Potential New Avian Species as Carriers of Diverse Circoviruses
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Study Procedures
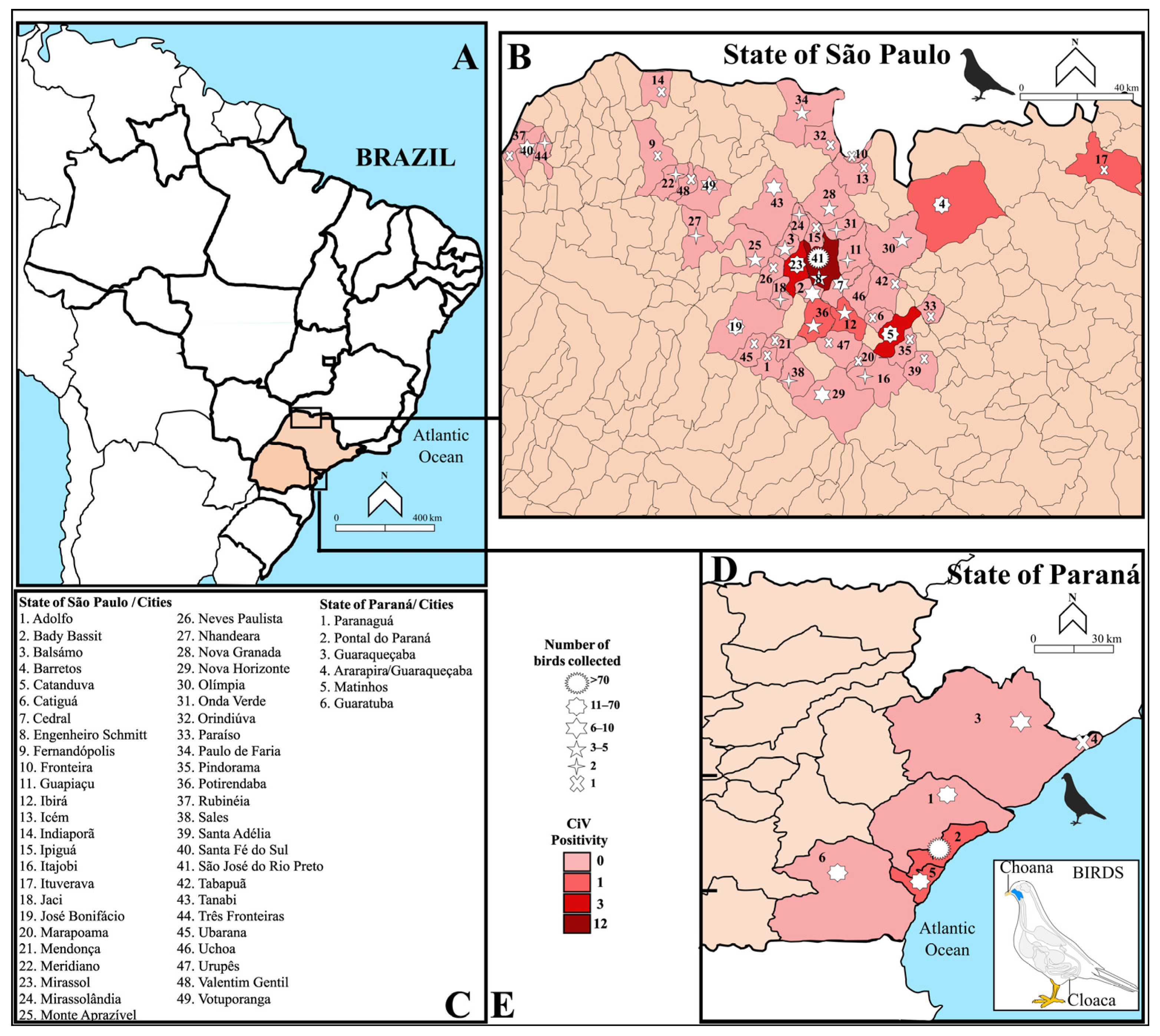
2.3. Samples from the Northwest Region of São Paulo
2.4. Samples from the Coast of Paraná
2.5. Total DNA Extraction
2.6. Nested Polymerase Chain Reaction (nPCR)
2.7. Sanger Sequencing
2.8. Isolation in Embryonic Eggs
2.9. Data Analysis
2.10. GenBank Accession Numbers
3. Results
3.1. Sample Characterization
3.2. Molecular Detection of CV
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BFDV | Pissitacine beak and feather disease circovirus |
| CaCV | Canary circovirus |
| Cap | Capsid protein |
| CV | Circovirus |
| GoCV | Goose circovirus |
| nPCR | Nested polymerase chain reaction |
| ORF | Open reading frame |
| PCV1 | Porcine circovirus 1 |
| PCV2 | Porcine circovirus 2 |
| PiCV | Pigeon circovirus |
| RaCV | Raven circovirus |
| Rep | Replicase protein |
| ssDNA | Single-stranded DNA |
References
- Chan, J.F.-W.; To, K.K.-W.; Chen, H.; Yuen, K.-Y. Cross-species transmission and emergence of novel viruses from birds. Curr. Opin. Virol. 2015, 10, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.F.-W.; To, K.K.-W.; Tse, H.; Jin, D.Y.; Yuen, K.Y. Interspecies transmission and emergence of novel viruses: Lessons from bats and birds. Trends Microbiol. 2013, 21, 544–555. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Wang, H.; Xu, J.; Lu, X.; Ni, P.; Yang, S.; Shen, Q.; Wang, X.; Li, W.; Wang, X.; et al. Unveiling the Virome of Wild Birds: Exploring CRESS-DNA Viral Dark Matter. Genome Biol. Evol. 2024, 16, evae206. [Google Scholar] [CrossRef]
- Todd, D.; Scott, A.N.J.; Fringuelli, E.; Shivraprasad, H.L.; Gavier-Widen, D.; Smyth, J.A. Molecular characterization of novel circoviruses from finch and gull. Avian Pathol. 2007, 36, 75–81. [Google Scholar] [CrossRef]
- Woods, L.W.; Latimer, K.S.; Barr, B.C.; Niagro, F.D.; Campagnoli, R.P.; Nordhausen, R.W.; Castro, A.E. Circovirus-like infection in a pigeon. J. Vet. Diagn. Investig. 1993, 5, 609–612. [Google Scholar] [CrossRef]
- Sheykhi, A.; Sheikhi, N.; Charkhkar, S.; Brujeni, G.N. Detection and Characterization of Circovirus in Canary Flocks. Avian Dis. 2018, 62, 137–142. [Google Scholar] [CrossRef]
- Soike, D.; Kohler, B.; Albrecht, K. A circovirus-like infection in geese related to a runting syndrome. Avian Pathol. 1999, 28, 199–202. [Google Scholar] [CrossRef]
- Todd, D. Circoviruses: Immunosuppressive threats to avian species: A review. Avian Pathol. 2000, 29, 373–394. [Google Scholar] [CrossRef]
- Todd, D.; McNulty, M.; Adair, B.; Allan, G. Animal circoviruses. Adv. Virus Res. 2001, 57, 1–70. [Google Scholar] [CrossRef]
