The Requirement of Turkey Herpesvirus (HVT) Glycoprotein C During Natural Infection in Chickens and Turkeys
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. Viruses
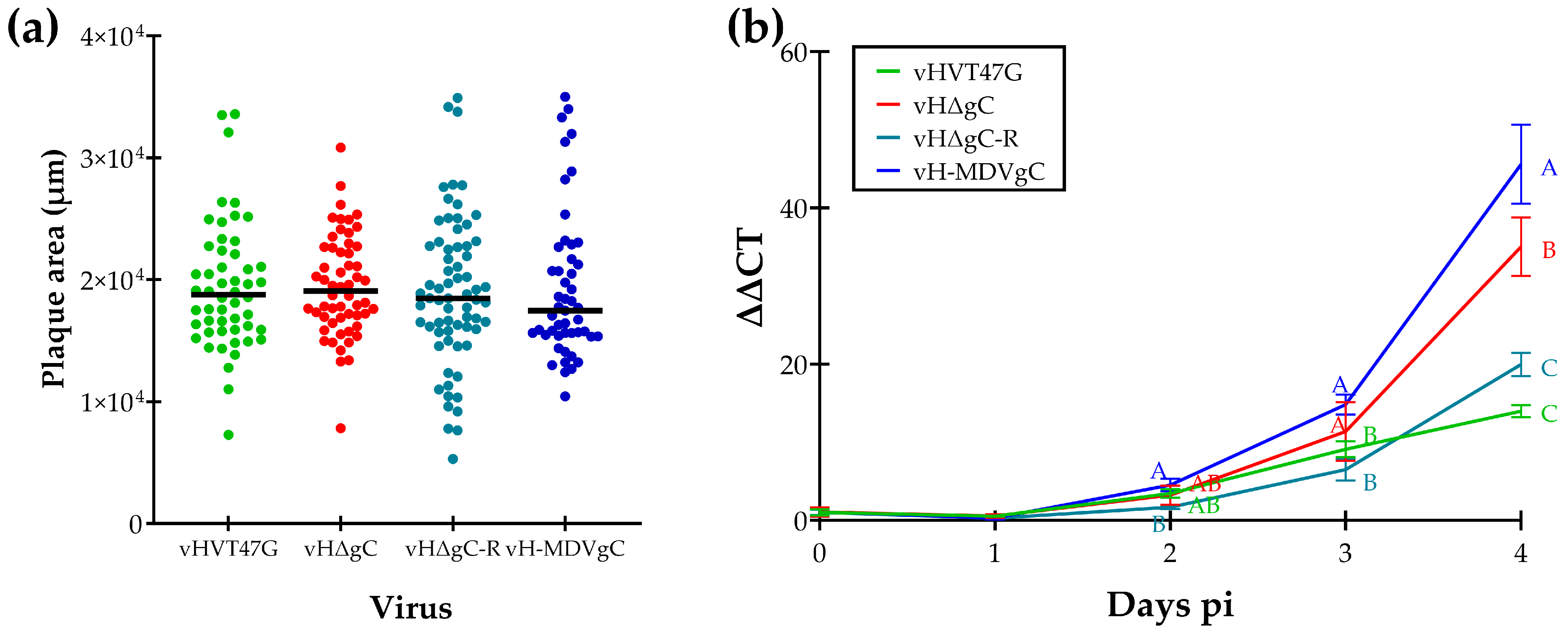
2.3. Measurement of Plaque Areas
2.4. Viral Replication Kinetics in Cell Culture
2.5. Ethics Statement
2.6. Animal Experiments
2.7. Viral Replication Kinetics in Turkeys
2.8. Direct Fluorescence and Immunofluorescence Assays (IFA) of Feather Follicles (FFs)
3. Results
3.1. Replication of vHVTs in Cell Culture
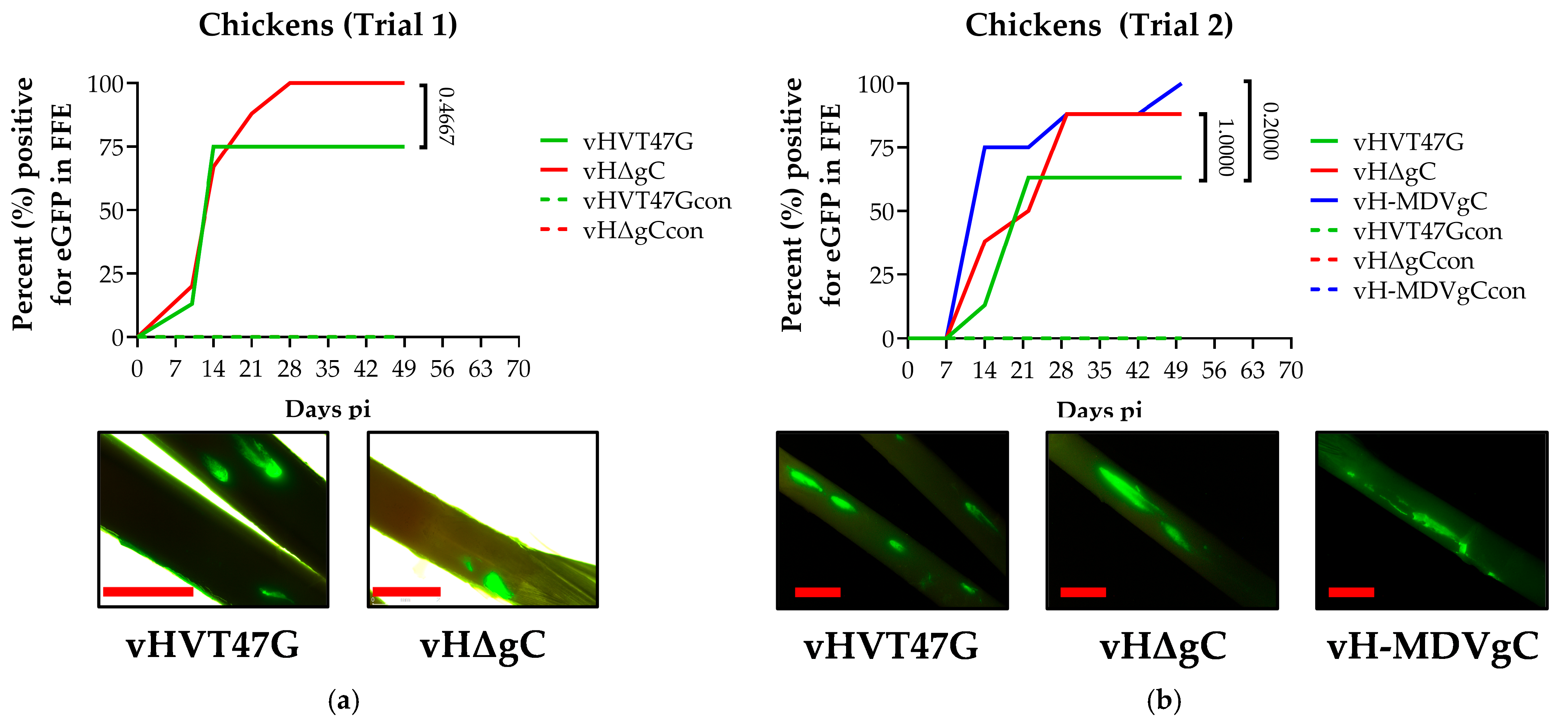
3.2. Replication and Transmission of vHVTs in Chickens
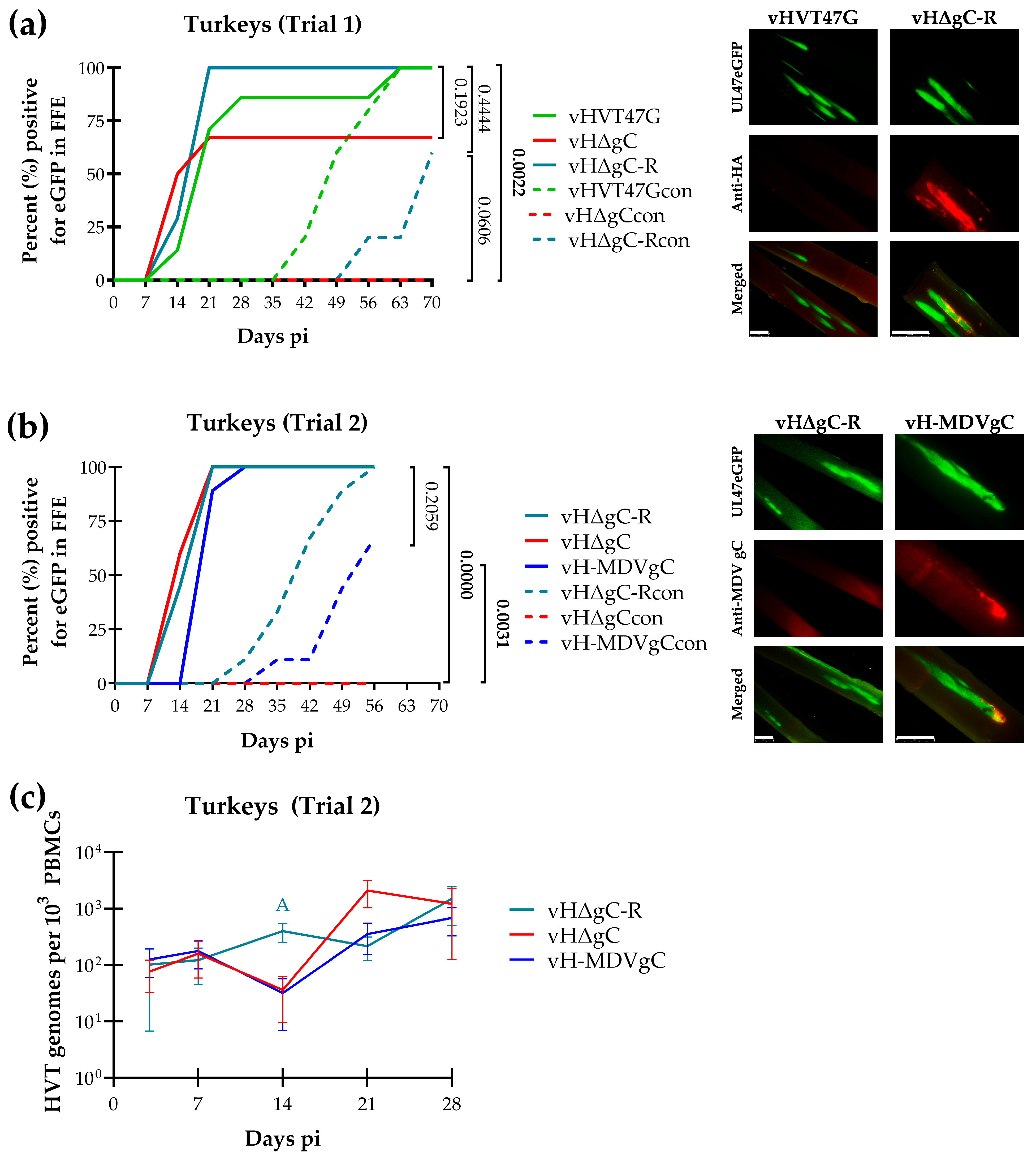
3.3. Replication and Transmission of vHVTs in Turkeys
4. Discussion
4.1. HVT Can Efficiently Transmit in Turkeys, but Not in Chickens
4.2. HVT gC Is Required for HVT Transmission in Turkeys
4.3. MDV gC Can Compensate for HVT gC in Transmission in Turkeys
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CECs | Chicken embryo cells |
| FFs | Feather follicles |
| GaAHV | Gallid alphaherpesvirus |
| gC | Glycoprotein C |
| HVT | Turkey herpesvirus |
| IFA | Immunofluorescence assay |
| MD | Marek’s disease |
| MDV | Marek’s disease virus |
| MgC | Membrane glycoprotein C |
| p.i. | Post-infection |
| r | recombinant |
| SgC | Secreted glycoprotein C |
| v | Virus |
References
- Witter, R.L. Marek’s disease research--history and perspectives. Poult. Sci. 1971, 50, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Purchase, H.G.; Okazaki, W. Effect of vaccination with herpesvirus of turkeys (HVT) on horizontal spread of Marek’s disease herpesvirus. Avian Dis. 1971, 15, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Witter, R.L. The changing landscape of Marek’s disease. Avian Pathol. 1998, 27, S46–S53. [Google Scholar] [CrossRef]
