Repellent Activity of DEET Combined with Botanical Compounds Against Amblyomma sculptum Nymphs: Laboratory and Field Evaluations
Abstract
1. Introduction
2. Materials and Methods
2.1. Ticks
2.2. Test Compounds
2.3. Laboratory Bioassays (Petri Dish Assay—One Choice)
2.3.1. Eugenol, Methyl Eugenol, and 1,8-Cineole
2.3.2. Eugenol, Methyl Eugenol, and Their Binary Combinations with DEET
2.4. Field Bioassays (Sock Assay)
2.5. Data Analysis
3. Results
3.1. Laboratory Assays
Eugenol, Methyl Eugenol, and 1,8-Cineole
3.2. Binary Combinations of Eugenol, Methyl Eugenol, and DEET
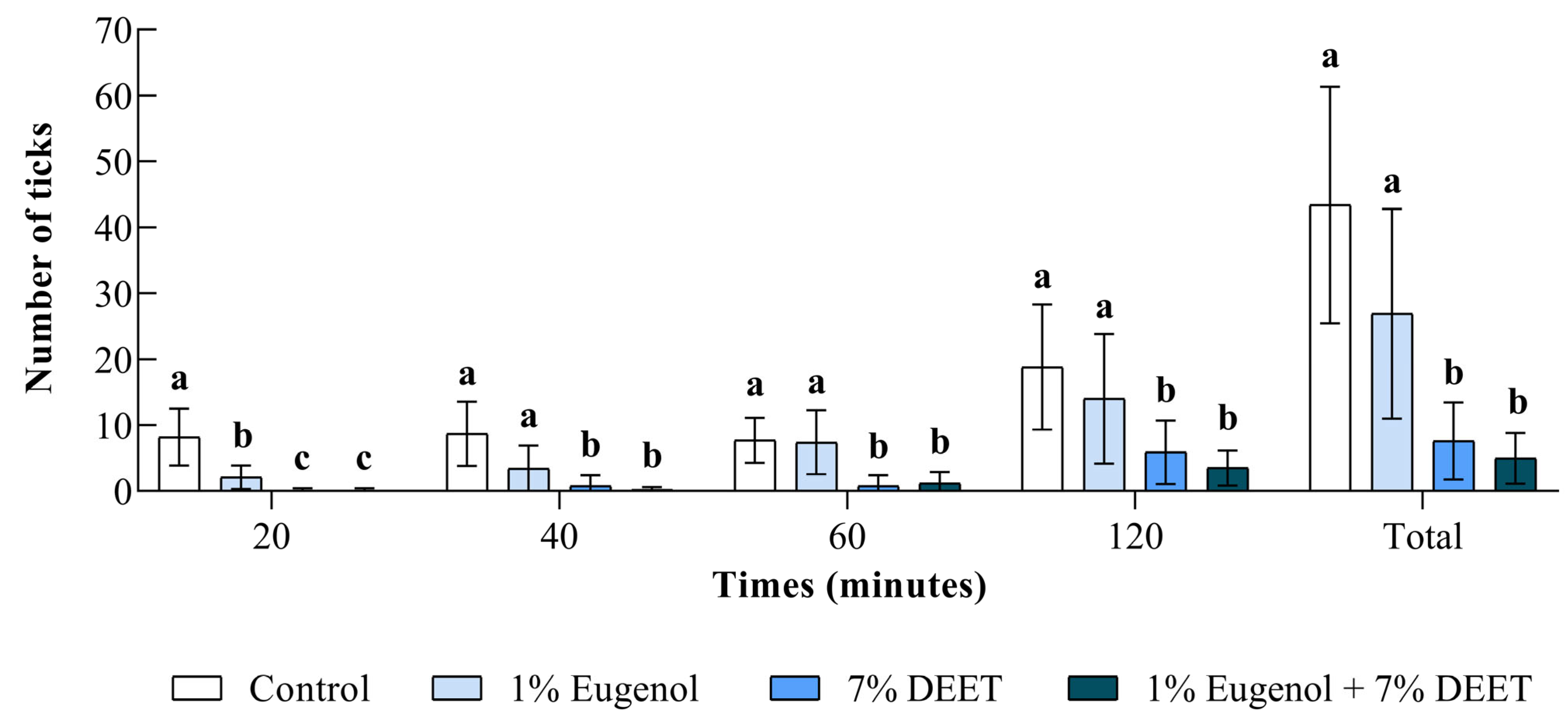
3.3. Field Bioassays
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| BSF | Brazilian spotted fever |
| CEP | Research ethics committee (Comitê de Ética em Pesquisa) |
| CEUA | Animal use ethics committee (Comissão de Ética no Uso de Animais) |
| DEET | N, N-diethyl-3-methylbenzamide |
| EO(s) | Essential oil(s) |
| EVZ | School of veterinary medicine and animal science (Escola de Veterinária e Zootecnia) |
| PAHO | Pan American Health Organization |
| PPE | Personal protective equipment |
| RH | Relative humidity |
| SBP | Brazilian society of parasitology (Sociedade Brasileira de Parasitologia) |
| SD | Standard deviation |
| UFG | Federal University of Goiás |
References
- Nava, S.; Beati, L.; Labruna, M.B.; Cáceres, A.G.; Mangold, A.J.; Guglielmone, A.A. Reassessment of the taxonomic status of Amblyomma cajennense (Fabricius, 1787) with the description of three new species, Amblyomma tonelliae n. sp., Amblyomma interandinum n. sp. and Amblyomma patinoi n. sp., and reinstatement of Amblyomma mixtum Koch, 1844, and Amblyomma sculptum Berlese, 1888 (Ixodida: Ixodidae). Ticks Tick Borne Dis. 2014, 5, 252–276. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, V.S.; Pina, F.T.B.; Barros, J.C.; Garcia, M.V.; Andreotti, R. Carrapato-estrela (Amblyomma sculptum): Ecologia, biologia, controle e importância. In Comunicado Técnico; Embrapa Gado de Corte: Campo Grande, Brazil, 2015. [Google Scholar]
- Barros-Battesti, D.M.; Onofrio, V.C.; Labruna, M.B.; Pinter, A.; Giacomin, F.; Battesti, D.M.; Barros, G.D.C. Carrapatos de Importância Médico-Veterinária da Região Neotropical: Um Guia Ilustrado Para Identificação de Espécies, 1st ed.; Instituto Butantan: São Paulo, Brazil, 2006. [Google Scholar]
- Camargo-Neves, V.; Vieira, A.; Souza, C.; Labruna, M.; Mayo, R.; Souza, S. Manual de Vigilância Acarológica do Estado de São Paulo, Brasil; Secretaria de Estado da Saúde: São Paulo, Brazil, 2004.
