-vaccination-advances-and-challenges-rabies-elimination-bolivia (accessed on 19 May 2025).
- Villegas Anze, F.A. The History of Rabies in Bolivia. In History of Rabies in the Americas: From the Pre-Columbian to the Present; Rupprecht, C.E., Ed.; Fascinating Life Sciences; Springer: Cham, Switzerland, 2024; Volume II, pp. 271–302. [Google Scholar] [CrossRef]
- Millien, M.F.; Duclair, H.R.; Suprême, F.; Augustin, P.D. A brief history of rabies in Haiti until the adoption of the One Health approach for its epidemiological surveillance and control. One Health Implement. Res. 2023, 3, 148–160. [Google Scholar] [CrossRef]
- Sarrameda, M.P.; Recuenco, S.E. The History of Rabies in Venezuela. In History of Rabies in the Americas: From the Pre-Columbian to the Present; Rupprecht, C.E., Ed.; Fascinating Life Sciences; Springer: Cham, Switzerland, 2024; Volume II, pp. 241–255. [Google Scholar] [CrossRef]
- Salazar, R.; Brunker, K.; Díaz, E.W.; Zegarra, E.; Monroy, Y.; Baldarrago, G.N.; Borrini-Mayorí, K.; De la Puente-León, M.; Palmalux, N.; Nichols, J.; et al. Genomic characterization of a dog-mediated rabies outbreak in El Pedregal, Arequipa, Peru. PLoS Negl. Trop. Dis. 2025, 19, e0012396. [Google Scholar] [CrossRef]
- Gilbert, A.T. The ecological range and principles of wildlife rabies virus perpetuation in the Americas. In History of Rabies in the Americas: From the Pre-Columbian to the Present, Volume I, Insights into Specific Cross-Cutting Aspects of the Disease in the Americas; Rupprecht, C.E., Ed.; Fascinating Life Sciences; Springer: Cham, Switzerland, 2023; pp. 61–75. [Google Scholar] [CrossRef]
- Leslie, M.J.; Messenger, S.; Rohde, R.E.; Smith, J.; Cheshier, R.; Hanlon, C.; Rupprecht, C.E. Bat-associated rabies virus in Skunks. Emerg. Infect. Dis. 2006, 12, 1274–1277. [Google Scholar] [CrossRef] [PubMed]
- Kuzmin, I.V.; Shi, M.; Orciari, L.A.; Yager, P.A.; Velasco-Villa, A.; Kuzmina, N.A.; Streicker, D.G.; Bergman, D.L.; Rupprecht, C.E. Molecular inferences suggest multiple host shifts of rabies viruses from bats to mesocarnivores in Arizona during 2001–2009. PLoS Pathog. 2012, 8, e1002786. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, A.T.; Van Pelt, L.I.; Hastings, L.A.; Gigante, C.M.; Orciari, L.A.; Kelley, S.; Fitzpatrick, K.; Condori, R.E.C.; Li, Y.; Brunt, S.; et al. Reemergence of a Big Brown Bat Lyssavirus rabies Variant in Striped Skunks in Flagstaff, Arizona, USA, 2021–2023. Vector Borne Zoonotic Dis. 2024, 24, 552–562. [Google Scholar] [CrossRef]
- Seetahal, J.F.R.; Vokaty, A.; Vigilato, M.A.N.; Carrington, C.V.F.; Pradel, J.; Louison, B.; Sauers, A.V.; Roopnarine, R.; Arrebato, J.C.G.; Millien, M.F.; et al. Rabies in the Caribbean: A Situational Analysis and Historic Review. Trop. Med. Infect. Dis. 2018, 3, 89. [Google Scholar] [CrossRef]
- Pawan, J.L. The transmission of paralytic rabies in Trinidad by the vampire bat (Desmodus rotundus murinus Wagner 1840). Ann. Trop. Med. Parasitol. 1936, 30, 101–129. [Google Scholar] [CrossRef]
- Metivier, H.V.M. Paralytic Rabies in Livestock. J. Comp. Path Therap. 1935, 48, 245–260. [Google Scholar] [CrossRef]
- PanAmerican Health Organization (PAHO). Regional Information System for Epidemiological Surveillance of Rabies (SIRVERA). 2025. Available online: http://sirvera.panaftosa.org.br/login (accessed on 19 May 2025).
- Vigilato, M.A.N.; Cosivi, O.; Knobi, T.; Clavijo, A.; Silva, H.M.T. Rabies update for Latin America and the Caribbean. Emerg. Infect. Dis. 2013, 19, 678–679. [Google Scholar] [CrossRef]
- Farias, L.; Caminha, I.; Perdigão Neto, L.V.; Cavalcanti, L.P.G. Human Rabies during the COVID-19 Pandemic: Insights into Rabies Worldwide and Brazil. Rev. Soc. Bras. Med. Trop. 2024, 57, e003002024. [Google Scholar] [CrossRef]
- Seetahal, J.F.R.; Sanchez-Vazquez, M.J.; Vokaty, A.; Carrington, C.V.F.; Mahabir, R.; Adesiyun, A.A.; Rupprecht, C.E. Of bats and livestock: The epidemiology of rabies in Trinidad, West Indies. Vet. Microbiol. 2019, 228, 93–100. [Google Scholar] [CrossRef]
- Bennett, S.P.; GWGarcia Lampkin, P. The buffalypso: The water buffalo of Trinidad and Tobago. Ital. J. Anim. Sci. 2007, 6, 179–183. [Google Scholar] [CrossRef][Green Version]
- Rastogi, L.; Youssef, F.G.; Gonzalez, F.O. Beef Type Buffalo of Trinidad: Beefalypso. World Rev. Anim. Prod. 1978, 13, 53–62. [Google Scholar]
- MacLachlan, E.E. Some notes on the Water Buffalo. Proceedings of the Caribbean Veterinary Association, 1959, Trinidad and Tobago.
- Bennett, S.P.P. News-West Indian Buffalypso Plays New Role as a Beef Producer. J. Am. Vet. Med. Assoc. 1974, 164, 17. [Google Scholar]
- Besson, G.A. Sweet Sorrow: The Timeline of Sugar in Trinidad and Tobago. The Caribbean History Archives. 2018. Available online: https://caribbeanhistoryarchives.blogspot.com/2018/12/sweet-sorrow-timeline-of-sugar-in.html (accessed on 19 May 2025).
- Mohammed, A.D.; Persad, A.; Mohammed, R.; Lambie, N.; Sieuchand, S. A status report on the water buffalo (Bubalus bubalis Linnaeus, 1758) industry in Trinidad. Trop Agric. 2017, 94, 200–208. [Google Scholar]
- Fosgate, G.T.; Diptee, M.D.; Ramnanan, A.; Adesiyun, A.A. Brucellosis in domestic water buffalo (Bubalus bubalis) of Trinidad and Tobago with comparative epidemiology to cattle. Trop. Anim. Health Prod. 2011, 43, 1479–1486. [Google Scholar] [CrossRef]
- Fosgate, G.T.; Adesiyun, A.A.; Hird, D.W.; Johnson, W.O.; Hietala, S.K.; Schurig, G.G.; Ryan, J.; Diptee, M.D. Evaluation of brucellosis RB51 vaccine for domestic water buffalo (Bubalus bubalis) in Trinidad. Prev. Vet. Med. 2003, 58, 211–225. [Google Scholar] [CrossRef]
- Diptee, M.D.; Adesiyun, A.A.; Asgarali, Z.; Campbell, M.; Adone, R. Serologic responses, biosafety and clearance of four dosages of Brucella abortus strain RB51 in 6-10 months old water buffalo (Bubalus bubalis). Vet. Immunol. Immunopathol. 2006, 109, 43–55. [Google Scholar] [CrossRef]
- Diptee, M.D.; Asgarali, Z.; Campbell, M.; Fosgate, G.; Adesiyun, A.A. Post-exposure serological and bacteriological responses of water buffalo (Bubalus bubalis) to Brucella abortus biovar 1 following vaccination with Brucella abortus strain RB51. Rev Sci Tech. 2007, 26, 669–678. [Google Scholar] [CrossRef]
- Ramnanan, A.; Diptee, M.; Asgarali, Z.; Campbell, M.; Adesiyun, A.A. Serological and bacteriological responses of water buffalo (Bubalus bubalis) vaccinated with two doses of Brucella abortus strain RB51 vaccine. Trop. Anim. Health Prod. 2012, 44, 1451–1458. [Google Scholar] [CrossRef]
- Diptee, M.D.; Adesiyun, A.A.; Asgarali, Z.; Campbell, M.; Fosgate, G.T. Evaluation of cell-mediated immune responses and bacterial clearance in 6-10 months old water buffalo (Bubalus bubalis) experimentally vaccinated with four dosages of commercial Brucella abortus strain RB51 vaccine. Vet. Immunol. Immunopathol. 2005, 106, 209–220. [Google Scholar] [CrossRef]
- Bianchi, R.M.; Panziera, W.; de Galiza, G.J.N.; Kommers, G.D.; Fighera, R.A. Rabies Outbreak in Buffaloes in Rio Grande Do Sul, Brazil. Ciên Rural. 2017, 47, e20160523. [Google Scholar] [CrossRef][Green Version]
- Animal Production and Health Divison. Veterinary Diagnostic Laboratory, Annual Report, 1997–1998; Trinidad Ministry of Agriculture Land and Fisheries: Chaguanas, Trinidad and Tobago, 1998. [Google Scholar]
- Jha, V.C.; Dhakal, M.; Pun, M.B.; Jha, V.K.; Aryal, T.; Dhakal, P.R.; Manahndar, S.; Sato, T.; Ide, S.; Morita, Y.; et al. Buffalo rabies in Nepal. Vet Rec. 2004, 154, 540. [Google Scholar] [PubMed]