- Franzo, G.; Segalés, J. Circoviruses (Circoviridae). Encycl. Virol. 2021, 1, 182–192. [Google Scholar] [CrossRef]
- Mankertz, A. Circoviruses. Encycl. Virol. 2008, 1, 513–519. [Google Scholar] [CrossRef]
- Breitbart, M.; Delwart, E.; Rosario, K.; Segalés, J.; Varsani, A. ICTV Virus Taxonomy Profile: Circoviridae. J. Gen. Virol. 2017, 98, 1997. [Google Scholar] [CrossRef]
- Steinfeldt, T.; Finsterbusch, T.; Mankertz, A. Rep and Rep′ Protein of Porcine circovirus Type 1 Bind to the Origin of Replication In Vitro. Virology 2001, 291, 152–160. [Google Scholar] [CrossRef]
- Faurez, F.; Dory, D.; Grasland, B.; Jestin, A. Replication of porcine circoviruses. Virol. J. 2009, 6, 60. [Google Scholar] [CrossRef]
- Tischer, I.; Gelderblom, H.; Vettermann, W.; Koch, M.A. A very small porcine virus with circular single-stranded DNA. Nature 1982, 295, 64–66. [Google Scholar] [CrossRef]
- Ritchie, B.W.; Niagro, F.D.; Lukert, P.D.; Steffens, W.L.; Latimer, K.S. Characterization of a new virus from cockatoos with psittacine beak and feather disease. Virology 1989, 171, 83–88. [Google Scholar] [CrossRef]
- Schmidt, R.E. Circovirus in pigeons. J. Assoc. Avian Vet. 1992, 6, 204. [Google Scholar] [CrossRef]
- Phenix, K.V.; Weston, J.H.; Ypelaar, I.; Lavazza, A.; Smyth, J.A.; Todd, D.; Wilcox, G.E.; Raidal, S.R. Nucleotide sequence analysis of a novel circovirus of canaries and its relationship to other members of the genus Circovirus of the family Circoviridae. J. Gen. Virol. 2001, 82, 2805–2809. [Google Scholar] [CrossRef]
- Todd, D.; Weston, J.; Soike, D.; Smyth, J. Genome Sequence Determinations and Analyses of Novel Circoviruses from Goose and Pigeon. Virology 2001, 286, 354–362. [Google Scholar] [CrossRef]
- Stewart, M.E.; Perry, R.; Raidal, S.R. Identification of a novel circovirus in Australian ravens (Corvus coronoides) with feather disease. Avian Pathol. 2006, 35, 86–92. [Google Scholar] [CrossRef]
- Develey, P.F. Bird Conservation in Brazil: Challenges and practical solutions for a key megadiverse country. Perspect. Ecol. Conserv. 2021, 19, 171–178. [Google Scholar] [CrossRef]
- Halami, M.; Nieper, H.; Müller, H.; Johne, R. Detection of a novel circovirus in mute swans (Cygnus olor) by using nested broad-spectrum PCR. Virus Res. 2008, 132, 208–212. [Google Scholar] [CrossRef]
- Shortridge, K.; Butterfield, W.; Webster, R.; Campbell, C. Campbell, Isolation and characterization of influenza A viruses from avian species in Hong Kong. Bull. World Health Organ. 1977, 55, 15. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC2366618/ (accessed on 6 February 2025). [PubMed]
- Thrusfield, M.; Christley, R.; Brown, H.; Diggle, P.J.; French, N.; Howe, K.; Kelly, L.; O’Connor, A.; Sargeant, J.; Wood, H. Veterinary Epidemiology, 4th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2017; pp. 270–295. [Google Scholar] [CrossRef]