- Labruna, M.B.; Kerber, C.E.; Ferreira, F.; Faccini, J.L.H.; De Waal, D.T.; Gennari, S.M. Risk factors to tick infestations and their occurrence on horses in the state of São Paulo, Brazil. Vet. Parasitol. 2001, 97, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Araújo, R.P.; Navarro, M.B.M.A.; Cardoso, T.A.O. Febre maculosa no Brasil: Estudo da mortalidade para a vigilância epidemiológica. Cad. Saúde Coletiva 2016, 24, 339–346. [Google Scholar] [CrossRef]
- Gerardi, M.; Ramírez-Hernández, A.; Binder, L.C.; Krawczak, F.S.; Gregori, F.; Labruna, M.B. Comparative susceptibility of different populations of Amblyomma sculptum to Rickettsia rickettsii. Front. Physiol. 2019, 10, 653. [Google Scholar] [CrossRef]
- de Paula, L.G.F.; do Nascimento, R.M.; Franco, A.d.; Szabó, M.P.J.; Labruna, M.B.; Monteiro, C.; Krawczak, F.S. Seasonal dynamics of Amblyomma sculptum: A review. Parasites Vectors 2022, 15, 193. [Google Scholar] [CrossRef]
- Ministério da Saúde. Situação Epidemiológica da Febre Maculosa—Brasil, 2007 a 2025; Ministério da Saúde: Brasília, Brazil, 2025. Available online: https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/f/febre-maculosa/situacao-epidemiologica/situacao-epidemiologica-da-febre-maculosa-brasil-2007-2025 (accessed on 24 April 2025).
- De La Fuente, J.; Estrada-Pena, A.; Venzal, J.M.; Kocan, K.M.; Sonenshine, D.E. Overview: Ticks as vectors of pathogens that cause disease in humans and animals. Front. Biosci. 2008, 13, 6938–6946. [Google Scholar] [CrossRef]
- Leal, W.S. The enigmatic reception of DEET—The gold standard of insect repellents. Curr. Opin. Insect Sci. 2014, 6, 93–98. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R. Repellence of essential oils and selected compounds against ticks—A systematic review. Acta Trop. 2018, 179, 47–54. [Google Scholar] [CrossRef]
- Agnew, J.; Gorzelski, A.; Zhu, J.; Romero, A. Coconut fatty acids exhibit strong repellency and week-long efficacy against several urban pest arthropods of the southwestern United States. Pest Manag. Sci. 2023, 79, 3511–3519. [Google Scholar] [CrossRef]
- Barcelos, B.R.; Coelho, N.G.S.S.; Barrozo, M.M.; Vale, F.L.; Teixeira, A.L.C.; Souza, L.M.P.; Zeringóta, V.; Monteiro, C.; Eugenio, C.U.O.; Obara, M.T. Do commercial insect repellents provide protection against the tick Amblyomma sculptum (Acari: Ixodidae)? Pathogens 2024, 13, 9. [Google Scholar] [CrossRef]
- Soares, S.F.; Braga, R.S.; Ferreira, L.L.; Louly, C.C.B.; Sousa, L.A.D.; Borges, L.M.F. Repellent activity of DEET against Amblyomma cajennense (Acari: Ixodidae) nymphs submitted to different laboratory bioassays. Rev. Bras. Parasitol. Vet. 2010, 19, 12–17. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ferreira, L.L.; Oliveira Filho, J.G.; Mascarin, G.M.; León, A.A.P.; Borges, L.M.F. In vitro repellency of DEET and β-citronellol against the ticks Rhipicephalus sanguineus sensu lato and Amblyomma sculptum. Vet. Parasitol. 2017, 239, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.L.; Sarria, A.L.F.; de Oliveira Filho, J.G.; de Silva, F.O.; Powers, S.J.; Caulfield, J.C.; Pickett, J.A.; Birkett, M.A.; Borges, L.M.F. Identification of a non-host semiochemical from tick-resistant donkeys (Equus asinus) against Amblyomma sculptum ticks. Ticks Tick Borne Dis. 2019, 10, 621–627. [Google Scholar] [CrossRef]
- Ferreira, L.L.; Sarria, A.L.F.; de Oliveira Filho, J.G.; de Silva, F.O.; Ferraz, A.L.L.; Mascarin, G.M. Attract or repel Amblyomma sculptum ticks: Screening of semiochemicals. Vet. Parasitol. 2020, 278, 109036. [Google Scholar] [CrossRef]
- Schaefer, C.; Peters, P.W.J. Intrauterine diethyltoluamide exposure and fetal outcome. Reprod. Toxicol. 1992, 6, 175–176. [Google Scholar] [CrossRef]
- Dugas, J.; Nieuwenhuijsen, M.J.; Martinez, D.; Iszatt, N.; Nelson, P.; Elliott, P. Use of biocides and insect repellents and risk of hypospadias. Occup. Environ. Med. 2010, 67, 196–200. [Google Scholar] [CrossRef]
- Sociedade Brasileira de Pediatria (SBP). Repelentes e Outras Medidas Protetoras Contra Insetos na Infância. Guia Prático de Atualização; SBP: Rio de Janeiro, Brazil, 2020; Volume 7, pp. 1–11. Available online: https://www.sbp.com.br/imprensa/detalhe/nid/repelentes-e-outras-medidas-protetoras-contra-insetos-na-infancia/ (accessed on 24 April 2025).