- El-Tholoth, M.; El-Beskawy, M.; Hamed, M.F. Identification and genetic characterization of rabies virus from Egyptian water buffaloes (Bubalus bubalis) bitten by a fox. Virus Dis. 2015, 26, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Gill, G.S.; Singh, B.B.; Dhand, N.K.; Aulakh, R.S.; Sandhu, B.S.; Ward, M.P.; Brookes, V.J. Estimation of the incidence of animal rabies in Punjab, India. PLoS ONE 2019, 14, e0222198. [Google Scholar] [CrossRef]
- Zhang, K.S.; Guo, J.H.; Xu, Z.F.; Xiang, M.; Wu, B.; Chen, H.C. Diagnosis and molecular characterization of rabies virus from a buffalo in China: A case report. Virol. J. 2011, 8, 101. [Google Scholar] [CrossRef]
- Roy, B.K.; Huda, N. Phenotypic Assessments of Cattle and Buffalo through Body Linear Measurements and their Correlations. J. Adv. Vet. Res. 2021, 11, 237–242. [Google Scholar]
- Goodwin, G.G.; Greenhall, A.M. A Review of the Bats of Trinidad and Tobago: Descriptions, Rabies Infection, and Ecology. Bull AMNH 1961, 122, 3. [Google Scholar]
- Persad, A.; Charles, R.; Adesiyun, A.A. Frequency of Toxoplasmosis in Water Buffalo (Bubalus bubalis) in Trinidad. Vet. Med. Int. 2011, 2011, 705358. [Google Scholar] [CrossRef]
- Kissoon, C. Mystery: Smuggled Animals Run Wild in Cedros. Trinidad and Tobago, Daily Express Newspapers, 19 September 2018. [Google Scholar]
- Santoo, S. Stray Cattle Problem: Here’s What to Do. Trinidad Daily Express Newspapers, 4 April 2019. [Google Scholar]
- Illamani, V.; Mahesh, K. A Case Study of Rabies in a Buffalo in Chennai, Tamil Nadu, India. Res. J. Pharm. Biol. Chem. Sci. 2014, 5, 841–845. [Google Scholar]
- Müller, T.; Freuling, C.M. Chapter 6—Rabies in terrestrial animals. In Rabies, 4th ed.; Fooks, A.R., Jackson, A.C., Eds.; Academic Press: Boston, MA, USA, 2020; pp. 195–230. [Google Scholar]
- Rupprecht, C.E.; Bannazadeh Baghi, H.; Del Rio Vilas, V.J.; Gibson, A.D.; Lohr, F.; Meslin, F.X.; Seetahal, J.F.R.; Shervell, K.; Gamble, L. Historical, current and expected future occurrence of rabies in enzootic regions. Rev. Sci. Tech. 2018, 37, 729–739. [Google Scholar] [CrossRef]
- Glosser, J.W.; Yarnell, E.P. Rabies control on Guam. Public Health Rep. 1970, 85, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, D.M.; Middleton, C.R.; Sawa, T.R.; Christensen, C.C.; Kobayashi, G.Y. Rabid bat diagnosed in Hawaii. Hawaii Med. J. 1992, 51, 181–185. [Google Scholar] [PubMed]
- Constantine, D.G. Geographic translocation of bats: Known and potential problems. Emerg. Infect. Dis. 2003, 9, 17–21. [Google Scholar] [CrossRef]
- Wardle, R.N. Australia Free from Rabies. Science 1956, 124, 1156. [Google Scholar] [CrossRef]
- Cowling, D.C.; Ungar, B.; Baird, C.W. Rabies, a cat and the quarantine laws. Med. J. Aust. 1965, 2, 81–82. [Google Scholar] [PubMed]
- Skerman, B.S. The prevention of rabies. Med. J. Aust. 1964, 2, 514–515. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Rabies. Med. J. Aust. 1969, 1, 1093–1095. [Google Scholar] [CrossRef]
- Nixon, J.; Pearn, J.; McGarn, F. Dog bite injuries to children: Potential rabies threat to Australia. Med. J. Aust. 1980, 1, 175–176. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.S.; Geering, W.A. Preparedness for human rabies. Med. J. Aust. 1977, 2, 235. [Google Scholar] [CrossRef]
- Faine, S. Of course it couldn’t happen here. Med. J. Aust. 1979, 2, 343. [Google Scholar] [CrossRef]
- CDC. Imported human rabies—Australia, 1987. MMWR 1988, 37, 351–353. [Google Scholar] [PubMed]
- Faoagali, J.L.; De Buse, P.; Strutton, G.M.; Samaratunga, H. A case of rabies. Med. J. Aust. 1988, 149, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Bucens, M.; O’Connor, L. Rabies—It can happen here. Med. J. Aust. 1988, 149, 576–577. [Google Scholar] [CrossRef] [PubMed]
- Bek, M.D.; Smith, W.T.; Levy, M.H.; Sullivan, E.; Rubin, G.L. Rabies case in New South Wales, 1990: Public health aspects. Med. J. Aust. 1992, 156, 596–597, 600. [Google Scholar] [CrossRef]
- McColl, K.A.; Gould, A.R.; Selleck, P.W.; Hooper, P.T.; Westbury, H.A.; Smith, J.S. Polymerase chain reaction and other laboratory techniques in the diagnosis of long incubation rabies in Australia. Aust. Vet. J. 1993, 70, 84–89. [Google Scholar] [CrossRef]
- Grattan-Smith, P.J.; O’Regan, W.J.; Ellis, P.S.; O’Flaherty, S.J.; McIntyre, P.B.; Barnes, C.J. Rabies. A second Australian case, with a long incubation period. Med. J. Aust. 1992, 156, 651–654. [Google Scholar] [CrossRef]
- Forman, A.J. The threat of rabies introduction and establishment in Australia. Aust. Vet. J. 1993, 70, 81–83. [Google Scholar] [CrossRef]
- Gale, A.E. Rabies and its prevention. Med. J. Aust. 1994, 161, 175. [Google Scholar] [CrossRef]
- Selvey, L.A.; Wells, R.M.; McCormack, J.G.; Ansford, A.J.; Murray, K.; Rogers, R.J.; Lavercombe, P.S.; Selleck, P.; Sheridan, J.W. Infection of humans and horses by a newly described morbillivirus. Med. J. Aust. 1995, 162, 642–645. [Google Scholar] [CrossRef]
- Speare, R.; Skerratt, L.; Foster, R.; Berger, L.; Hooper, P.; Lunt, R.; Blair, D.; Hansman, D.; Goulet, M.; Cooper, S. Australian bat lyssavirus infection in three fruit bats from north Queensland. Commun. Dis. Intell. 1997, 21, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Skerratt, L.F.; Speare, R.; Berger, L.; Winsor, H. Lyssaviral infection and lead poisoning in black flying foxes from Queensland. J. Wildl. Dis. 1998, 34, 355–361. [Google Scholar] [CrossRef]
- Hooper, P.T.; Lunt, R.A.; Gould, A.R.; Samaratunga, H.; Hyatt, A.D.; Gleeson, L.J.; Rodwell, B.J.; Rupprecht, C.E.; Smith, J.S.; Murray, P.K. A new lyssavirus—The first endemic rabies-related virus recognized in Australia. Bull. L’institut Pasteur 1997, 95, 209–218. [Google Scholar] [CrossRef]
- Gould, A.R.; Hyatt, A.D.; Lunt, R.; Kattenbelt, J.A.; Hengstberger, S.; Blacksell, S.D. Characterisation of a novel lyssavirus isolated from Pteropid bats in Australia. Virus Res. 1998, 54, 165–187. [Google Scholar] [CrossRef] [PubMed]
- Garner, G.; Bunn, C. Update on surveillance for Australian bat Lyssavirus. Australas. Epidemiol. 1997, 4, 27–30. [Google Scholar]
- Crerar, S.; Longbottom, H.; Rooney, J.; Thornber, P. Human health aspects of a possible Lyssavirus in a black flying fox. Commun. Dis. Intell. 1996, 20, 325. [Google Scholar] [CrossRef]
- Lyssavirus Expert Group (LEG). Prevention of human lyssavirus infection. Commun. Dis. Intell. 1996, 20, 505–507. [Google Scholar] [CrossRef]
- Skull, S.A.; Krause, V.; Dalton, C.B.; Roberts, L.A. A retrospective search for lyssavirus in humans in the Northern Territory. Aust. N. Z. J. Public Health 1999, 23, 305–308. [Google Scholar] [CrossRef]
- Allworth, A.; Murray, K.; Morgan, J. A human case of encephalitis due to a lyssavirus recently identified in fruit bats. Commun. Dis. Intell. 1996, 20, 504. [Google Scholar] [CrossRef]
- Samaratunga, H.; Searle, J.W.; Hudson, N. Non-rabies Lyssavirus human encephalitis from fruit bats: Australian bat Lyssavirus (pteropid Lyssavirus) infection. Neuropathol. Appl. Neurobiol. 1998, 24, 331–335. [Google Scholar] [CrossRef]
- Torvaldsen, S.; Watson, T. Rabies prophylaxis in Western Australia: The impact of Australian bat lyssavirus. Commun. Dis. Intell. 1998, 22, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Hanna, J.N.; Carney, I.K.; Smith, G.A.; Tannenberg, A.E.; Deverill, J.E.; Botha, J.A.; Serafin, I.L.; Harrower, B.J.; Fitzpatrick, P.F.; Searle, J.W. Australian bat lyssavirus infection: A second human case, with a long incubation period. Med. J. Aust. 2000, 172, 597–599. [Google Scholar] [CrossRef]
