Spatial Distribution of Equid Exposure to Rickettsia spp. in Goiás State, Midwestern Brazil
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Locations and Sampling Calculation
2.2. Serological Testing
2.3. Statistical and Risk Factors Analysis
2.4. Blood Samples and Ticks
2.5. DNA Extraction and Molecular Detection of Rickettsia
3. Results
3.1. Collected Samples and Serology of Equids—Samples from Agrodefesa
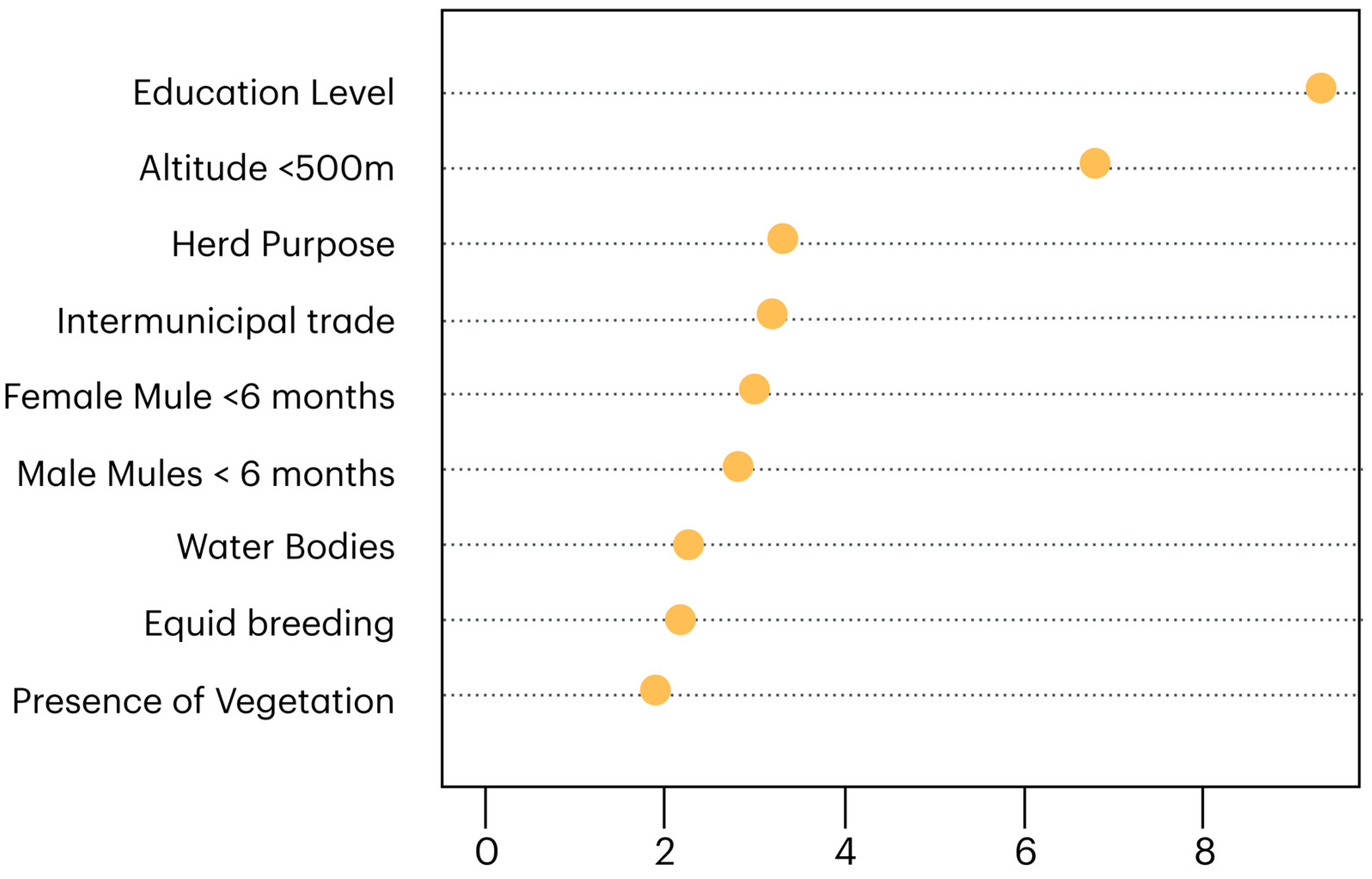
3.2. Risk Factors
3.3. Tick Identification and Serology of Equids—Field Expedition
3.4. Molecular Detection of Rickettsia spp.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El Karkouri, K.; Ghigo, E.; Raoult, D.; Fournier, P.-E. Genomic evolution and adaptation of arthropod-associated Rickettsia. Sci. Rep. 2022, 12, 3807. [Google Scholar] [CrossRef] [PubMed]
- Merhej, V.; Angelakis, E.; Socolovschi, C.; Raoult, D. Genotyping, evolution and epidemiological findings of Rickettsia species. Infect. Genet. Evol. 2014, 25, 122–137. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.; Blanton, L.S.; Walker, D.H. Rickettsiae as emerging infectious agents. Clin. Lab. Med. 2017, 37, 383–400. [Google Scholar] [CrossRef]
- Labruna, M.B. Ecology of Rickettsia in South America. Ann. N. Y. Acad. Sci. 2009, 1166, 156–166. [Google Scholar] [CrossRef]
- Souza, Z.Ê.S.; Moraes, B.V.; Krawczak, F.S.; Zulzke, L.; Carvalho, T.V.; Sousa, A.O.; Agopian, R.G.; Marcili, A.; Labruna, M.B.; Moraes-Filho, J. Detection of anti-Rickettsia rickettsii antibodies in dogs living in a neglected área in São Paulo, SP, Brazil. Arq. Bras. Med. Vet. Zootec. 2020, 72, 2141–2147. [Google Scholar] [CrossRef]
- Richardson, E.A.; Roe, R.M.; Apperson, C.S.; Ponnusamy, L. Rickettsia amblyommatis in ticks: A review of distribution, pathogenicity, and diversity. Microorganisms 2023, 11, 493. [Google Scholar] [CrossRef]
- Szabó, M.P.J.; Pinter, A.; Labruna, M.B. Ecology, biology and distribution of spotted-fever tick vectors in Brazil. Front. Cell. Infect. Microbiol. 2013, 3, 27. [Google Scholar] [CrossRef]