- Parecer Técnico nº 2/2009—Avaliação Toxicológica de Ingredientes Ativos Utilizados em Produtos Repelentes de Insetos de uso Tópico; ANVISA—Agência Nacional de Vigilância Sanitária: Brasília, Brazil, 2009.
- Resolução RDC nº 19, de 10 de Abril de 2013; ANVISA—Agência Nacional de Vigilância Sanitária: Brasília, Brazil, 2013. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2013/rdc0019_10_04_2013.html (accessed on 24 April 2025).
- Kamsuk, K.; Choochote, W.; Chaithong, U.; Jitpakdi, A.; Tippawangkosol, P.; Riyong, D.; Pitasawat, B. Effectiveness of Zanthoxylum piperitum-derived essential oil as an alternative repellent under laboratory and field applications. Parasitol. Res. 2007, 100, 339–345. [Google Scholar] [CrossRef]
- Liu, F.; Xia, X.; Liu, N. Molecular basis of N,N-diethyl-3-methylbenzamide (DEET) in repelling the common bed bug, Cimex lectularius. Front. Physiol. 2017, 8, 1–13. [Google Scholar] [CrossRef]
- Adenubi, O.T.; Ahmed, A.S.; Fasina, F.O.; McGaw, L.J.; Eloff, J.N.; Naidoo, V. Pesticidal plants as a possible alternative to synthetic acaricides in tick control: A systematic review and meta-analysis. Ind. Crops Prod. 2018, 123, 779–806. [Google Scholar] [CrossRef]
- Khan, A.M.A.; Abbas, R.Z.; Sindhu, Z.D.; Mahmood, M.S. In vitro acaricidal and repellent effects of Amomum subulatum essential oil against Hyalomma ticks. Punjab Univ. J. Zool. 2023, 38, 211–219. [Google Scholar] [CrossRef]
- Koc, S.; Cengiz, A.; Polat, B.; Kokten, S.K.; Gultekin, Z.N.; Caliskan, C.; Tufan-Cetin, O.; Cetin, H. Evaluating the repellent effects of major essential oil components (Lamiaceae) on brown dog tick Rhipicephalus sanguineus sensu lato using the larval repellent activity test. Vet. Parasitol. 2025, 333, 110361. [Google Scholar] [CrossRef] [PubMed]
- Nota Técnica nº 1/2016—DEET em Produtos Cosméticos; Ministério da Saúde: Brasília, Brazil, 2016. Available online: https://www.gov.br/saude/pt-br/assuntos/notas-tecnicas/2016/nota-tecnica-1-2016-deet-em-produtos-cosmeticos (accessed on 24 April 2025).
- Nerio, L.S.; Olivero-Verbel, J.; Stashenko, E. Repellent activity of essential oils: A review. Bioresour. Technol. 2010, 101, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Kim, H.K.; Ahn, Y.J. Acaricidal activity of clove bud oil compounds against Dermatophagoides farinae and Dermatophagoides pteronyssinus (Acari: Pyroglyphidae). J. Agric. Food Chem. 2003, 51, 885–889. [Google Scholar] [CrossRef]
- Pålsson, K.; Jaenson, T.G.T.; Bæckström, P.; Borg-Karlson, A.K. Tick repellent substances in the essential oil of Tanacetum vulgare. J. Med. Entomol. 2008, 45, 88–93. [Google Scholar] [CrossRef]
- Martinez-Velazquez, M.; Castillo-Herrera, G.A.; Rosario-Cruz, R.; Flores-Fernandez, J.M.; Lopez-Ramirez, J.; Hernandez-Gutierrez, R.; Lugo-Cervantes, E.D.C. Acaricidal effect and chemical composition of essential oils extracted from Cuminum cyminum, Pimenta dioica and Ocimum basilicum against the cattle tick Rhipicephalus (Boophilus) microplus (Acari: Ixodidae). Parasitol. Res. 2011, 108, 481–487. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; Khalil, N.S.; Azeem, M.; Taher, E.A.; Göransson, U.; Pålsson, K.; Borg-Karlson, A.K. Chemical composition and repellency of essential oils from four medicinal plants against Ixodes ricinus nymphs (Acari: Ixodidae). J. Med. Entomol. 2012, 49, 1067–1075. [Google Scholar] [CrossRef]
- Zeringóta, V.; Senra, T.O.S.; Calmon, F.; Maturano, R.; Faza, A.P.; Catunda-Junior, F.E.A.; Monteiro, C.M.O.; Carvalho, M.G.; Daemon, E. Repellent activity of eugenol on larvae of Rhipicephalus microplus and Dermacentor nitens (Acari: Ixodidae). Parasitol. Res. 2013, 112, 2675–2679. [Google Scholar] [CrossRef]