- Warrilow, D.; Smith, I.L.; Harrower, B.; Smith, G.A. Sequence analysis of an isolate from a fatal human infection of Australian bat lyssavirus. Virology 2002, 297, 109–119. [Google Scholar] [CrossRef]
- Scott, J.G. Australian bat lyssavirus: The public health response to an emerging infection. Med. J. Aust. 2000, 172, 573–574. [Google Scholar] [CrossRef]
- Hooper, P.T.; Fraser, G.C.; Foster, R.A.; Storie, G.J. Histopathology and immunohistochemistry of bats infected by Australian bat lyssavirus. Aust. Vet. J. 1999, 77, 595–599. [Google Scholar] [CrossRef]
- McCall, B.J.; Epstein, J.H.; Neill, A.S.; Heel, K.; Field, H.; Barrett, J.; Smith, G.A.; Selvey, L.A.; Rodwell, B.; Lunt, R. Potential exposure to Australian bat lyssavirus, Queensland, 1996-1999. Emerg. Infect. Dis. 2000, 6, 259–264. [Google Scholar] [CrossRef]
- Gould, A.R.; Kattenbelt, J.A.; Gumley, S.G.; Lunt, R.A. Characterisation of an Australian bat lyssavirus variant isolated from an insectivorous bat. Virus Res. 2002, 89, 1–28. [Google Scholar] [CrossRef]
- Warrilow, D.; Harrower, B.; Smith, I.L.; Field, H.; Taylor, R.; Walker, C.; Smith, G.A. Public health surveillance for Australian bat lyssavirus in Queensland, Australia, 2000–2001. Emerg. Infect. Dis. 2003, 9, 262–264. [Google Scholar] [CrossRef]
- Field, H.; McCall, B.; Barrett, J. Australian bat lyssavirus infection in a captive juvenile black flying fox. Emerg. Infect. Dis. 1999, 5, 438–440. [Google Scholar] [CrossRef]
- Thompson, G.K. Veterinary surgeon’s guide to Australian bat lyssavirus. Aust. Vet. J. 1999, 77, 710–712. [Google Scholar] [CrossRef]
- McColl, K.A.; Tordo, N.; Aguilar Setién, A.A. Bat lyssavirus infections. Rev. Sci. Tech. 2000, 19, 177–196. [Google Scholar] [CrossRef]
- Mackenzie, J.S.; Field, H.E. Emerging encephalitogenic viruses: Lyssaviruses and henipaviruses transmitted by frugivorous bats. Arch. Virol. Suppl. 2004, 18, 97–111. [Google Scholar] [CrossRef]
- Guyatt, K.J.; Twin, J.; Davis, P.; Holmes, E.C.; Smith, G.A.; Smith, I.L.; Mackenzie, J.S.; Young, P.L. A molecular epidemiological study of Australian bat lyssavirus. J. Gen. Virol. 2003, 84, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, J.S.; Field, H.E.; Guyatt, K.J. Managing emerging diseases borne by fruit bats (flying foxes), with particular reference to henipaviruses and Australian bat lyssavirus. J. Appl. Microbiol. 2003, 94, 59S–69S. [Google Scholar] [CrossRef] [PubMed]
- Field, H.; Mackenzie, J.; Daszak, P. Novel viral encephalitides associated with bats (Chiroptera)—Host management strategies. Arch. Virol. Suppl. 2004, 18, 113–121. [Google Scholar] [CrossRef]
- Anonymous. Rabies and bat lyssavirus infection. NSW Public Health Bull. 2002, 13, 27. [Google Scholar] [CrossRef]
- McQuiston, J.H.; Yager, P.A.; Smith, J.S.; Rupprecht, C.E. Epidemiologic characteristics of rabies virus variants in dogs and cats in the United States, 1999. J. Am. Vet. Med. Assoc. 2001, 218, 1939–1942. [Google Scholar] [CrossRef]
- Wallace, R.M.; Gilbert, A.; Slate, D.; Chipman, R.; Singh, A.; Cassie Wedd Blanton, J.D. Right place, wrong species: A 20-year review of rabies virus cross species transmission among terrestrial mammals in the United States. PLoS ONE 2014, 9, e107539. [Google Scholar] [CrossRef]
- McColl, K.A.; Chamberlain, T.; Lunt, R.A.; Newberry, K.M.; Westbury, H.A. Susceptibility of domestic dogs and cats to Australian bat lyssavirus (ABLV). Vet. Microbiol. 2007, 123, 15–25. [Google Scholar] [CrossRef]
- McCall, B.J.; Field, H.E.; Smith, G.A.; Storie, G.J.; Harrower, B.J. Defining the risk of human exposure to Australian bat lyssavirus through potential non-bat animal infection. Comm. Dis. Intell. 2005, 29, 201–204. [Google Scholar] [CrossRef]
- Ewald, B.; Durrheim, D. Australian Bat Lyssavirus: Examination of post-exposure treatment in NSW. NSW Public Health Bull. 2008, 19, 104–107. [Google Scholar] [CrossRef]
- Craig, A.T.; Mannes, T.F.; Gupta, L. Audit of post-exposure treatment to prevent lyssavirus infection in Sydney South West Area Health Service, 2005–2007. NSW Public Health Bull. 2009, 20, 86–89. [Google Scholar] [CrossRef]
- Tack, D.M.; Holman, R.C.; Folkema, A.M.; Mehal, J.M.; Blanton, J.D.; Sejvar, J.J. Trends in encephalitis-associated deaths in the United States, 1999–2008. Neuroepidemiology 2014, 43, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Huppatz, C.; Kelly, P.M.; Levi, C.; Dalton, C.; Williams, D.; Durrheim, D.N. Encephalitis in Australia, 1979–2006: Trends and aetiologies. Commun. Dis. Intell. Q. Rep. 2009, 33, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Moore, P.R.; Jansen, C.C.; Graham, G.C.; Smith, I.L.; Craig, S.B. Emerging tropical diseases in Australia. Part 3. Australian bat lyssavirus. Ann. Trop. Med. Parasitol. 2010, 104, 613–621. [Google Scholar] [CrossRef]
- Kardamanidis, K.; Cashman, P.; Durrheim, D.N. Travel and non-travel associated rabies post exposure treatment in New South Wales residents, Australia, 2007–2011: A cross-sectional analysis. Travel. Med. Infect. Dis. 2013, 11, 421–426. [Google Scholar] [CrossRef]
- Francis, J.R.; Nourse, C.; Vaska, V.L.; Calvert, S.; Northill, J.A.; McCall, B.; Mattke, A.C. Australian Bat Lyssavirus in a child: The first reported case. Pediatrics 2014, 133, e1063-7. [Google Scholar] [CrossRef]
- Quinn, E.K.; Massey, P.D.; Cox-Witton, K.; Paterson, B.J.; Eastwood, K.; Durrheim, D.N. Understanding human—Bat interactions in NSW, Australia: Improving risk communication for prevention of Australian bat lyssavirus. BMC Vet. Res. 2014, 10, 144. [Google Scholar] [CrossRef]
- Francis, J.R.; McCall, B.J.; Hutchinson, P.; Powell, J.; Vaska, V.L.; Nourse, C. Australian bat lyssavirus: Implications for public health. Med. J. Aust. 2014, 201, 647–649. [Google Scholar] [CrossRef] [PubMed]
- Young, M.K.; El Saadi, D.; McCall, B.J. Preventing Australian bat lyssavirus: Community knowledge and risk perception of bats in South East Queensland. Vector Borne Zoonotic Dis. 2014, 14, 284–290. [Google Scholar] [CrossRef]
- Crockford, C.N.; Dean, A.J.; Reid, S.; Dean, J.H. Conservation Values and Risk of Handling Bats: Implications for One Health Communication. Ecohealth 2018, 15, 682–687. [Google Scholar] [CrossRef]
- Field, H.E. Evidence of Australian bat lyssavirus infection in diverse Australian bat taxa. Zoonoses Public Health 2018, 65, 742–748. [Google Scholar] [CrossRef]
- Barrett, J.; Höger, A.; Agnihotri, K.; Oakey, J.; Skerratt, L.F.; Field, H.E.; Meers, J.; Smith, C. An unprecedented cluster of Australian bat lyssavirus in Pteropus conspicillatus indicates pre-flight flying fox pups are at risk of mass infection. Zoonoses Public Health 2020, 67, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, R.; Cox-Witton, K.; Field, H.; Skerratt, L.F.; Barrett, J. Australian Bat Lyssavirus: Analysis of National Bat Surveillance Data from 2010 to 2016. Viruses 2021, 13, 189. [Google Scholar] [CrossRef]
- Steele, S.G.; Booy, R.; Manocha, R.; Mor, S.M.; Toribio, J.L.M.L. Towards One Health clinical management of zoonoses: A parallel survey of Australian general medical practitioners and veterinarians. Zoonoses Public Health 2021, 68, 88–102. [Google Scholar] [CrossRef]
- Uren, A.M.; Young, M.K. Field testing Australian bat lyssavirus risk communication resources. Health Promot. J. Austr. 2024, 35, 1067–1075. [Google Scholar] [CrossRef]