- Witter, R.L.; Solomon, J.J. Epidemiology of a herpesvirus of turkeys: Possible sources and spread of infection in turkey flocks. Infect. Immun. 1971, 4, 356–361. [Google Scholar] [CrossRef]
- Witter, R.L.; Solomon, J.J. Experimental infection of turkeys and chickens with a herpesvirus of turkeys (HVT). Avian Dis. 1972, 16, 34–44. [Google Scholar] [CrossRef]
- Banfield, B.W.; Leduc, Y.; Esford, L.; Visalli, R.J.; Brandt, C.R.; Tufaro, F. Evidence for an interaction of herpes simplex virus with chondroitin sulfate proteoglycans during infection. Virology 1995, 208, 531–539. [Google Scholar] [CrossRef]
- Mettenleiter, T.C. Glycoprotein gIII deletion mutants of pseudorabies virus are impaired in virus entry. Virology 1989, 171, 623–625. [Google Scholar] [CrossRef]
- Rue, C.A.; Ryan, P. Characterization of pseudorabies virus glycoprotein C attachment to heparan sulfate proteoglycans. J. Gen. Virol. 2002, 83, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Osterrieder, N. Construction and characterization of an equine herpesvirus 1 glycoprotein C negative mutant. Virus Res. 1999, 59, 165–177. [Google Scholar] [CrossRef]
- Allen, G.P.; Coogle, L.D. Characterization of an equine herpesvirus type 1 gene encoding a glycoprotein (gp13) with homology to herpes simplex virus glycoprotein C. J. Virol. 1988, 62, 2850–2858. [Google Scholar] [CrossRef]
- Eisenberg, R.J.; de Leon, M.P.; Friedman, H.M.; Fries, L.F.; Frank, M.M.; Hastings, J.C.; Cohen, G.H. Complement component C3b binds directly to purified glycoprotein C of herpes simplex virus types 1 and 2. Microb. Pathog. 1987, 3, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Huemer, H.P.; Larcher, C.; den Hurk, D.L.-V.; Babiuk, L.A. Species selective interaction of Alphaherpesvirinae with the “unspecific” immune system of the host. Arch. Virol. 1993, 130, 353–364. [Google Scholar] [CrossRef]