- Lunguinho, A.S.; Cardoso, M.G.; Ferreira, V.R.F.; Konig, I.F.M.; Gonçalves, R.R.P.; Brandão, R.M.; Caetano, A.R.S.; Nelson, D.L.; Remedio, R.N. Acaricidal and repellent activity of the essential oils of Backhousia citriodora, Callistemon viminalis and Cinnamodendron dinisii against Rhipicephalus spp. Vet. Parasitol. 2021, 300, 109594. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Feng, Y.; Du, S.; Jia, L. Contact toxicity and repellent efficacy of essential oil from aerial parts of Melaleuca bracteata and its major compositions against three kinds of insects. J. Essent. Oil Bear. Plants 2021, 24, 349–359. [Google Scholar] [CrossRef]
- Alanazi, A.D.; Ben Said, M.; Shater, A.F.; Al-Sabi, M.N.S. Acaricidal, larvicidal, and repellent activity of Elettaria cardamomum essential oil against Hyalomma anatolicum ticks infesting Saudi Arabian cattle. Plants 2022, 11, 1221. [Google Scholar] [CrossRef]
- Alimi, D.; Hajri, A.; Jallouli, S.; Sebai, H. Toxicity, repellency, and anti-cholinesterase activities of bioactive molecules from clove buds Syzygium aromaticum L. as an ecological alternative in the search for control of Hyalomma scupense (Acari: Ixodidae). Heliyon 2023, 9, e18899. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhang, J.; Shen, L.; Wang, L.; Qian, C.; Lyu, H.; Yi, C.; Cai, J.; Chen, X.; Wen, X.; et al. Eugenol derivatives: Strong and long-lasting repellents against both undisturbed and disturbed red imported fire ants. J. Pest Sci. 2023, 96, 327–344. [Google Scholar] [CrossRef]
- Harris, R. Progress with superficial mycoses using essential oils. Int. J. Aromather. 2002, 12, 83–91. [Google Scholar] [CrossRef]
- Kamatou, G.P.; Vermaak, I.; Viljoen, A.M. Eugenol—From the remote Maluku Islands to the international marketplace: A review of a remarkable and versatile molecule. Molecules 2012, 17, 6953–6981. [Google Scholar] [CrossRef]
- Salehi, B.; Sharifi-Rad, J.; Quispe, C.; Llaique, H.; Villalobos, M.; Smeriglio, A.; Trombetta, D.; Ezzat, S.M.; Salem, M.A.; Zayed, A.; et al. Insights into Eucalyptus genus chemical constituents, biological activities and health-promoting effects. Trends Food Sci. Technol. 2019, 91, 609–624. [Google Scholar] [CrossRef]
- Soares, J.F.; Soares, H.S.; Barbieri, A.M.; Labruna, M.B. Experimental infection of the tick Amblyomma cajennense, Cayenne tick, with Rickettsia rickettsii, the agent of Rocky Mountain spotted fever. Med. Vet. Entomol. 2012, 26, 139–151. [Google Scholar] [CrossRef]
- Resende, J.D.S.A.; Daemon, E.; Monteiro, C.M.O.; Maturano, R.; Azevedo Prata, M.C.; Rodrigues, A.F.S.F. Toxicity of solvents and surfactants to Amblyomma cajennense (Fabricius, 1787) (Acari: Ixodidae) and Dermacentor nitens (Neumann, 1897) (Acari: Ixodidae) larvae. Exp. Parasitol. 2012, 131, 139–142. [Google Scholar] [CrossRef]
- Barrozo, M.M.; Zeringóta, V.; Borges, L.M.F.; Moraes, N.; Benz, K.; Farr, A.; Zhu, J.J. Repellent and acaricidal activity of coconut oil fatty acids and their derivative compounds and catnip oil against Amblyomma sculptum. Vet. Parasitol. 2021, 300, 109591. [Google Scholar] [CrossRef]
- Bissinger, B.W.; Apperson, C.S.; Sonenshine, D.E.; Watson, D.W.; Roe, R.M. Efficacy of the new repellent BioUD® against three species of ixodid ticks. Exp. Appl. Acarol. 2009, 48, 239–250. [Google Scholar] [CrossRef]
- Paula, L.G.F.; Zeringóta, V.; Sampaio, A.L.N.; Bezerra, G.P.; Barreto, A.L.G.; Santos, A.A.; Miranda, V.C.; Paula, W.V.F.; Neves, L.C.; Secchis, M.V.; et al. Seasonal dynamics of Amblyomma sculptum in two areas of the Cerrado biome, midwestern Brazil, where human cases of rickettsiosis have been reported. Exp. Appl. Acarol. 2021, 84, 215–225. [Google Scholar] [CrossRef]
- Neves, L.C.; Paula, W.V.F.; Paula, L.G.F.; Silva, B.B.F.; Dias, S.A.; Pereira, B.G.; Silva, B.S.A.; Sevá, A.P.; Dantas-Torres, F.; Labruna, M.B.; et al. Detection of Rickettsia spp. in animals and ticks in midwestern Brazil, where human cases of rickettsiosis were reported. Animals 2023, 13, 1288. [Google Scholar] [CrossRef] [PubMed]