- Labruna, M.B.; Kasai, N.; Ferreira, F.; Faccini, J.L.H.; Gennari, S.M. Seasonal dynamics of ticks (Acari: Ixodidae) on horses in the state of São Paulo, Brazil. Vet. Parasitol. 2002, 105, 65–77. [Google Scholar] [CrossRef]
- Ueno, T.E.H.; Costa, F.B.; Moraes-Filho, J.; Agostinho, W.C.; Fernandes, W.R.; Labruna, M.B. Experimental infection of horses with Rickettsia rickettsii. Parasites Vectors 2016, 9, 1–11. [Google Scholar] [CrossRef]
- Halliday, J.E.B.; Meredith, A.L.; Knobel, D.L.; Shaw, D.J.; Bronsvoort, B.M.C.; Cleaveland, S. A framework for evaluating animals as sentinels for infectious disease surveillance. J. R. Soc. Interface 2007, 4, 973–984. [Google Scholar] [CrossRef]
- Ministério da Saúde. Casos confirmados e óbitos por Febre Maculosa. Brasil, Grandes Regiões e Unidades Federadas (infecção) 2007–2025. Sistema de Informação de Agravos de Notificação, Brazil. 2025. Available online: https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/f/febre-maculosa/situacao-epidemiologica (accessed on 13 February 2025).
- Paula, L.G.F.; Zeringóta, V.; Sampaio, A.L.N.; Bezerra, G.P.; Barreto, A.L.G.; Santos, A.A.; Miranda, V.C.; Paula, W.V.F.; Neves, L.C.N.; Secchis, M.V.; et al. Seasonal dynamics of Amblyomma sculptum in two areas of the Cerrado biome midwestern Brazil, where human cases of rickettsiosis have been reported. Exp. Appl. Acarol. 2021, 84, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Instituto Brasileiro de Geografia e Estatística. Available online: https://www.ibge.gov.br (accessed on 1 October 2024).
- Coutinho, L.M. O conceito de bioma. Acta Bot. Bras. 2006, 20, 13–23. [Google Scholar] [CrossRef]
- Françoso, R.D.; Brandão, R.; Nogueira, C.C.; Salmona, Y.B.; Machado, R.B.; Colli, G.R. Habitat loss and the effectiveness of protected areas in the Cerrado biodiversity hotspot. Nat. Conserv. 2015, 13, 35–40. [Google Scholar] [CrossRef]
- Brasil. Governo de Goiás. Conheça Goiás—Geografia. 2023. Available online: https://goias.gov.br/geografia (accessed on 1 October 2024).