- Philadelpho, N.A.; Chacón, R.D.; Forero, A.J.D.; Guimarães, M.B.; Astolfi-Ferreira, C.S.; Ferreira, A.J.P. Detection of aves polyomavirus 1 (APyV) and beak and feather disease virus (BFDV) in exotic and native Brazilian Psittaciformes. Braz. J. Microbiol. 2022, 53, 1665–1673. [Google Scholar] [CrossRef] [PubMed]
- Stenzel, T.; Koncicki, A. The epidemiology, molecular characterization and clinical pathology of circovirus infections in pigeons–Current knowledge. Veter-Q. 2017, 37, 166–174. [Google Scholar] [CrossRef]
- Todd, D.; Duchatel, J.; Bustin, J.C.; Scullion, F.T.; Scullion, M.G.; Scott, A.N.J.; Curry, A.; Ball, N.W.; Smyth, J.A. Detection of pigeon circovirus in cloacal swabs: Implications for diagnosis, epidemiology and control. Veter. Rec. 2006, 159, 314–317. [Google Scholar] [CrossRef]
- Abadie, J.; Nguyen, F.; Groizeleau, C.; Amenna, N.; Fernandez, B.; Guereaud, C.; Guigand, L.; Robart, P.; Lefebvre, B.; Wyers, M. Pigeon circovirus infection: Pathological observations and suggested pathogenesis. Avian Pathol. 2001, 30, 149–158. [Google Scholar] [CrossRef]
- Loiko, M.R.; Junqueira, D.M.; Varela, A.P.M.; Tochetto, C.; Scheffer, C.M.; Lima, D.A.; Morel, A.P.; Cerva, C.; Paim, W.P.; Mayer, F.Q.; et al. Columbid circoviruses detected in free ranging pigeons from Southern Brazil: Insights on PiCV evolution. Arch. Virol. 2018, 163, 3083–3090. [Google Scholar] [CrossRef]
- Tischer, I.; Mields, W.; Wolff, D.; Vagt, M.; Griem, W. Studies on epidemiology and pathogenicity of porcine circovirus. Arch. Virol. 1986, 91, 271–276. [Google Scholar] [CrossRef]
- Duriez, O.; Sassi, Y.; Le Gall-Ladevèze, C.; Giraud, L.; Straughan, R.; Dauverné, L.; Terras, A.; Boulinier, T.; Choquet, R.; Van De Wiele, A.; et al. Highly pathogenic avian influenza affects vultures’ movements and breeding output. Curr. Biol. 2023, 33, 3766–3774.e3. [Google Scholar] [CrossRef]
- Ducatez, M.F.; Tarnagda, Z.; Tahita, M.C.; Sow, A.; de Landtsheer, S.; Londt, B.Z.; Brown, I.H.; Osterhaus, A.D.; Fouchier, R.A.; Ouedraogo, J.-B.B.; et al. Genetic Characterization of HPAI (H5N1) Viruses from Poultry and Wild Vultures, Burkina Faso, Emerg. Infect. Dis. 2007, 13, 611. [Google Scholar] [CrossRef] [PubMed]
- Werther, K.; Raso, T.; Durigon, E.; Latimer, K.S.; Campagnoli, R. Psittacine Beak and Feather Disease in Brazil. In Revista Brasileira de Ciência Avícola; APINCO Foundation of Poultry Science and Technology: Salvador, Brazil, 1999; pp. 85–88. [Google Scholar]