- Bissinger, B.W.; Apperson, C.S.; Watson, D.W.; Arellano, C.; Sonenshine, D.E.; Roe, R.M. Novel field assays and the comparative repellency of BioUD®, DEET and permethrin against Amblyomma americanum. Med. Vet. Entomol. 2011, 25, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Martins, T.F.; Onofrio, V.C.; Barros-Battesti, D.M.; Labruna, M.B. Nymphs of the genus Amblyomma (Acari: Ixodidae) of Brazil: Descriptions, redescriptions, and identification key. Ticks Tick Borne Dis. 2010, 1, 75–99. [Google Scholar] [CrossRef]
- Suzin, A.; Rodrigues, V.S.; Ramos, V.N.; Szabó, M.P.J. Comparing scapular morphology of Amblyomma sculptum and Amblyomma dubitatum nymphs allows a fast and practical differential diagnosis of ticks in highly infested areas with dominance of these two species. Exp. Appl. Acarol. 2022, 86, 455–463. [Google Scholar] [CrossRef]
- Del-Fabbro, S.D.; Nazzi, F. From chemistry to behavior: Molecular structure and bioactivity of repellents against Ixodes ricinus ticks. PLoS ONE 2013, 8, e67832. [Google Scholar] [CrossRef]
- Wang, H.V.; Pickett, L.J.; Faraone, N. Repellent and acaricidal activities of basil (Ocimum basilicum) essential oils and rock dust against Ixodes scapularis and Dermacentor variabilis ticks. Exp. Appl. Acarol. 2022, 86, 583–598. [Google Scholar] [CrossRef]
- Senra, T.O.S.; Calmon, F.; Zeringóta, V.; Monteiro, C.M.O.; Maturano, R.; Matos, R.S.; Melo, D.; Gomes, G.A.; Carvalho, M.G.; Daemon, E. Investigation of activity of monoterpenes and phenylpropanoids against immature stages of Amblyomma cajennense and Rhipicephalus sanguineus (Acari: Ixodidae). Parasitol. Res. 2013, 112, 3471–3476. [Google Scholar] [CrossRef]
- Vale, F.L.; Paula, L.G.F.; Vieira, M.; Alves, S.G.A.; Moraes Junior, N.R.; Filgueiras, M.D.; Teixeira, W.F.P.; Rizzo, P.V.; Freitas, F.M.C.; Ferreira, L.L.; et al. Binary combinations of thymol, carvacrol and eugenol for Amblyomma sculptum control: Evaluation of in vitro synergism and effectiveness under semi-field conditions. Ticks Tick Borne Dis. 2021, 12, 101816. [Google Scholar] [CrossRef]
- Lopez, A.D.; Whyms, S.; Luker, H.A.; Galvan, C.J.; Holguin, F.O.; Hansen, I.A. Repellency of essential oils and plant-derived compounds against Aedes aegypti mosquitoes. Insects 2025, 16, 51. [Google Scholar] [CrossRef]
- Moemenbellah-Fard, M.D.; Shahriari-Namadi, M.; Kelidari, H.R.; Nejad, Z.B.; Ghasemi, H.; Osanloo, M. Chemical composition and repellent activity of nine medicinal essential oils against Anopheles stephensi, the main malaria vector. Int. J. Trop. Insect Sci. 2021, 41, 1325–1332. [Google Scholar] [CrossRef]
- Dadé, M.M.; Daniele, M.; Reyes-Novelo, E.; Rodriguez-Vivas, R.I. Lethal and repellent effect of amitraz, eugenol and thymol against Triatoma infestans, the main vector of Trypanosoma cruzi in South America. Med. Vet. Entomol. 2023, 37, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Lv, N.; Zhao, M.; Hao, L.; Zhou, X.; Chen, H.; Zhou, H. Eugenol and carboxymethyl cellulose-derived nanocoating with insect repellent and long-term antibacterial activity. Ind. Crops Prod. 2022, 190, 115902. [Google Scholar] [CrossRef]
- Wen, C.; Chen, J.; He, Y.; Wang, F.; Qian, C.; Wen, J.; Wen, X.; Wang, C. Electrophysiological and behavioral responses of red imported fire ants (Hymenoptera: Formicidae) to an essential balm and its components. Pest Manag. Sci. 2020, 77, 1971–1980. [Google Scholar] [CrossRef]
- Ali, A.; Cantrell, C.L.; Khan, I.A. A new in vitro bioassay system for the discovery and quantitative evaluation of mosquito repellents. J. Med. Entomol. 2017, 54, 1328–1336. [Google Scholar] [CrossRef]
- Brown, H.A.; Minott, D.A.; Ingram, C.W.; Williams, L.A.D. Biological activities of the extracts and constituents of pimento, Pimenta dioica L. against the southern cattle tick, Boophilus microplus. Int. J. Trop. Insect Sci. 1998, 18, 9–16. [Google Scholar] [CrossRef]