- O’Shea, T.J.; Bowen, R.A.; Stanley, T.R.; Shankar, V.; Rupprecht, C.E. Variability in seroprevalence of rabies virus neutralizing antibodies and associated factors in a Colorado population of big brown bats (Eptesicus fuscus). PLoS ONE 2014, 9, e86261. [Google Scholar] [CrossRef]
- Serra-Cobo, J.; López-Roig, M.; Lavenir, R.; Abdelatif, E.; Boucekkine, W.; Elharrak, M.; Harif, B.; El Ayachi, S.; Salama, A.A.; Nayel, M.A.; et al. Active sero-survey for European bat lyssavirus type-1 circulation in North African insectivorous bats. Emerg. Microbes Infect. 2018, 7, 213. [Google Scholar] [CrossRef]
- Costa, L.J.; Andrade, F.A.; Uieda, W.; Martorelli, L.F.; Kataoka, A.P.; Fernandes, M.E. Serological investigation of rabies virus neutralizing antibodies in bats captured in the eastern Brazilian Amazon. Trans. R. Soc. Trop. Med. Hyg. 2013, 107, 684–689. [Google Scholar] [CrossRef]
- 506Kuzmin, I.V.; Niezgoda, M.; Carroll, D.S.; Keeler, N.; Hossain, M.J.; Breiman, R.F.; Ksiazek, T.G.; Rupprecht, C.E. Lyssavirus surveillance in bats, Bangladesh. Emerg. Infect. Dis. 2006, 12, 486–488. [Google Scholar] [CrossRef]
- Reynes, J.M.; Molia, S.; Audry, L.; Hout, S.; Ngin, S.; Walston, J.; Bourhy, H. Serologic evidence of lyssavirus infection in bats, Cambodia. Emerg. Infect. Dis. 2004, 10, 2231–2234. [Google Scholar] [CrossRef]
- Harris, S.L.; Aegerter, J.N.; Brookes, S.M.; McElhinney, L.M.; Jones, G.; Smith, G.C.; Fooks, A.R. Targeted surveillance for European bat lyssaviruses in English bats (2003–2006). J. Wildl. Dis. 2009, 45, 1030–1041. [Google Scholar] [CrossRef]
- Picard-Meyer, E.; Servat, A.; Wasniewski, M.; Gaillard, M.; Borel, C.; Cliquet, F. Bat rabies surveillance in France: First report of unusual mortality among serotine bats. BMC Vet. Res. 2017, 13, 387. [Google Scholar] [CrossRef] [PubMed]
- de Thoisy, B.; Bourhy, H.; Delaval, M.; Pontier, D.; Dacheux, L.; Darcissac, E.; Donato, D.; Guidez, A.; Larrous, F.; Lavenir, R.; et al. Bioecological Drivers of Rabies Virus Circulation in a Neotropical Bat Community. PLoS Negl. Trop. Dis. 2016, 10, e0004378. [Google Scholar] [CrossRef] [PubMed]
- Zieger, U.; Cheetham, S.; Santana, S.E.; Leiser-Miller, L.; Matthew-Belmar, V.; Goharriz, H.; Fooks, A.R. Natural exposure of bats in Grenada to rabies virus. Infect. Ecol. Epidemiol. 2017, 7, 1332935. [Google Scholar] [CrossRef]
- Mélade, J.; McCulloch, S.; Ramasindrazana, B.; Lagadec, E.; Turpin, M.; Pascalis, H.; Goodman, S.M.; Markotter, W.; Dellagi, K. Serological Evidence of Lyssaviruses among Bats on Southwestern Indian Ocean Islands. PLoS ONE 2016, 11, e0160553. [Google Scholar] [CrossRef]
- Reynes, J.M.; Andriamandimby, S.F.; Razafitrimo, G.M.; Razainirina, J.; Jeanmaire, E.M.; Bourhy, H.; Heraud, J.M. Laboratory surveillance of rabies in humans, domestic animals, and bats in Madagascar from 2005 to 2010. Adv. Prev. Med. 2011, 2011, 727821. [Google Scholar] [CrossRef]
- Tyem, D.A.; Dogonyaro, B.B.; Woma, T.A.; Ngoepe, E.C.; Sabeta, C.T. Sero-Surveillance of Lyssavirus Specific Antibodies in Nigerian Fruit Bats (Eidolon helvum). Trop. Med. Infect. Dis. 2017, 2, 26. [Google Scholar] [CrossRef]
- Arguin, P.M.; Murray-Lillibridge, K.; Miranda, M.E.; Smith, J.S.; Calaor, A.B.; Rupprecht, C.E. Serologic evidence of Lyssavirus infections among bats, the Philippines. Emerg. Infect. Dis. 2002, 8, 258–262. [Google Scholar] [CrossRef]
- Hirsbrunner, A.; Rodriguez-Duran, A.; Jarvis, J.A.; Rudd, R.J.; Davis, A.D. Detection of rabies viral neutralizing antibodies in the Puerto Rican Brachyphylla cavernarum. Infect. Ecol. Epidemiol. 2020, 10, 1840773. [Google Scholar] [CrossRef]
- Vázquez, S.; Ibáñez, C.; Juste, J.; Echevarria, J.E. EBLV1 circulation in natural bat colonies of Eptesicus serotinus: A six year survey. Dev. Biol. 2006, 125, 257–261. [Google Scholar] [PubMed]
- Lumlertdacha, B.; Boongird, K.; Wanghongsa, S.; Wacharapluesadee, S.; Chanhome, L.; Khawplod, P.; Hemachudha, T.; Kuzmin, I.; Rupprecht, C.E. Survey for bat lyssaviruses, Thailand. Emerg. Infect. Dis. 2005, 11, 232–236. [Google Scholar] [CrossRef]
- Seetahal, J.F.R.; Greenberg, L.; Satheshkumar, P.S.; Sanchez-Vazquez, M.J.; Legall, G.; Singh, S.; Ramkissoon, V.; Schountz, T.; Munster, V.; Oura, C.A.L.; et al. The Serological Prevalence of Rabies Virus-Neutralizing Antibodies in the Bat Population on the Caribbean Island of Trinidad. Viruses 2020, 12, 178. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.T.; Nguyen, T.T.; Noguchi, A.; Nguyen, D.V.; Ngo, G.C.; Thong, V.D.; Olowokure, B.; Inoue, S. Bat lyssaviruses, northern Vietnam. Emerg. Infect. Dis. 2014, 20, 161–163. [Google Scholar] [CrossRef] [PubMed]
- Hayman, D.T.S.; Luis, A.D.; Restif, O.; Baker, K.S.; Fooks, A.R.; Leach, C.; Horton, D.L.; Suu-Ire, R.; Cunningham, A.A.; Wood, J.L.N.; et al. Maternal antibody and the maintenance of a lyssavirus in populations of seasonally breeding African bats. PLoS ONE 2018, 13, e0198563. [Google Scholar] [CrossRef]
- Ameh, V.O.; Wu, G.; Goharriz, H.; Shipley, R.; Fooks, A.R.; Sabeta, C.T.; McElhinney, L.M. Serum Neutralization Profiles of Straw-Colored Fruit Bats (Eidolon helvum) in Makurdi (Nigeria), against Four Lineages of Lagos Bat Lyssavirus. Viruses 2021, 13, 2378. [Google Scholar] [CrossRef]
- Tidemann, C.R.; Vardon, M.J. Pests, pestilence, pollen and pot roasts: The need for community based management of flying foxes in Australia. Aust. Biol. 1997, 10, 77–83. [Google Scholar]
- Vora, N.M.; Osinubi, M.O.V.; Davis, L.; Abdurrahman, M.; Adedire, E.B.; Akpan, H.; Aman-Oloniyo, A.F.; Audu, S.W.; Blau, D.; Dankoli, R.S.; et al. Bat and Lyssavirus Exposure among Humans in Area that Celebrates Bat Festival, Nigeria, 2010 and 2013. Emerg. Infect. Dis. 2020, 26, 1399–1408. [Google Scholar] [CrossRef]
- Morgan, C.N.; Wallace, R.M.; Vokaty, A.; Seetahal, J.F.R.; Nakazawa, Y.J. Risk Modeling of Bat Rabies in the Caribbean Islands. Trop. Med. Infect. Dis. 2020, 5, 35. [Google Scholar] [CrossRef]
- Pieracci, E.G.; Pearson, C.M.; Wallace, R.M.; Blanton, J.D.; Whitehouse, E.R.; Ma, X.; Stauffer, K.; Chipman, R.B.; Olson, V. Trends in Human Rabies Deaths and Exposures—United States, 1938–2018. MMWR 2019, 68, 524–528. [Google Scholar] [CrossRef]
- Ma, X.; Bonaparte, S.; Corbett, P.; Orciari, L.A.; Gigante, C.M.; Kirby, J.D.; Chipman, R.B.; Fehlner-Gardiner, C.; Thang, C.; Cedillo, V.G.; et al. Rabies surveillance in the United States during 2021. J. Am. Vet. Med. Assoc. 2023, 261, 1045–1053. [Google Scholar] [CrossRef]
- Davis, A. Death Due to Possible Rabies Infection Reported in Western Nebraska. 9 September 2024. Available online: https://nebraska.tv/news/local/nebraska-health-departments-respond-to-death-from-potential-rabies-infection (accessed on 19 May 2025).
- Minnesota Department of Health. State Reports Rare Death from Rabies. 27 September 2024. Available online: https://www.health.state.mn.us/news/pressrel/2024/rabies092724.html (accessed on 19 May 2025).
- Hassan, C. A California Art Teacher Died from Rabies After an Encounter with a Bat in Her Classroom. 3 December 2024. Available online: https://edition.cnn.com/2024/12/03/health/rabies-death-california-art-teacher-bat/index.html (accessed on 19 May 2025).
- Ladd, S. Northern Kentuckian with Rabies Dies. 27 December 2024. Available online: https://kentuckylantern.com/briefs/northern-kentuckian-with-rabies-dies/ (accessed on 19 May 2025).