- Araújo, A.V.; Andery, D.A.; Ferreira, F.C.; Ortiz, M.C.; Marques, M.V.R.; Marín, S.Y.; Vilela, D.; Resende, J.; Resende, M.; Donatti, R.V.; et al. Diagnosis of Beak and Feather Disease in Native Brazilian Psittacines. Braz. J. Poult. Sci. 2015, 17, 451–458. [Google Scholar] [CrossRef]
- Twentyman, C.; Alley, M.; Meers, J.; Cooke, M.; Duignan, P. Circovirus-like infection in a southern black-backed gull (Larus dominicanus). Avian Pathol. 1999, 28, 513–516. [Google Scholar] [CrossRef] [PubMed]
- Smyth, J.A.; Todd, D.; Scott, A.; Beckett, A.; Twentyman, C.M.; Bröjer, C.; Uhlhorn, H.; Gavier-Widen, D. Identification of circovirus infection in three species of gull. Veter. Rec. 2006, 159, 212–214. [Google Scholar] [CrossRef]
- Louten, J. Virus Transmission and Epidemiology. In Essential Human Virology; Academic Press: Cambridge, MA, USA, 2016; p. 71. [Google Scholar] [CrossRef]
| Order | Family | Scientific Name | Number of Birds |
|---|---|---|---|
| - | - | - | 1 |
| Accipitriformes | Accipitridae | Gampsonyx swainsonii | 2 |
| Geranoaetus albicaudatus | 2 | ||
| Harpia harpyja | 1 | ||
| Ictinia plumbea | 1 | ||
| Rupornis magnirostris | 19 | ||
| Anseriformes | Anatidae | Alopochen aegyptiaca | 1 |
| Cairina moschata | 5 | ||
| Dendrocygna autumnalis | 1 | ||
| Nomonyx dominicus | 1 | ||
| Spatula querquedula | 1 | ||
| Anhimidae | Anhima cornuta | 1 | |
| Caprimulgiformes | Caprimulgidae | Nyctidromus albicollis | 3 |
| Cariamiformes | Cariamidae | Cariama cristata | 22 |
| Cathartiformes | Cathartidae | Coragyps atratus | 19 |
| Charadriiformes | Charadriidae | Vanellus chilensis | 2 |
| Columbiformes | Columbidae | Columba livia | 10 |
| Columbina | 2 | ||
| Patagioenas picazuro | 26 | ||
| Zenaida auriculata | 6 | ||
| Coraciiformes | Momotidae | Momotus momota | 1 |
| Cuculiformes | Cuculidae | Guira guira | 1 |
| Falconiformes | Falconidae | Caracara Plancus | 10 |
| Falco sparverius | 5 | ||
| Milvago chimachima | 1 | ||
| Galináceos | Phasianidae | Pavo cristatus | 1 |
| Nyctibiiformes | Nyctibiidae | Nyctibius griseus | 3 |
| Passeriformes | Hirundinidae | Pygochelidon sp. | 1 |
| Icteridae | Gnorimopsar chopi | 1 | |
| Icterus pyrrhopterus | 1 | ||
| Thraupidae | Sicalis flaveola | 1 | |
| Thraupis sayaca | 1 | ||
| Tyrannidae | Pitangus sulphuratus | 4 | |
| Tyrannus melancholicus | 1 | ||
| Tyrannus savana | 1 | ||
| Pelecaniformes | Ardeidae | Ardea alba | 1 |
| Bubulcus-ibis | 3 | ||
| Butorides striata | 1 | ||
| Nycticorax nycticorax | 3 | ||
| Syrigma sibilatrix | 2 | ||
| Threskiornithidae | Mesembrinibis cayennensis | 1 | |
| Theristicus caudatus | 8 | ||
| Piciformes | Picidae | Colaptes campestres | 3 |
| Melanerpes candidus | 1 | ||
| Ramphastidae | Pteroglossus castanotis | 1 | |
| Pteroglossus sp. | 1 | ||
| Ramphastos toco | 24 | ||
| Psittaciformes | Psittacidae | Amazona aestiva | 5 |