- Diaz, E.L.; Camberos, E.P.; Herrera, G.A.C.; Espinosa, M.E.; Andrews, H.E.; Buelnas, N.A.P.; Ortega, A.G.; Velázquez, M.M. Development of essential oil-based phyto-formulations to control the cattle tick Rhipicephalus microplus using a mixture design approach. Exp. Parasitol. 2019, 201, 26–33. [Google Scholar] [CrossRef]
- İnceboz, T.; Erkan, G.; Türkoğlu, G.C.; Sarıışık, A.M.; Bakırcı, S.; Üner, S.; Üner, A. In vivo and in vitro tick repellent properties of cotton fabric. Text. Res. J. 2015, 85, 2071–2082. [Google Scholar] [CrossRef]
- Behle, R.W.; Flor-Weiler, L.B.; Bharadwaj, A.; Stafford, K.C. A formulation to encapsulate nootkatone for tick control. J. Med. Entomol. 2011, 48, 1120–1127. [Google Scholar] [CrossRef]
- Oliveira, J.L.; Campos, E.V.R.; Germano-Costa, T.; Lima, R.; Vechia, J.F.D.; Soares, S.T.; Andrade, D.J.; Gonçalves, K.C.; Nascimento, J.; Polanczyk, R.A.; et al. Association of zein nanoparticles with botanical compounds for effective pest control systems. Pest Manag. Sci. 2019, 75, 1855–1865. [Google Scholar] [CrossRef]
- Caballero-Gallardo, K.; Pino-Benitez, N.; Pajaro-Castro, N.; Stashenko, E.; Olivero-Verbel, J. Plants cultivated in Chocó, Colombia, as source of repellents against Tribolium castaneum (Herbst). J. Asia-Pac. Entomol. 2014, 17, 753–759. [Google Scholar] [CrossRef]
- Novato, T.P.; Milhomem, M.N.; Marchesini, P.B.C.; Coutinho, A.L.; Silva, I.S.; Perinotto, W.M.S.; Prata, M.C.A.; Ferreira, L.L.; Lopes, W.D.Z.; Costa-Júnior, L.M.; et al. Acaricidal activity of carvacrol and thymol on acaricide-resistant Rhipicephalus microplus (Acari: Ixodidae) populations and combination with cypermethrin: Is there cross-resistance and synergism? Vet. Parasitol. 2022, 310, 109787. [Google Scholar] [CrossRef] [PubMed]
- Tavares, C.P.; Sousa, I.C.; Gomes, M.N.; Miró, V.; Virkel, G.; Lifschitz, A.; Costa-Junior, L.M. Combination of cypermethrin and thymol for control of Rhipicephalus microplus: Efficacy evaluation and description of an action mechanism. Ticks Tick Borne Dis. 2022, 13, 101874. [Google Scholar] [CrossRef] [PubMed]
- Gonzaga, B.C.F.; Moraes, N.R.; Gomes, G.W.; Coutinho, A.L.; Vale, F.L.; Sousa, L.J.M.P.; Marrero, L.; Rodrigues, D.C.; Prata, M.C.A.; Marchesini, P.; et al. Combination of synthetic acaricides with (E)-cinnamaldehyde to control Rhipicephalus microplus. Exp. Appl. Acarol. 2022, 88, 191–207. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.L.C.; Marreto, L.C.N.L.; Vale, F.L.; Sousa, L.J.M.P.; Gonzaga, B.C.F.; Silva, I.S.; Santos, E.F.; Lopes, F.F.S.; Morais, S.M.M.; Lopes, W.D.Z.; et al. Combinations of amitraz with essential oils from Lippia sidoides and Thymus vulgaris, thymol and thymol acetate for Rhipicephalus microplus control: Studies under laboratory and field conditions. Vet. Parasitol. 2023, 321, 109997. [Google Scholar] [CrossRef]
- Le Mauff, A.; Cartereau, A.; Plantard, O.; Taillebois, E.; Thany, S.H. Effect of the combination of DEET and flupyradifurone on the tick Ixodes ricinus: Repellency bioassay and pharmacological characterization using microtransplantation of synganglion membranes. Ticks Tick Borne Dis. 2023, 14, 102079. [Google Scholar] [CrossRef]
- Burtis, J.C.; Ford, S.L.; Parise, C.M.; Eisen, R.J.; Eisen, L. Efficacy of unregulated minimum risk tick repellent products evaluated with Ixodes scapularis nymphs in a human skin bioassay. Parasites Vectors 2024, 17, 1–8. [Google Scholar] [CrossRef]
- Menon, K.S.; Brown, A.E. Exposure of children to DEET and other topically applied insect repellents. Am. J. Ind. Med. 2005, 47, 91–97. [Google Scholar] [CrossRef]
- Legeay, S.; Clere, N.; Apaire-Marchais, V.; Faure, S.; Lapied, B. Unusual modes of action of the repellent DEET in insects highlight some human side effects. Eur. J. Pharmacol. 2018, 825, 92–98. [Google Scholar] [CrossRef]