- Richardson, R. After Michigan Patient Dies of Rabies from a Transplanted Kidney, Donor’s Other Recipients Get Preventive Shots. 26 March 2025. Available online: https://www.nbcnews.com/health/health-news/rabies-organ-transplant-death-michigan-rcna198265 (accessed on 19 May 2025).
- Boldbaatar, B.; Inoue, S.; Sugiura, N.; Noguchi, A.; Orbina, J.R.C.; Demetria, C.; Miranda, M.E.; Yamada, A. Rapid Detection of Rabies Virus by Reverse Transcription Loop-Mediated Isothermal Amplification. Jpn. J. Infect. Dis. 2009, 62, 187–191. [Google Scholar] [CrossRef]
- Faye, M.; Abd El Wahed, A.; Faye, O.; Kissenkötter, J.; Hoffmann, B.; Sall, A.A.; Faye, O. A Recombinase Polymerase Amplification Assay for Rapid Detection of Rabies Virus. Sci. Rep. 2021, 11, 3131. [Google Scholar] [CrossRef] [PubMed]
- Naji, E.; Fadajan, Z.; Afshar, D.; Fazeli, M. Comparison of Reverse Transcription Loop-Mediated Isothermal Amplification Method with SYBR Green Real-Time RT-PCR and Direct Fluorescent Antibody Test for Diagnosis of Rabies. Jpn. J. Infect. Dis. 2020, 73, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Mei, H.; Zhou, J.; Zhou, M.; Han, H.; Zhao, L. Early Diagnosis of Rabies Virus Infection by RPA-CRISPR Techniques in a Rat Model. Arch. Virol. 2021, 166, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Saitou, Y.; Kobayashi, Y.; Hirano, S.; Mochizuki, N.; Itou, T.; Ito, F.H.; Sakai, T. A Method for Simultaneous Detection and Identification of Brazilian Dog- and Vampire Bat-Related Rabies Virus by Reverse Transcription Loop-Mediated Isothermal Amplification Assay. J. Virol. Methods 2010, 168, 13–17. [Google Scholar] [CrossRef]
- Klyuchnikova, E.; Gladkikh, A.; Iunikhina, O.; Sbarzaglia, V.; Drobot, E.; Popova, M.; Lyapun, I.; Arbuzova, T.; Galkina, I.; Sharova, A.; et al. Genomic and Clinical Analysis of a Fatal Human Lyssavirus irkut Case: Evidence for a Natural Focus in the Russian Far East. Viruses 2025, 17, 769. [Google Scholar] [CrossRef]
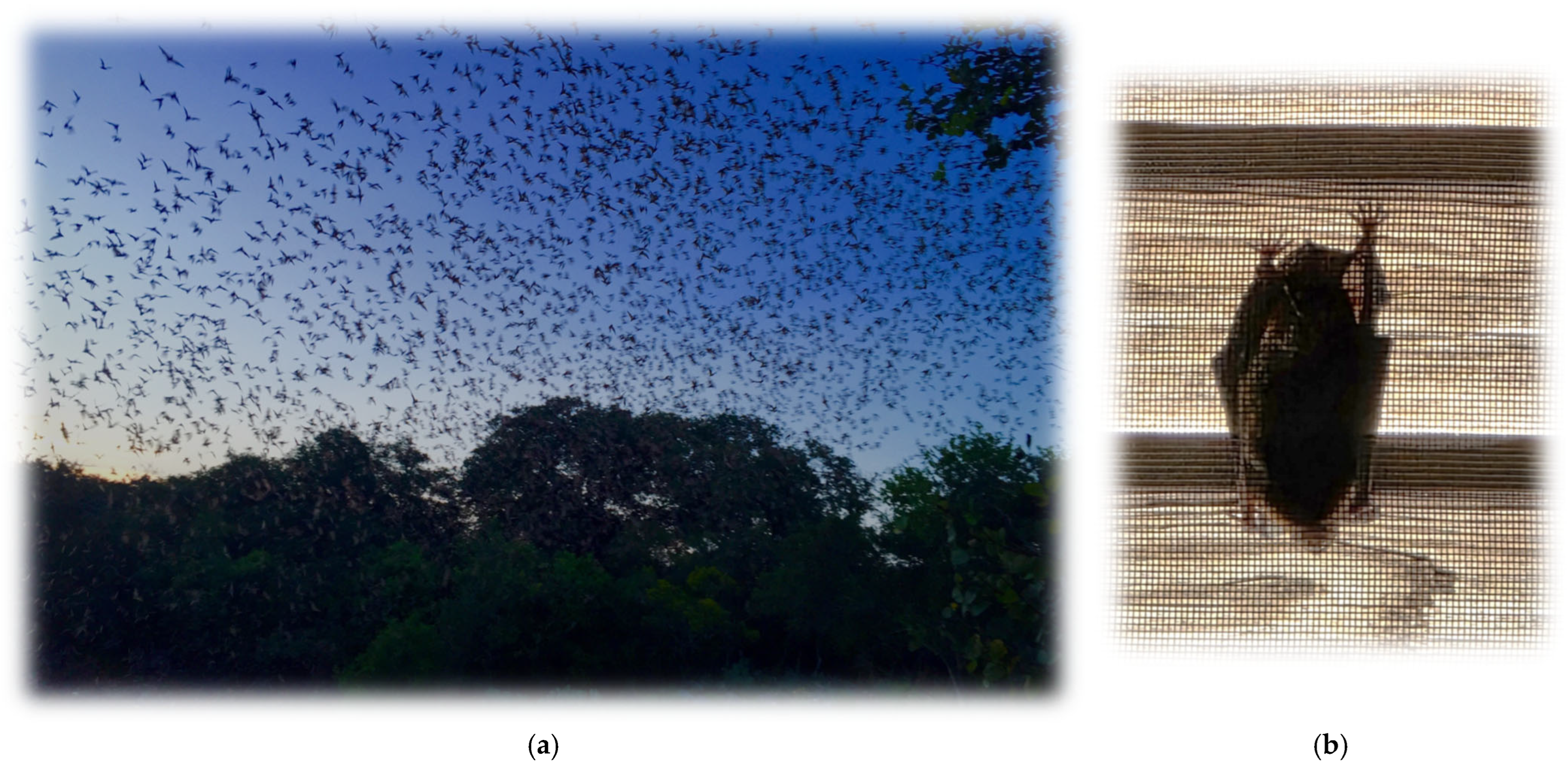

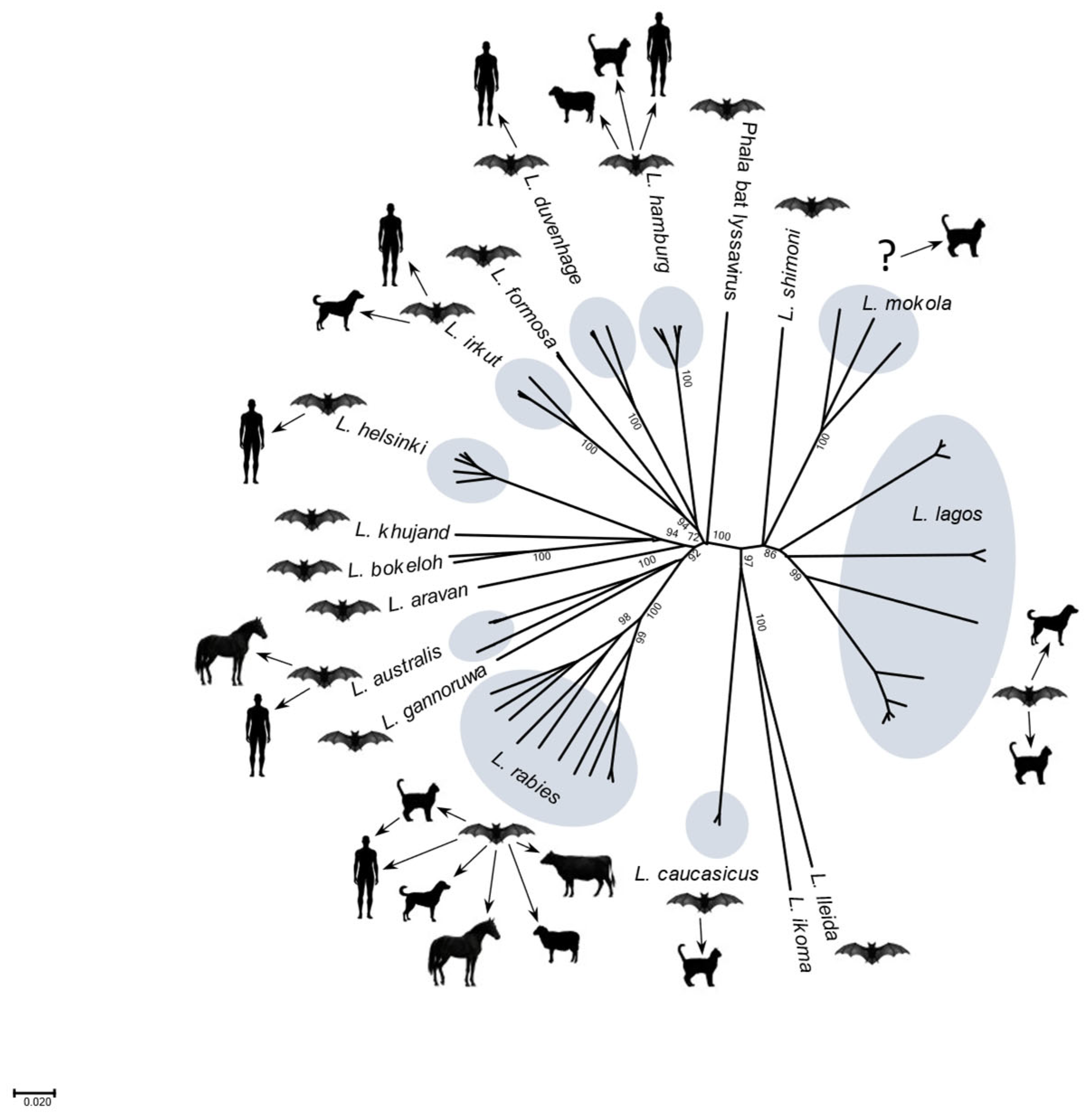
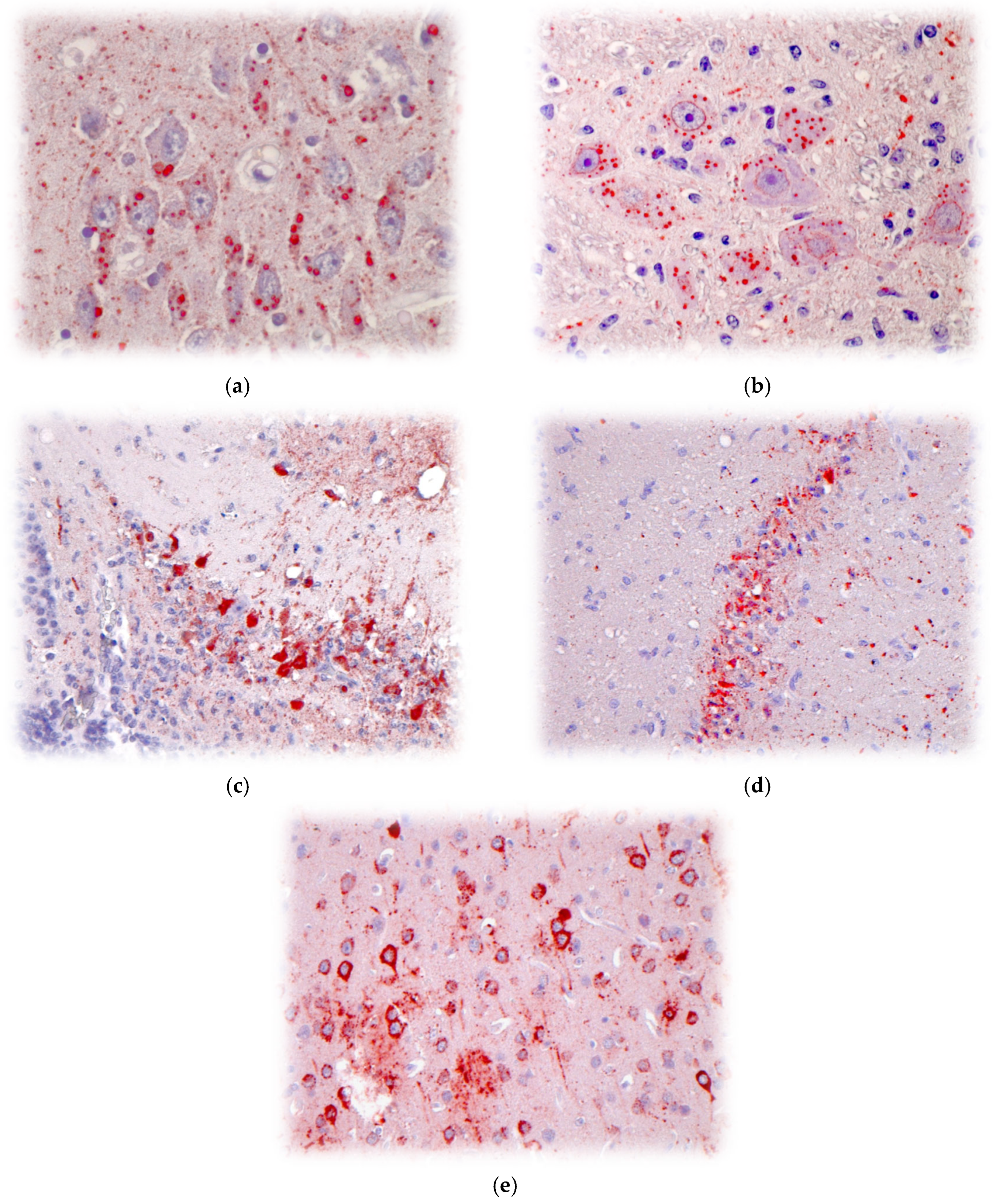
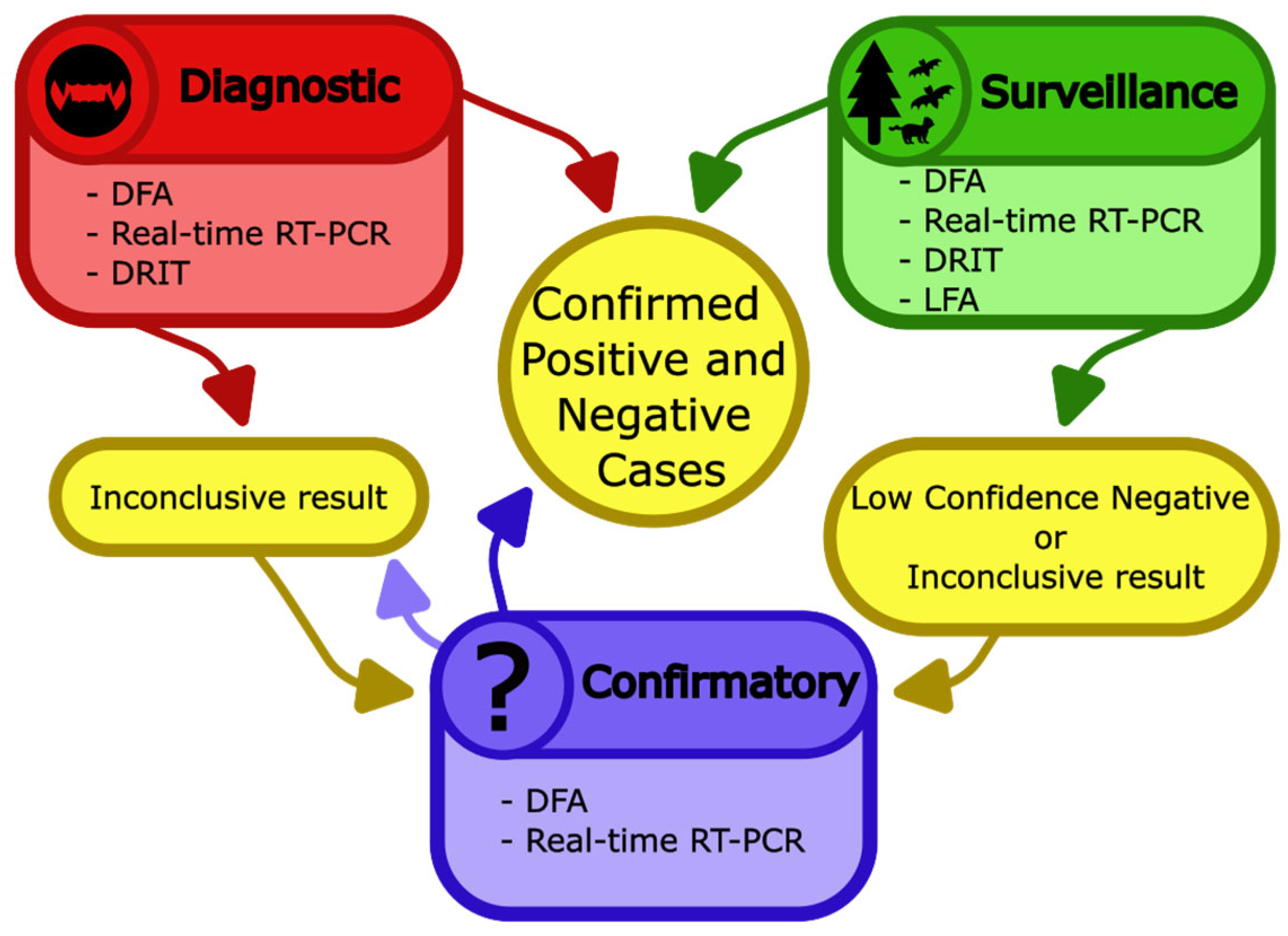
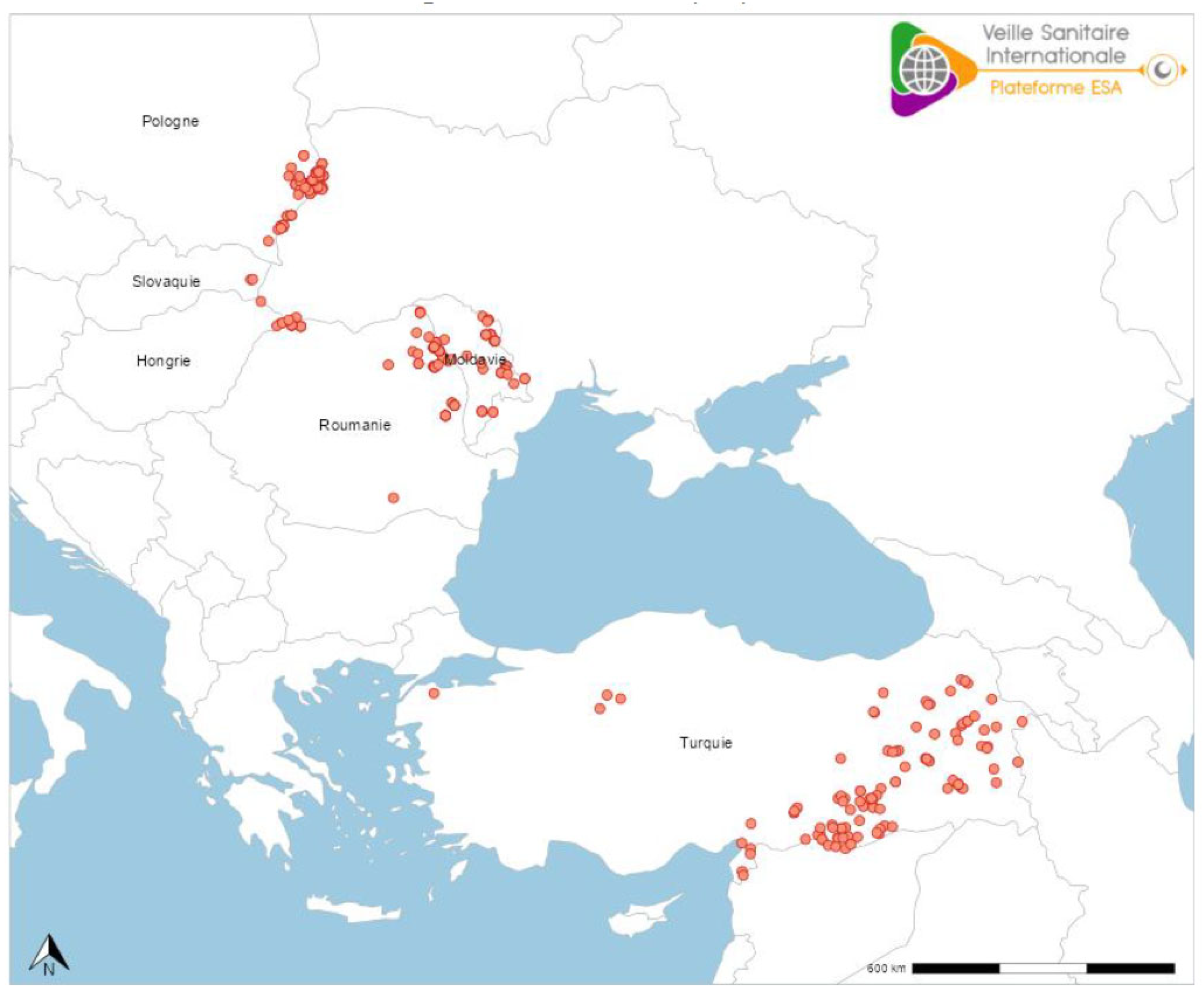

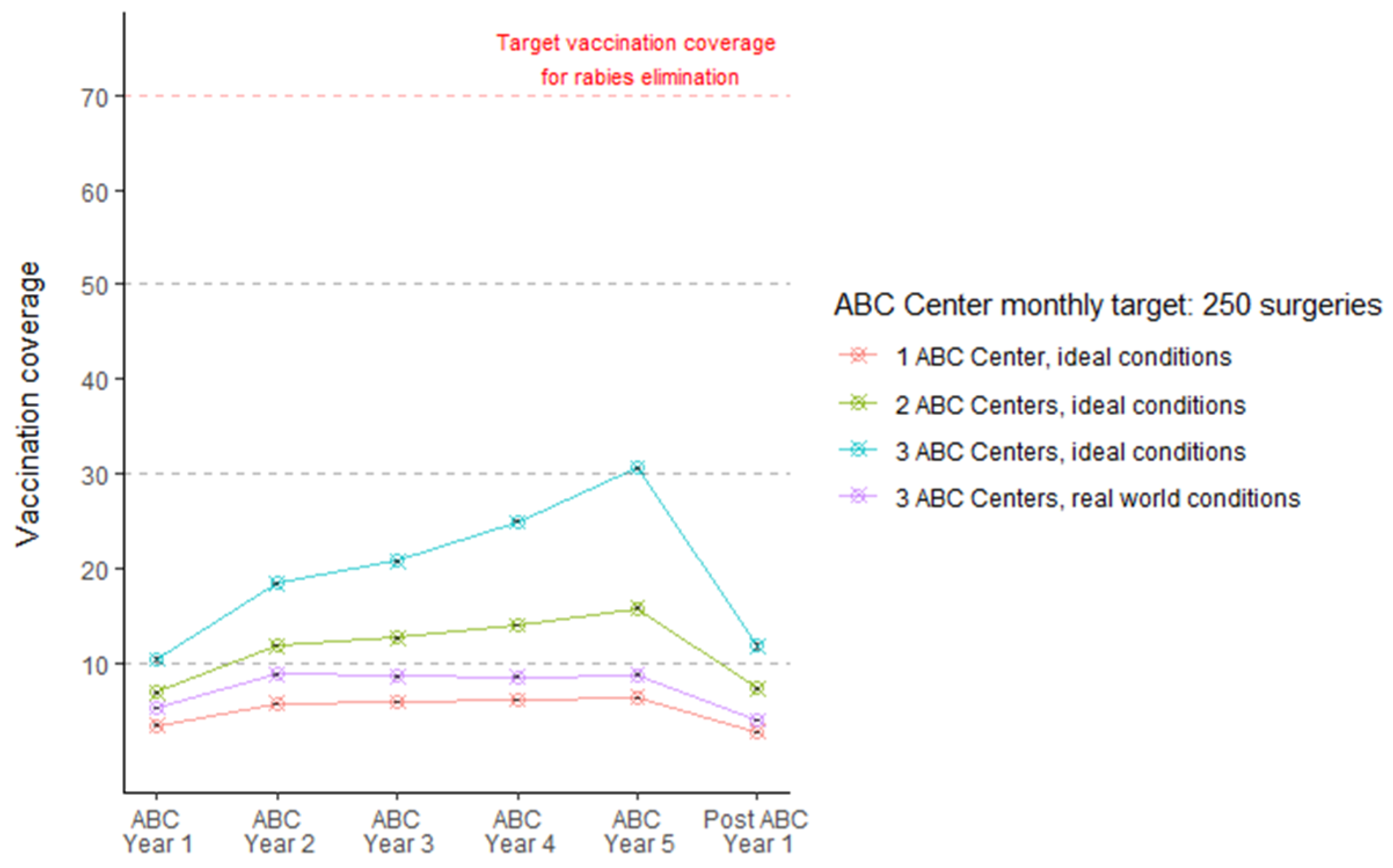
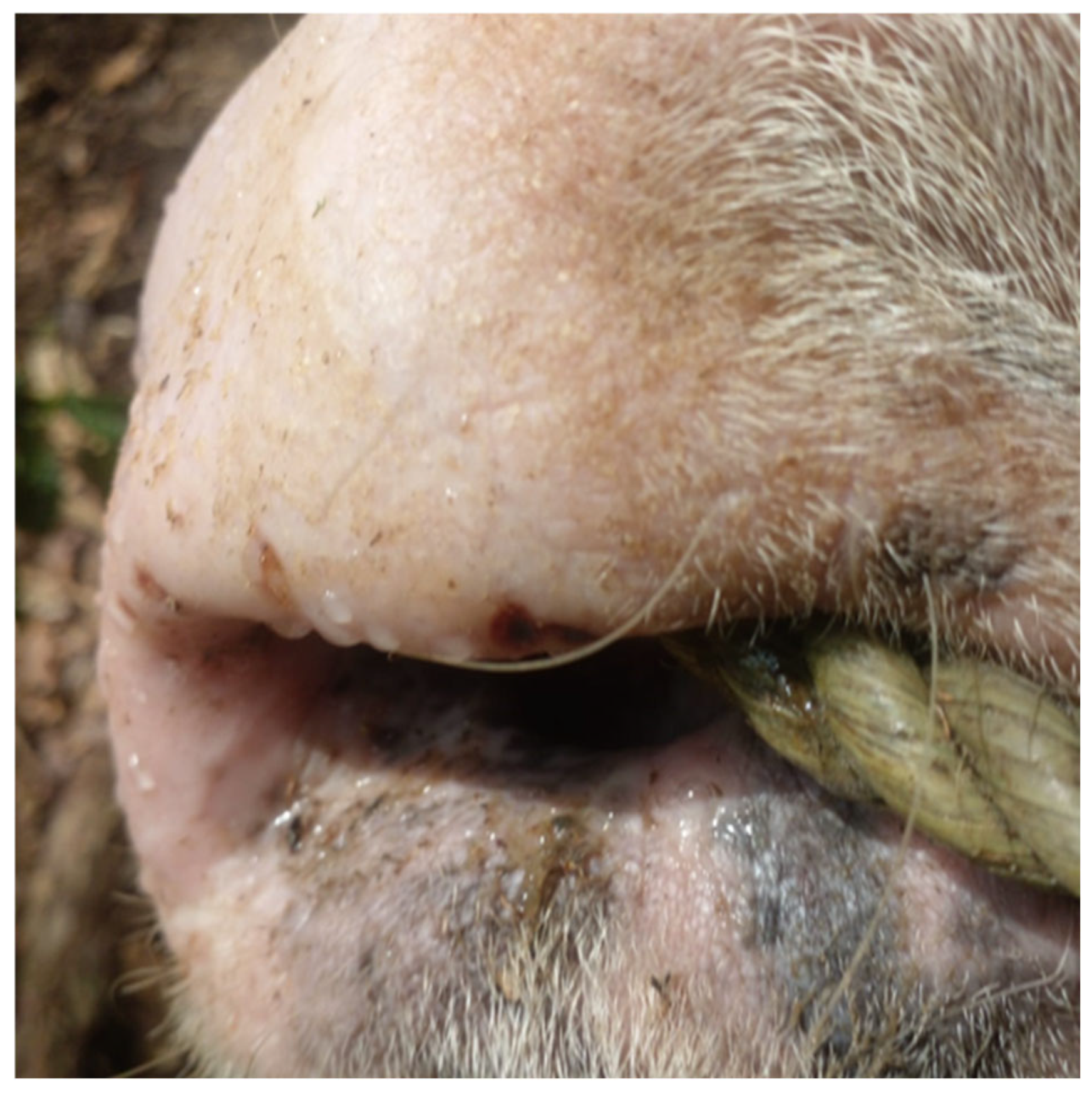


| Lyssavirus (Phylogroup) | Common Name | Reservoirs | Year and Localities | Spillover Infections | References |