| Amazona amazonica | 2 | ||
| Ara ararauna | 13 | ||
| Aratinga auricapillus | 3 | ||
| Brotogeris chiriri | 20 | ||
| Eupsittula aurea | 24 | ||
| Psittacara leucophthalmus | 83 | ||
| Strigiformes | Strigidae | Athene cunicularia | 23 |
| Megascops choliba | 3 | ||
| Pulsatrix perspicillata | 1 | ||
| Strix virgata | 5 | ||
| Tytonidae | Tyto furcata | 21 | |
| Tinamiformes | Tinamidae | Rhynchotus rufescens | 1 |
| Trochiliformes | Trochilidae | - | 1 |
| Total | 413 |
| Order | Family | Scientific Name | Number of Birds |
|---|---|---|---|
| Charadriiformes | - | - | 1 |
| Charadriidae | Charadrius sp. | 1 | |
| Pluvialis dominica | 2 | ||
| Pluvialis squatarola | 1 | ||
| Laridae | Larus dominicanus | 38 | |
| Lorus dominicanus | 1 | ||
| Rynchops niger | 3 | ||
| Sterna hirundo | 2 | ||
| Sterna sp. | 2 | ||
| Thalasseus acuflavidus | 3 | ||
| Thalasseus maximus | 2 | ||
| Scolopacidae | Calidris canutus | 1 | |
| Stercorariidae. | Stercorarius sp. | 2 | |
| Pelecaniformes | Threskiornithidae | Phimosus infuscatus | 3 |
| Procellariiformes | Diomedeidae | Thalassarche chlororhynchos | 1 |
| Procellariidae | Calonectris sp. | 11 | |
| Daption capense | 1 | ||
| Pterodroma sp. | 2 | ||
| Puffinus puffinus | 11 | ||
| Sphenisciformes | Spheniscidae | Spheniscus magellanicus | 50 |
| Suliformes | Fregatidae | Fregata magnificens | 11 |
| Phalacrocoracidae | Phalacrocorax brasilianus | 7 | |
| Sulidae | Sula leucogaster | 32 | |
| Total | 188 |
| Region | Species | City | Positive Samples | Development Stage | Identity (%) | Viral Subtype |
|---|---|---|---|---|---|---|
| Northwest São Paulo state | Caracara plancus | São José do Rio Preto | Cloacal | Juveniles | 98.02% | columbid circovirus |
| Caracara plancus | Barretos | Cloacal | Juveniles | n | ||
| Caracara plancus | Mirassol | Cloacal | Juveniles | 97.58% | pigeon circovirus | |
| Coragyps atratus | São José do Rio Preto | Oropharyngeal and cloacal | Juveniles | 95.05% | columbid circovirus | |
| Coragyps atratus | São José do Rio Preto | Oropharyngeal and cloacal | Adults | 99.58% | porcine circovirus 2 | |
| Coragyps atratus | São José do Rio Preto | Cloacal | Adults | 97.10% | porcine circovirus 2 | |
| Coragyps atratus | Catanduva | Cloacal | Juveniles | 99.09% | columbid circovirus | |
| Columba livia | São José do Rio Preto | Oropharyngeal and cloacal | Adults | 93.18% | pigeon circovirus | |
| Columba livia | São José do Rio Preto | Oropharyngeal and cloacal | Adults | 99.55% | pigeon circovirus | |
| Columba livia | São José do Rio Preto | Oropharyngeal and cloacal | Adults | 99.52% | columbid circovirus | |
| Columba livia | Mirassol | Oropharyngeal | Adults | 99.03% | columbid circovirus | |