- Consulta de Cosméticos Registrados: Repelentes; Agência Nacional de Vigilância Sanitária (ANVISA): Brasília, Brazil, 2025. Available online: https://consultas.anvisa.gov.br/#/cosmeticos/registrados/q/?nomeProduto=repelente (accessed on 23 February 2025).
- Ikawati, S.; Himawan, T.; Abadi, A.L.; Tarno, H. Thermostability, photostability, and toxicity of clove oil nanoparticles against Cryptolestes ferrugineus (Stephens) (Coleoptera: Laemophloeidae). Biodiversitas 2020, 21, 4764–4771. [Google Scholar] [CrossRef]
- Costa, J.R.S.; Vale, T.L.; Silva, G.F.; Silva, N.C.S.; Lima, A.S.; Costa-Júnior, L.M.; Luz, H.R. Encapsulation of carvacrol and thymol with yeast cell wall and its repellent activity against Amblyomma sculptum and Rhipicephalus sanguineus (sensu lato). Exp. Appl. Acarol. 2024, 92, 555–565. [Google Scholar] [CrossRef]
- Taveira, S.F.; Marreto, R.N.; Monteiro, C.M.O.; Lul, W.C.; Oliveira, G.A.R.; Pereira, G.; Souza, B.S.; Damaceno, G.B.R.; Cardoso, G. Composição e Método Para Reduzir a Permeação Cutânea de Substâncias Repelentes na Pele, Aumentando a Segurança e Eficácia de Formulações Repelentes de Mosquitos e Carrapatos. Brazil Patent BR10202301238, 2023. [Google Scholar]



| Evaluation Time | Control (Ethanol) | Control (Blank) | 2% Eugenol | 2% Methyl Eugenol | 2% 1,8-Cineole | |
|---|---|---|---|---|---|---|
| Minutes | 1 | 56.7 ± 27.4 | 53.3 ± 19.0 | 70.0 * ± 20.4 | 73.3 * ± 18.3 | 53.3 ± 30.3 |
| 15 | 50.0 ± 7.5 | 45.0 ± 11.8 | 81.7 * ± 13.9 | 85.0 * ± 21.7 | 56.7 ± 34.2 | |
| 30 | 50.0 ± 13.9 | 40.0 ± 19.0 | 75.0 * ± 11.8 | 83.3 * ± 0.0 | 65.0 * ± 27.4 | |
| Hours | 1 | 45.0 ± 20.4 | 50.0 ± 13.9 | 90.0 * ± 9.1 | 76.7 * ± 14.9 | 51.7 ± 27.9 |
| 2 | 46.7 ± 21.7 | 33.3 ± 23.6 | 86.7 * ± 14.9 | 73.3 * ± 13.9 | 55.0 ± 19.0 | |
| 3 | 50.0 ± 14.9 | 38.3 ± 23.6 | 88.3 * ± 9.1 | 88.3 * ± 7.5 | 76.7 * ± 19.0 | |
| 4 | 51.7 ± 14.9 | 41.7 ± 16.7 | 83.3 * ± 0.0 | 70.0 * ± 18.3 | 61.7 ± 19.0 | |
| 24 | 50.0 ± 14.9 | 51.7 ± 11.8 | 73.3 * ± 14.9 | 85.0 * ± 14.9 | 56.7 ± 14.9 | |
| 48 | 55.0 ± 7.5 | 63.3 * ± 19.0 | 66.7 * ± 27.4 | 75.0 * ± 21.7 | 65.0 * ± 16.7 | |
| 72 | 41.7 ± 25.3 | 63.3 * ± 19.0 | 60.0 ± 19.0 | 73.3 * ± 18.3 | 55.0 ± 22.4 | |
| 96 | 55.0 ± 30.3 | 50.0 ± 13.9 | 61.7 ± 25.3 | 55.0 ± 21.4 | 53.3 ± 21.7 | |
| 168 | 48.3 ± 30.3 | 70.0 * ± 14.9 | 71.7 * ± 23.6 | 61.7 ± 16.7 | 55.0 ± 29.8 | |
| Mean repellency (%) ± standard deviation | 50.0 ± 4.5 | 50.0 ± 8.0 | 75.7 ± 8.2 | 78.3 ± 9.8 | 58.8 ± 5.2 | |
| Evaluation Time | Control (Ethanol) | Control (Blank) | 1% Methyl Eugenol | 2% Methyl Eugenol | 7% Deet | 7% Deet + 1% Methyl Eugenol | 7% Deet + 2% Methyl Eugenol | |
|---|---|---|---|---|---|---|---|---|
| Minutes | 1 | 56.7 ± 22.5 | 51.7 ± 22.8 | 78.3 * ± 17.7 | 86.7 * ± 15.3 | 83.3 * ± 17.6 | 96.7 * ± 7.0 | 100.0 * ± 0.0 |
| 15 | 51.7 ± 16.6 | 41.7 ± 18.0 | 83.3 * ± 15.7 | 91.7 * ± 8.8 | 85.0 * ± 18.3 | 93.3 * ± 8.6 | 100.0 * ± 0.0 | |
| 30 | 48.3 ± 21.4 | 40.0 ± 22.5 | 85.0 * ± 14.6 | 91.7 * ± 8.8 | 91.7 * ± 14.2 | 96.7 * ± 7.0 | 96.7 * ± 10.5 | |
| Hours | 1 | 48.3 ± 20.0 | 53.3 ± 15.3 | 85.0 * ± 16.6 | 96.7 * ± 7.0 | 91.7 * ± 11.8 | 90.0 * ± 17.9 | 100.0 * ± 0.0 |
| 2 | 56.7 ± 19.6 | 41.7 ± 21.2 | 88.3 * ± 13.7 | 71.7 * ± 20.9 | 89.7 * ± 14.3 | 90.0 * ± 17.9 | 95.0 * ± 11.2 | |
| 3 | 48.3 ± 12.3 | 28.1 ± 21.0 | 88.3 * ± 13.7 | 86.7 * ± 13.1 | 88.3 * ± 13.7 | 88.3 * ± 17.7 | 95.0 * ± 8.1 | |
| 4 | 53.3 ± 17.2 | 48.3 ± 22.8 | 93.3 * ± 16.1 | 90.0 * ± 16.1 | 85.0 * ± 20.0 | 90.0 * ± 17.9 | 90.0 * ± 14.1 | |
| 24 | 40.0 ± 17.9 | 48.3 ± 14.6 | 71.7 * ± 23.6 | 83.3 * ± 13.6 | 68.3 * ± 24.2 | 71.7 * ± 29.4 | 73.3 * ± 29.6 | |
| 48 | 43.3 ± 11.7 | 55.0 ± 20.9 | 76.7 * ± 28.5 | 65.0 * ± 16.6 | 55.0 ± 15.8 | 68.3 * ± 34.6 | 63.3 * ± 36.7 | |