|---|---|---|---|---|---|
| Lyssavirus aravan (I) | Aravan virus (ARAV) | Insectivorous bats (e.g., Myotis blythi) | 1991, Central Asia (e.g., southern Kyrgyzstan) | ? | [49,50,51,52,53] |
| Lyssavirus australis (I) | Australian bat lyssavirus (ABLV) | Frugivorous and insectivorous bats (e.g., Pteropus, Saccolaimus, etc.) | 1995, Australia | Humans, horses | [54,55,56] |
| Lyssavirus bokeloh (I) | Bokeloh bat lyssavirus (BBLV) | Insectivorous bats (e.g., Myotis, Pipistrellus spp.) | 2009, Europe (e.g., Germany, France, Poland, etc.) | ? | [57,58,59] |
| Lyssavirus caucasicus (III) | West Caucasian bat virus (WCBV) | Insectivorous bats (e.g., Miniopterus schreibersii) | 2002, Europe (e.g., Krasnodar region of Russia, Italy, etc.) | Cats | [60,61] |
| Lyssavirus duvenhage (I) | Duvenhage virus (DUVV) | African bats (e.g., Nycteris thebaica) | 1970, Sub-Saharan Africa (e.g., South Africa, Kenya, Zimbabwe, etc.) | Humans | [36,37,62,63,64,65] |
| Lyssavirus formosa (I) | Taiwan bat lyssavirus (TWBLV) | Insectivorous bats (e.g., Pipistrellus spp.) | 2016, Taiwan | ? | [66] |
| Lyssavirus gannoruwa (I) | Gannoruwa bat lyssavirus (GBLV) | Fruit bats (e.g., Pteropus spp.) | 2014, Sri Lanka | ? | [67] |
| Lyssavirus hamburg (I) | European bat lyssavirus 1 (EBLV1) | Insectivorous bats (e.g., Eptesicus, Myotis, Rhinolophus spp., etc.) | 1950s, Europe (e.g., Belgium, Denmark, France, Germany, Netherlands, Poland, Russia, Spain, Ukraine, etc.) | Humans, Cats, Sheep, Marten | [68,69,70,71,72,73,74,75,76,77,78,79] |
| Lyssavirus helsinki (I) | European bat lyssavirus 2 (EBLV2) | Insectivorous bats (e.g., Myotis spp., etc.) | 1985, Europe (e.g., Finland, Netherlands, Switzerland, UK, etc.) | Humans | [43,80,81,82,83,84,85] |
| Lyssavirus ikoma (III) | Ikoma lyssavirus (IKOV) | Unknown | 2009, Tanzania | Civet | [86] |
| Lyssavirus irkut (I) | Irkut virus (IRKV) | Insectivorous bats (e.g., Murina leucogaster) | 2002, Eurasia (e.g., Irkutsk, east Siberia region of Russia, China) | Humans, Dog | [52,60,87,88] |
| Lyssavirus khujand (I) | Khujand virus (KHUV) | Insectivorous bats (e.g., Myotis spp.) | 2001, Central Asia (e.g., northern Tajikistan) | ? | [52] |
| Lyssavirus kotalahti (I) | Kotalahti bat lyssavirus (KBLV) | Insectivorous bats (e.g., Myotis spp.) | 2017, Finland | ? | [89,90] |