- Huemer, H.P.; Nowotny, N.; Crabb, B.S.; Meyer, H.; Hubert, P.H. gp13 (EHV-gC): A complement receptor induced by equine herpesviruses. Virus Res. 1995, 37, 113–126. [Google Scholar] [CrossRef]
- Smiley, L.; Seidel, C.; Friedman, H.M. Glycoprotein-C of herpes-simplex virus type I functions as a C3b receptor. Clin. Res. 1984, 32, A384. [Google Scholar]
- Jarosinski, K.W.; Margulis, N.G.; Kamil, J.P.; Spatz, S.J.; Nair, V.K.; Osterrieder, N. Horizontal transmission of Marek’s disease virus requires US2, the UL13 protein kinase, and gC. J. Virol. 2007, 81, 10575–10587. [Google Scholar] [CrossRef]
- Vega-Rodriguez, W.; Xu, H.; Ponnuraj, N.; Akbar, H.; Kim, T.; Jarosinski, K.W. The requirement of glycoprotein C (gC) for interindividual spread is a conserved function of gC for avian herpesviruses. Sci. Rep. 2021, 11, 7753. [Google Scholar] [CrossRef] [PubMed]
- Moffat, J.F.; Zerboni, L.; Kinchington, P.R.; Grose, C.; Kaneshima, H.; Arvin, A.M. Attenuation of the vaccine Oka strain of varicella-zoster virus and role of glycoprotein C in alphaherpesvirus virulence demonstrated in the SCID-hu mouse. J. Virol. 1998, 72, 965–974. [Google Scholar] [CrossRef]
- Xu, H.; Vega-Rodriguez, W.; Campos, V.; Jarosinski, K.W. mRNA splicing of UL44 and secretion of Alphaherpesvirinae glycoprotein C (gC) Is conserved among the Mardiviruses. Viruses 2024, 16, 782. [Google Scholar] [CrossRef] [PubMed]