- Pádua, B.R.; Dias, R.A.; Fioravanti, M.C.S.; Borsanelli, A.C. Seroprevalence and risk factors associated with equine infectious anemia in the state of Goiás, Brazil. Prev. Vet. Med. 2022, 209, 105781. [Google Scholar] [CrossRef]
- Horta, M.C.; Labruna, M.B.; Sangioni, L.A.; Vianna, M.C.B.; Gennari, M.S.; Galvão, M.A.M. Prevalence of antibodies to spotted fever group rickettsiae in humans and domestic animals in a Brazilian spotted fever endemic area in the state of S.o Paulo, Brazil: Serological evidence for infection by Rickettsia rickettsii and another spotted fever groups Rickettsia. Am. J. Trop. Med. Hyg. 2004, 71, 93–97. [Google Scholar]
- Labruna, M.B.; Horta, M.C.; Aguiar, D.M.; Cavalcante, G.T.; Pinter, A.; Gennari, S.M.; Camargo, C.M.A. Prevalence of Rickettsia infection in dogs from the urban and rural areas of Monte Negro municipality, western Amazon, Brazil. Vector Borne Zoonotic Dis. 2007, 7, 249–255. [Google Scholar] [CrossRef]
- Barbieri, A.R.M.; Filho, J.M.; Nieri-Bastos, F.A.; Souza, J.C.; Szabó, M.P.J.; Labruna, M.B. Epidemiology of Rickettsia sp. strain Atlantic rainforest in a spotted fever endemic area of southern Brazil. Ticks Tick-Borne Dis. 2014, 5, 848–853. [Google Scholar] [CrossRef]
- Szabó, M.P.J.; Nieri-Bastos, F.A.; Spolidorio, M.G.; Martins, T.F.; Barbieri, A.M.; Labruna, M.B. In vitro isolation from Amblyomma ovale (Acari: Ixodidae) and ecologiacal aspects of the Atlantic rainforest Rickettsia, the causative agent of a new spotted fever rickettsiosis in Brazil. Parasitology 2013, 140, 719–728. [Google Scholar] [CrossRef]
- R core team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, 2022. Available online: https://www.R-project.org/ (accessed on 11 September 2024).
- Terassini, F.A.; Barbieri, F.S.; Albuquerque, S.; Szabó, M.P.J.; Camargo, L.M.A.; Labruna, M.M. Comparison of two methods for collecting free-living ticks in the Amazonian Forest. Ticks Tick-Borne Dis. 2010, 1, 194–196. [Google Scholar] [CrossRef]
- de Paula, L.G.F.; do Nascimento, R.M.; Franco, A.D.; Szabó, M.P.J.; Labruna, M.B.; Monteiro, C.; Krawczak, F.S. Seasonal dynamics of Amblyomma sculptum: A review. Parasites Vectors 2022, 15, 193. [Google Scholar] [CrossRef]
- Barros-Battesti, D.M.; Arzua, M.; Bechara, G.H. Carrapatos de Importância Médico-Veterinária da Região Neotropical: Um Guia Ilustrado para Identificacão de Espécies; Vox/ICTTD-3,/Butantan: São Paulo, Brazil, 2006; p. 223. Available online: https://repositorio.butantan.gov.br/handle/butantan/3153 (accessed on 2 April 2024).
- Martins, T.F.; Onofrio, V.C.; Barros-Battesti, D.M.; Labruna, M.B. Nymphs of the genus Amblyomma (Acari: Ixodidae) of Brazil: Descriptions, redescriptions, and identification Key. Ticks Tick-Borne Dis. 2010, 1, 75–99. [Google Scholar] [CrossRef] [PubMed]
- Dantas-Torres, F.; Martins, T.F.; Murioz-Leal, S.; Onofrio, V.C.; Barros-Battesti, D.M. Ticks (Ixodida: Argasidae, Ixodidae) of Brazil: Updated species checklist and taxonomic keys. Ticks Tick-Borne Dis. 2019, 10, 101252. [Google Scholar] [CrossRef] [PubMed]
- Martins, T.F.; Barbieri, A.R.M.; Costa, F.B.; Terassini, F.A.; Camargo, L.M.A.; Peterka, C.R.L.; Pacheco, R.d.C.; Dias, R.A.; Nunes, P.H.; Marcili, A.; et al. Geographical distribution of Amblyomma cajennense (sensu lato) ticks (Parasitiformes: Ixodidae) in Brazil, with description of the nymph of A. cajennense (sensu stricto). Parasites Vectors 2016, 9, 186. [Google Scholar] [CrossRef]