| Lyssavirus lagos (II) | Lagos bat virus (LBV) | African bats (e.g., Eidolon helvum, Epomophorus wahlbergi, Micropteropus pussilus, Rousettus aegyptiacus, etc.) | 1956, Sub-Saharan Africa (e.g., Central African Republic, Ethiopia, Ghana, Guinea, Kenya, Nigeria, Senegal, South Africa, Zimbabwe, etc.) | Cats, dogs, water mongoose | [29,91,92] |
| Lyssavirus lleida (III) | Lleida bat lyssavirus (LLEBV) | Insectivorous bats (e.g., Miniopterus spp. | Europe (e.g., Spain, France, etc.) | ? | [93,94,95,96] |
| Lyssavirus mokola (II) | Mokola virus (MOK) | Unknown, but small mammals suspected | 1968, Sub-Saharan Africa (e.g., Cameroon, Central African Republic, Ethiopia, Nigeria, South Africa, Zimbabwe, etc.) | Cats, dogs, humans, etc. | [30,31,97,98,99,100] |
| Lyssavirus rabies (I) | Rabies virus (RABV) | Bats, mesocarnivores, non-human primates | Believed to be recognized for millennia, throughout human history | In theory, all warm-blooded vertebrates | [101,102,103] |
| Lyssavirus shimoni (II) | Shimoni bat virus (SHIBV) | African bats (e.g., Macronycteris vittatus) | 2009, Kenya | ? | [104,105] |
| UNCL | Divača bat lyssavirus (DBLV) | Insectivorous bats (e.g., Myotis capaccinii) | 2014, Slovenia | ? | [106] |
| UNCL | Phala bat lyssavirus (PBLV) | Bat (e.g., Nycticeinops schlieffeni) | 2021, South Africa | ? | [107,108] |
| UNCL | Taiwan bat lyssavirus 2 (TWBLV2) | Bat (Nyctalus plancyi velutinus) | 2018, Taiwan | ? | [109] |
| UNCL | Matlo bat lyssavirus (MBLV) | African bats (e.g., Miniopterus natalensis) | 2015, South Africa | ? | [109] |
| Locality | WOAH—Rabies Free? | WOAH—Dog Rabies Free? | WHO—Carnivore Rabies Free? | WHO—Lyssavirus Free? |
|---|---|---|---|---|
| Australia | Yes | Yes | Yes | No |
| Canada | No | Yes | No | No |
| China | No | No | No | No |
| France | Yes | Yes | Yes | No |
| India | No | No | No | No |
| Japan | Yes | Yes | Yes | Yes |
| Nigeria | No | No | No | No |
| Uruguay | No | Yes | Yes | No |
| USA | No | Yes | No | No |
| Host Status | Management Considerations |
|---|---|
| Naïve, non-exposed, healthy | Pre-exposure vaccination (prime, later boost, based upon label recommendations, or 1 year later) |
| Naïve, exposed, healthy | Euthanize, or strict quarantine for ~3–6 months (may consider postexposure prophylaxis management if vaccines are licensed for such use, or with agricultural and public health approval) |