- Volkening, J.D.; Spatz, S.J.; Ponnuraj, N.; Akbar, H.; Arrington, J.V.; Vega-Rodriguez, W.; Jarosinski, K.W. Viral proteogenomic and expression profiling during productive replication of a skin-tropic herpesvirus in the natural host. PLoS Pathog. 2023, 19, e1011204. [Google Scholar] [CrossRef]
- Jarosinski, K.W.; Osterrieder, N. Marek’s disease virus expresses multiple UL44 (gC) variants through mRNA splicing that are all required for efficient horizontal transmission. J. Virol. 2012, 86, 7896–7906. [Google Scholar] [CrossRef]
- Jarosinski, K.W.; Osterrieder, N. Further analysis of Marek’s disease virus horizontal transmission confirms that UL44 (gC) and UL13 protein kinase activity are essential, while US2 is nonessential. J. Virol. 2010, 84, 7911–7916. [Google Scholar] [CrossRef] [PubMed]
- Schat, K.A.; Sellers, H.S. Cell-culture methods. In A Laboratory Manual for the Identification and Characterization of Avian Pathogens, 5th ed.; Dufour-Zavala, L., Swayne, D.E., Glisson, J.R., Pearson, J.E., Reed, W.M., Jackwood, M.W., Woolcock, P.R., Eds.; American Association of Avian Pathologists: Jacksonville, FL, USA, 2008; pp. 195–203. [Google Scholar]
- Jarosinski, K.W.; Arndt, S.; Kaufer, B.B.; Osterrieder, N. Fluorescently tagged pUL47 of Marek’s disease virus reveals differential tissue expression of the tegument protein in vivo. J. Virol. 2012, 86, 2428–2436. [Google Scholar] [CrossRef]
- Jarosinski, K.W.; Osterrieder, N.; Nair, V.K.; Schat, K.A. Attenuation of Marek’s disease virus by deletion of open reading frame RLORF4 but not RLORF5a. J. Virol. 2005, 79, 11647–11659. [Google Scholar] [CrossRef]
- Abramoff, M.D.; Magalhaes, P.J.; Ram, S.J. Image processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Harrison, B.; Cheetham, B.F.; Mahony, T.J.; Young, P.L.; Walkden-Brown, S.W. Differential amplification and quantitation of Marek’s disease viruses using real-time polymerase chain reaction. J. Virol. Methods 2004, 119, 103–113. [Google Scholar] [CrossRef]