| 72 | 45.0 ± 19.3 | 51.7 ± 20.0 | 71.7 * ± 11.2 | 61.7 ± 19.3 | 48.3 ± 25.4 | 73.3 * ± 23.8 | 76.7 * ± 19.6 | |
| 96 | 56.7 ± 26.3 | 56.7 ± 17.9 | 57.0 ± 17.1 | 66.7 * ± 20.8 | 43.3 ± 21.1 | 66.7 * ± 33.3 | 71.7 * ± 17.7 | |
| 168 | 42.8 ± 29.4 | 53.3 ± 29.2 | 51.7 ± 21.4 | 55.0 ± 22.3 | 60.0 ± 29.6 | 70.0 * ± 21.9 | 63.3 * ± 18.9 | |
| Mean repellency (%) ± standard deviation | 49.3 ± 4.2 | 47.5 ± 6.3 | 77.5 ± 3.7 | 78.9 ± 9.6 | 74.1 ± 3.9 | 82.9 ± 3.4 | 85.4 ± 2.4 | |
| Evaluation Time (Minutes) | Control (Ethanol) | Control (Blank) | 1% Eugenol | 2% Eugenol | 7% Deet | 7% Deet + 1% Eugenol | 7% Deet + 2% Eugenol | |
|---|---|---|---|---|---|---|---|---|
| Minutes | 1 | 53.3 ± 18.9 | 53.3 ± 25.8 | 73.3 * ± 22.5 | 85.0 * ± 14.6 | 83.3 * ± 17.6 | 98.3 * ± 5.3 | 93.3 * ± 8.6 |
| 15 | 60.0 ± 19.6 | 36.7 ± 15.3 | 68.3 * ± 16.6 | 88.3 * ± 13.7 | 85.0 * ± 18.3 | 86.7 * ± 18.9 | 90.0 * ± 11.7 | |
| 30 | 50.0 ± 24.8 | 41.7 ± 22.6 | 76.7 * ± 14.1 | 93.3 * ± 11.7 | 91.7 * ± 14.2 | 91.7 * ± 11.8 | 85.0 * ± 14.6 | |
| Hours | 1 | 41.7 ± 21.2 | 55.0 ± 13.7 | 86.7 * ± 15.3 | 91.7 * ± 11.8 | 91.7 * ± 11.8 | 91.7 * ± 8.8 | 83.3 * ± 22.2 |
| 2 | 55.0 ± 20.9 | 46.7 ± 13.1 | 91.7 * ± 14.2 | 88.3 * ± 13.7 | 89.7 * ± 14.3 | 93.3 * ± 8.6 | 88.3 * ± 13.7 | |
| 3 | 50.0 ± 11.1 | 33.1 ± 19.5 | 93.3 * ± 11.7 | 91.7 * ± 8.8 | 88.3 * ± 13.7 | 93.3 * ± 8.6 | 86.7 * ± 15.3 | |
| 4 | 50.0 ± 22.2 | 60.0 ± 14.1 | 85.0 * ± 18.3 | 81.7 * ± 21.4 | 85.0 * ± 20.0 | 95.0 * ± 8.1 | 88.3 * ± 15.8 | |
| 24 | 38.3 ± 23.6 | 41.7 ± 19.6 | 79.2 * ± 18.9 | 81.7 * ± 16.6 | 68.3 * ± 24.2 | 85.0 * ± 25.4 | 88.3 * ± 15.8 | |
| 48 | 41.7 ± 11.8 | 48.3 ± 20.0 | 68.8 * ± 9.2 | 81.7 * ± 18.3 | 55.0 ± 15.8 | 85.0 * ± 25.4 | 81.7 * ± 14.6 | |
| 72 | 43.3 ± 11.7 | 46.7 ± 13.1 | 70.0 * ± 18.9 | 77.5 * ± 15.7 | 48.3 ± 25.4 | 78.3 * ± 23.6 | 83.3 * ± 19.2 | |
| 96 | 46.7 ± 27.0 | 56.7 ± 16.1 | 62.2 ± 22.9 | 58.3 ± 23.9 | 43.3 ± 21.1 | 80.0 * ± 24.6 | 76.7 * ± 16.1 | |
| 168 | 41.2 ± 28.4 | 46.7 ± 13.1 | 74.2 * ± 31.0 | 75.8 * ± 21.0 | 60.0 ± 29.6 | 78.3 * ± 22.3 | 81.0 * ± 15.5 | |
| Mean repellency (%) ± standard deviation | 47.6 ± 6.8 | 47.2 ± 7.7 | 77.6 ± 9.6 | 82.9 ± 3.2 | 74.1 ± 3.9 | 88.1 ± 4.2 | 85.5 ± 4.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barrozo, M.M.; Chagas, H.D.F.; Damaceno, G.B.R.; Santos, E.F.; Carvalho, R.A.; Silva, I.S.; Vale, F.L.; Sousa, L.J.M.P.e.; Luz, H.R.; Ferreira, L.L.; et al. Repellent Activity of DEET Combined with Botanical Compounds Against Amblyomma sculptum Nymphs: Laboratory and Field Evaluations. Pathogens 2025, 14, 495. https://doi.org/10.3390/pathogens14050495
Barrozo MM, Chagas HDF, Damaceno GBR, Santos EF, Carvalho RA, Silva IS, Vale FL, Sousa LJMPe, Luz HR, Ferreira LL, et al. Repellent Activity of DEET Combined with Botanical Compounds Against Amblyomma sculptum Nymphs: Laboratory and Field Evaluations. Pathogens. 2025; 14(5):495. https://doi.org/10.3390/pathogens14050495
Chicago/Turabian StyleBarrozo, Mayara Macêdo, Haile Dean Figueiredo Chagas, Gabrielly Bernardes Rodrigues Damaceno, Emilly Faria Santos, Rafael Assunção Carvalho, Isabela Santos Silva, Francisca Letícia Vale, Lainny Jordana Martins Pereira e Sousa, Hermes Ribeiro Luz, Lorena Lopes Ferreira, and et al. 2025. "Repellent Activity of DEET Combined with Botanical Compounds Against Amblyomma sculptum Nymphs: Laboratory and Field Evaluations" Pathogens 14, no. 5: 495. https://doi.org/10.3390/pathogens14050495
APA StyleBarrozo, M. M., Chagas, H. D. F., Damaceno, G. B. R., Santos, E. F., Carvalho, R. A., Silva, I. S., Vale, F. L., Sousa, L. J. M. P. e., Luz, H. R., Ferreira, L. L., & Monteiro, C. (2025). Repellent Activity of DEET Combined with Botanical Compounds Against Amblyomma sculptum Nymphs: Laboratory and Field Evaluations. Pathogens, 14(5), 495. https://doi.org/10.3390/pathogens14050495