| Cariama cristata | São José do Rio Preto | Cloacal | Adults | 85.45% | pigeon circovirus | |
| Eupsittula aurea | Potirendaba | Oropharyngeal and cloacal | Adults | 91.90% | beak and feather disease virus | |
| Eupsittula aurea | São José do Rio Preto | Oropharyngeal and cloacal | Adults | n | ||
| Geranoaetus albicaudatus | Ituverava | Cloacal | Juveniles | n | ||
| Nyctibius griseus | Catanduva | Cloacal and blood | Adults | 81.30% | raven circovirus | |
| Nyctibius griseus | Catanduva | Cloacal | Adults | n | ||
| Patagioenas picazuro | São José do Rio Preto | Oropharyngeal | Juveniles | 98.02% | columbid circovirus | |
| Patagioenas picazuro | São José do Rio Preto | Oropharyngeal and cloacal | Adults | 98.00% | columbid circovirus | |
| Psittacara leucophthalmus | Mirassol | Oropharyngeal | Adults | 90.90% | beak and feather disease virus | |
| Psittacara leucophthalmus | Ibirá | Cloacal | Adults | 94.52% | beak and feather disease virus | |
| Tyto furcata | São José do Rio Preto | Cloacal | Adults | 77.27% | human associated cyclovirus 6 | |
| Paraná Coast | Sterna sp. | Matinhos | Oropharyngeal and cloacal | Juveniles | 83.30% | gull circovirus |
| Larus dominicanus | Pontal do Paraná | Cloacal | Juveniles | 85.60% | gull circovirus | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia, Y.L.N.L.; Gomes, A.J.C.; Neto, G.G.; Ando, N.F.; Rodrigues, C.S.; Cesario, R.A.; Domit, C.; Lima, F.H.; Ferreira, H.L.; Araújo, J.P., Jr.; et al. Potential New Avian Species as Carriers of Diverse Circoviruses. Pathogens 2025, 14, 540. https://doi.org/10.3390/pathogens14060540
Garcia YLNL, Gomes AJC, Neto GG, Ando NF, Rodrigues CS, Cesario RA, Domit C, Lima FH, Ferreira HL, Araújo JP Jr., et al. Potential New Avian Species as Carriers of Diverse Circoviruses. Pathogens. 2025; 14(6):540. https://doi.org/10.3390/pathogens14060540
Chicago/Turabian StyleGarcia, Yasmin Luisa Neves Lemes, Ana Júlia Chaves Gomes, Guilherme Guerra Neto, Natasha Fujii Ando, Camila Sanches Rodrigues, Richard Alegria Cesario, Camila Domit, Fábio Henrique Lima, Helena Lage Ferreira, João Pessoa Araújo, Jr., and et al. 2025. "Potential New Avian Species as Carriers of Diverse Circoviruses" Pathogens 14, no. 6: 540. https://doi.org/10.3390/pathogens14060540
APA StyleGarcia, Y. L. N. L., Gomes, A. J. C., Neto, G. G., Ando, N. F., Rodrigues, C. S., Cesario, R. A., Domit, C., Lima, F. H., Ferreira, H. L., Araújo, J. P., Jr., Silva, B. L. d., Spilki, F. R., Thomazelli, L. M., Gamon, T. H. M., Assis, I. B., Durigon, E. L., Oliveira, D. B. L., Costa, V. G. d., Calmon, M. d. F., & Rahal, P. (2025). Potential New Avian Species as Carriers of Diverse Circoviruses. Pathogens, 14(6), 540. https://doi.org/10.3390/pathogens14060540