| Previously vaccinated, non-exposed, healthy | Booster routinely, annually to triennially, according to vaccine label indications |
| Previously vaccinated, exposed, healthy | Booster immediately and observe for ~45 days and euthanize if compatible signs of viral encephalitis appear |
| Pregnant, naïve, healthy | Pre-exposure vaccination ad hoc |
| Pregnant, previously vaccinated, healthy | Booster during ~3rd trimester |
| Neonate, healthy, receiving colostrum from vaccinated dam | Pre-exposure vaccination at ~3–6 months |
| Neonate, healthy, from naïve healthy dam | Pre-exposure vaccination at first health check |
| Neonate, healthy, from rabid dam | Sedate dam (and later euthanize), consider emergency cesarean section, vaccinate neonate immediately (with a booster at 3 months), etc. using an abundance of caution with adequate PPE |
| Any animal with compatible clinical signs of rabies, regardless of age or vaccination status | Euthanize immediately and manage potentially exposed litter mates appropriately |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rupprecht, C.E.; Belsare, A.V.; Cliquet, F.; Mshelbwala, P.P.; Seetahal, J.F.R.; Wicker, V.V. The Challenge of Lyssavirus Infections in Domestic and Other Animals: A Mix of Virological Confusion, Consternation, Chagrin, and Curiosity. Pathogens 2025, 14, 586. https://doi.org/10.3390/pathogens14060586
Rupprecht CE, Belsare AV, Cliquet F, Mshelbwala PP, Seetahal JFR, Wicker VV. The Challenge of Lyssavirus Infections in Domestic and Other Animals: A Mix of Virological Confusion, Consternation, Chagrin, and Curiosity. Pathogens. 2025; 14(6):586. https://doi.org/10.3390/pathogens14060586
Chicago/Turabian StyleRupprecht, Charles E., Aniruddha V. Belsare, Florence Cliquet, Philip P. Mshelbwala, Janine F. R. Seetahal, and Vaughn V. Wicker. 2025. "The Challenge of Lyssavirus Infections in Domestic and Other Animals: A Mix of Virological Confusion, Consternation, Chagrin, and Curiosity" Pathogens 14, no. 6: 586. https://doi.org/10.3390/pathogens14060586
APA StyleRupprecht, C. E., Belsare, A. V., Cliquet, F., Mshelbwala, P. P., Seetahal, J. F. R., & Wicker, V. V. (2025). The Challenge of Lyssavirus Infections in Domestic and Other Animals: A Mix of Virological Confusion, Consternation, Chagrin, and Curiosity. Pathogens, 14(6), 586. https://doi.org/10.3390/pathogens14060586