- Nava, S.; Beati, L.; Labruna, M.B.; Cáceres, A.G.; Mangold, A.J.; Guglielmone, A.A. Reassessment of the taxonomic status of Amblyomma cajennense (Fabricius, 1787) with the description of three new species, Amblyomma tonelliae n. sp., Amblyomma interandinum n. sp. and Amblyomma patinoi n. sp., and reinstatement of Amblyomma mixtum Koch, 1. Ticks Tick-Borne Dis. 2014, 5, 252–276. [Google Scholar] [CrossRef]
- Clifford, C.M.; Anastos, G.; Elbl, A. The Larval Ixodid Ticks of the Eastern United States (Acarina-Ixodidae); Miscellaneous; Publications of the Entomological Society of America: College Park, MD, USA, 1961; Volume 2, pp. 213–237. [Google Scholar] [CrossRef]
- Sangioni, L.A.; Horta, M.C.; Vianna, M.C.; Gennari, S.M.; Soares, R.M.; Galvão, M.A.; Schumaker, T.T.; Ferreira, F.; Vidotto, O.; Labruna, M.B. Rickettsial infection in animals and Brazilian spotted fever endemicity. Emerg. Infect. Dis. 2005, 11, 265–270. [Google Scholar] [CrossRef]
- Labruna, M.B.; Whitworth, T.; Horta, M.C.; Bouyer, D.H.; Mcbride, J.W.; Pinter, A.; Popov, V.; Gennari, S.M.; Walker, D.H. Rickettsia species infecting Amblyomma cooperi Ticks from an area in the state of São Paulo Brazil, where Brazilian spotted fever is endemic. J. Clin. Microbiol. 2004, 42, 90–98. [Google Scholar] [CrossRef]
- Guedes, E.; Leite, R.C.; Prata, M.C.A.; Pacheco, R.C.; Walker, D.H.; Labruna, M.B. Detection of Rickettsia rickettsii in the tick Amblyomma cajennense in a new Brazilian spotted fever endemic area in the state of Minas Gerais. Mem. Inst. Oswaldo Cruz 2005, 100, 841–845. [Google Scholar] [CrossRef]
- Regnery, R.L.; Spruil, C.L.; Plikaytis, B.D. Genotipic identification of rickettsiae and estimation of intraspecific sequences divergence for portion of two Rickettsial gene. J. Bacteriol. 1991, 173, 1576–1589. [Google Scholar] [CrossRef]
- Vitorino, L.; Chelo, I.M.; Bacellar, F.; Zé-Zé, L. Rickettsiae phylogeny: A multigenic approach. Microbiology 2007, 153, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Mangold, A.J.; Bargues, M.D.; Mas-Coma, S. Mitochondrial 16S rDNA sequences and phylogenetic relationships of species of Rhipicephalus and other tick genera among Metastriata (Acari: Ixodidae). Parasitol. Res. 1998, 84, 478–484. [Google Scholar] [CrossRef]
- Neves, L.C.; Paula, W.V.F.; de Paula, L.G.F.; Silva, B.B.F.; Dias, S.A.; Pereira, B.G.; Silva, B.S.A.; Sevá, A.P.; Dantas-Torres, F.; Labruna, M.B.; et al. Detection of Rickettsia spp. in animals and ticks in midwestern Brazil, where human cases of rickettsiosis were reported. Animals 2023, 13, 1288. [Google Scholar] [CrossRef] [PubMed]
- Amorin Filho, E.F.; Costa, F.B.; Moraes-Filho, J.; Santos, A.C.G.; Vale, T.L.; Costa, A.P.; Silva, A.B.; Labruna, M.B.; Nogueira, R.M.S. Exposure of Baixadeiro horses to Rickettsia spp. and to ticks infected by Rickettsia amblyommatis in the Baixada Maranhense micro-region, Maranhão, Brazil. Ciência Rural 2018, 48, e20180002. [Google Scholar] [CrossRef]
- Souza, C.E.; Camargo, L.B.; Pinter, A.; Donalisio, M.R. High Seroprevalence for Rickettsia rickettsii in equines suggests risk of human infection in silent areas for the Brazilian Spotted Fever. PLoS ONE 2016, 11, e0153303. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, P.B.; Harvey, T.V.; Fehlberg, H.F.; Rocha, J.M.; Martins, T.F.; Acosta, I.C.L.; Labruna, M.B.; Faccini, J.L.H.; Albuquerque, G.R. Serologic and molecular survey of Rickettsia spp. in dogs, horses and ticks from the Atlantic rainforest of the state of Bahia, Brazil. Exp. Appl. Acarol. 2019, 78, 431–442. [Google Scholar] [CrossRef]