- Jarosinski, K.W.; Yunis, R.; O’Connell, P.H.; Markowski-Grimsrud, C.J.; Schat, K.A. Influence of genetic resistance of the chicken and virulence of Marek’s disease virus (MDV) on nitric oxide responses after MDV infection. Avian Dis. 2002, 46, 636–649. [Google Scholar] [CrossRef]
- Tischer, B.K.; Schumacher, D.; Chabanne-Vautherot, D.; Zelnik, V.; Vautherot, J.F.; Osterrieder, N. High-level expression of Marek’s disease virus glycoprotein C is detrimental to virus growth in vitro. J. Virol. 2005, 79, 5889–5899. [Google Scholar] [CrossRef]
- Witter, R.L.; Nazerian, K.; Purchase, H.G.; Burgoyne, G.H. Isolation from turkeys of a cell-associated herpesvirus antigenically related to Marek’s disease virus. Am. J. Vet. Res. 1970, 31, 525–538. [Google Scholar] [CrossRef]
- Okazaki, W.; Purchase, H.G.; Burmester, B.R. Protection against Marek’s disease by vaccination with a herpesvirus of turkeys. Avian Dis. 1970, 14, 413–429. [Google Scholar] [CrossRef]
- Cho, B.R.; Kenzy, S.G.; Haider, S.A. Horizontal transmission of turkey herpesvirus to chickens. 1. Preliminary observation. Poult. Sci. 1971, 50, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Vega-Rodriguez, W.; Ponnuraj, N.; Garcia, M.; Jarosinski, K.W. The requirement of glycoprotein C for interindividual spread Is functionally conserved within the Alphaherpesvirus genus (Mardivirus), but not the host (Gallid). Viruses 2021, 13, 1419. [Google Scholar] [CrossRef] [PubMed]
- Rispens, B.H.; van Vloten, H.; Mastenbroek, N.; Maas, H.J.; Schat, K.A. Control of Marek’s disease in the Netherlands. I. Isolation of an avirulent Marek’s disease virus (strain CVI 988) and its use in laboratory vaccination trials. Avian Dis. 1972, 16, 108–125. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.K.; Sharma, J.M.; Ahmad, J.; Reddy, D.N.; McMillen, J.K.; Cook, S.M.; Wild, M.A.; Schwartz, R.D. Protective efficacy of a recombinant herpesvirus of turkeys as an in ovo vaccine against Newcastle and Marek’s diseases in specific-pathogen-free chickens. Vaccine 1996, 14, 469–477. [Google Scholar] [CrossRef]
- Hein, R.; Koopman, R.; Garcia, M.; Armour, N.; Dunn, J.R.; Barbosa, T.; Martinez, A. Review of Poultry Recombinant Vector Vaccines. Avian Dis. 2021, 65, 438–452. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Vega-Rodriguez, W.; Van Etten, K.; Jarosinski, K. The Requirement of Turkey Herpesvirus (HVT) Glycoprotein C During Natural Infection in Chickens and Turkeys. Pathogens 2025, 14, 538. https://doi.org/10.3390/pathogens14060538
Xu H, Vega-Rodriguez W, Van Etten K, Jarosinski K. The Requirement of Turkey Herpesvirus (HVT) Glycoprotein C During Natural Infection in Chickens and Turkeys. Pathogens. 2025; 14(6):538. https://doi.org/10.3390/pathogens14060538
Chicago/Turabian StyleXu, Huai, Widaliz Vega-Rodriguez, Kathrine Van Etten, and Keith Jarosinski. 2025. "The Requirement of Turkey Herpesvirus (HVT) Glycoprotein C During Natural Infection in Chickens and Turkeys" Pathogens 14, no. 6: 538. https://doi.org/10.3390/pathogens14060538
APA StyleXu, H., Vega-Rodriguez, W., Van Etten, K., & Jarosinski, K. (2025). The Requirement of Turkey Herpesvirus (HVT) Glycoprotein C During Natural Infection in Chickens and Turkeys. Pathogens, 14(6), 538. https://doi.org/10.3390/pathogens14060538