- Neves, L.C.; Barreto, A.L.G.; Souza, M.X.; Martins, D.B.; Barbieri, A.R.M.; Serpa, M.C.A.; Muñoz-Leal, S.; Labruna, M.B.; Krawczak, F.S. Serosurvey on rickettsiae of the spotted fever group and Rickettsia bellii among dogs in the state of Goiás, Brazil. Braz. J. Vet. Parasitol. 2020, 29, e021419. [Google Scholar] [CrossRef]
- Paludo, R.L.d.R.; Paula, W.V.d.F.; Neves, L.C.; de Paula, L.G.F.; de Lima, N.J.; da Silva, B.B.F.; Pereira, B.G.; Padua, G.T.; Dantas-Torres, F.; Labruna, M.B.; et al. Rickettsial infection in ticks from a National Park in the Cerrado biome, midwestern Brazil. Pathogens 2024, 13, 13. [Google Scholar] [CrossRef]
- de Lima, N.J.; Pádua, G.T.; Cardoso, E.R.; Bittencourt, R.B.M.; Tavares, M.A.; Paula, W.V.d.F.; Neves, L.C.; Segovia, C.D.; Santos, G.C.; Serpa, M.C.d.A.; et al. Serological and molecular survey of rickettsial agents in wild boars (Sus. scrofa) from Midwestern Brazil. Animals 2024, 14, 2224. [Google Scholar] [CrossRef]
- Ueno, T.E.H.; Cutolo, A.A.; Martins, T.F.; Moraes-Filho, J.; Azevedo, S.S.; Labruna, M.B. Rickettsial infection in equids, opossums and ticks in the municipality of Monte Mor, state of São Paulo, Brazil. Braz. J. Vet. Parasitol. 2020, 29, e015420. [Google Scholar] [CrossRef]
- Costa, F.B.; da Costa, A.P.; Moraes-Filho, J.; Martins, T.F.; Soares, H.S.; Ramirez, D.G.; Dias, R.A.; Labruna, M.B. Rickettsia amblyommatis infecting ticks and exposure of domestic dogs to Rickettsia spp. in na Amazon-Cerrado transition region of northeastern Brazil. PLoS ONE 2017, 12, e0179163. [Google Scholar] [CrossRef]
- Alves, A.S.; Melo, A.L.T.; Amorin, M.V.; Borges, A.M.C.M.; Silva, L.G.; Martins, T.F.; Labruna, M.B.; Aguiar, D.M.; Pacheco, R.C. Seroprevalence of Rickettsia spp. in equids and molecular detection of ‘Candidatus Rickettsia amblyommii’ in Amblyomma cajennense sensu lato ticks from the pantanal region of Mato Grosso, Brazil. J. Med. Entomol. 2014, 51, 1242–1247. [Google Scholar] [CrossRef]
- Bermúdez, C.S.; Zaldívar, A.Y.; Spolidorio, M.G.; Moraes-Filho, J.; Miranda, R.J.; Caballero, C.M.; Mendoza, Y.; Labruna, M.B. Rickettsial infection in domestic mammals and their ectoparasites in El Valle de Antón, Coclé, Panamá. Vet. Parasitol. 2011, 177, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez, S.C.; Zaldívar, Y.; Domínguez, L.A.; Hernández, M.; de Antinori, M.E.B.; Krawczak, F.S. Rickettsia amblyommatis isolated from Amblyomma mixtum (Acari: Ixodida) from two sites in Panama. Ticks Tick-Borne Dis. 2021, 12, 101597. [Google Scholar] [CrossRef] [PubMed]
- Karpathy, S.E.; Slater, K.S.; Goldsmith, C.S.; Nicholson, W.L.; Paddock, C.D. Rickettsia amblyommatis sp. Nov., a spotted fever group Rickettsia associated with multiple species of Amblyomma ticks in North, Central and South America. Int. J. Syst. Evol. Microbiol. 2016, 66, 5236–5243. [Google Scholar] [CrossRef]
- Han, H.; Guo, X.; Yu, H. Variable selection using mean decrease accuracy and mean decrease gini based on random forest. In Proceedings of the 2016 7th IEEE International Conference on Software Engineering and Service Science (ICSESS), Beijing, China, 26–28 August 2016; pp. 219–224. [Google Scholar]
- Nicodemus, K.K. On the stability and ranking of predictors from random forest variable importance measures. Brief. Bioinform. 2011, 12, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Silveira, I.; Martins, T.F.; Olegário, M.M.; Peterka, C.; Guedes, E.; Ferreira, F.; Labruna, M.B. Rickettsial infection in animals, humans and ticks in Paulicéia, Brazil. Zoon. Publ. Health 2015, 62, 525–533. [Google Scholar] [CrossRef]
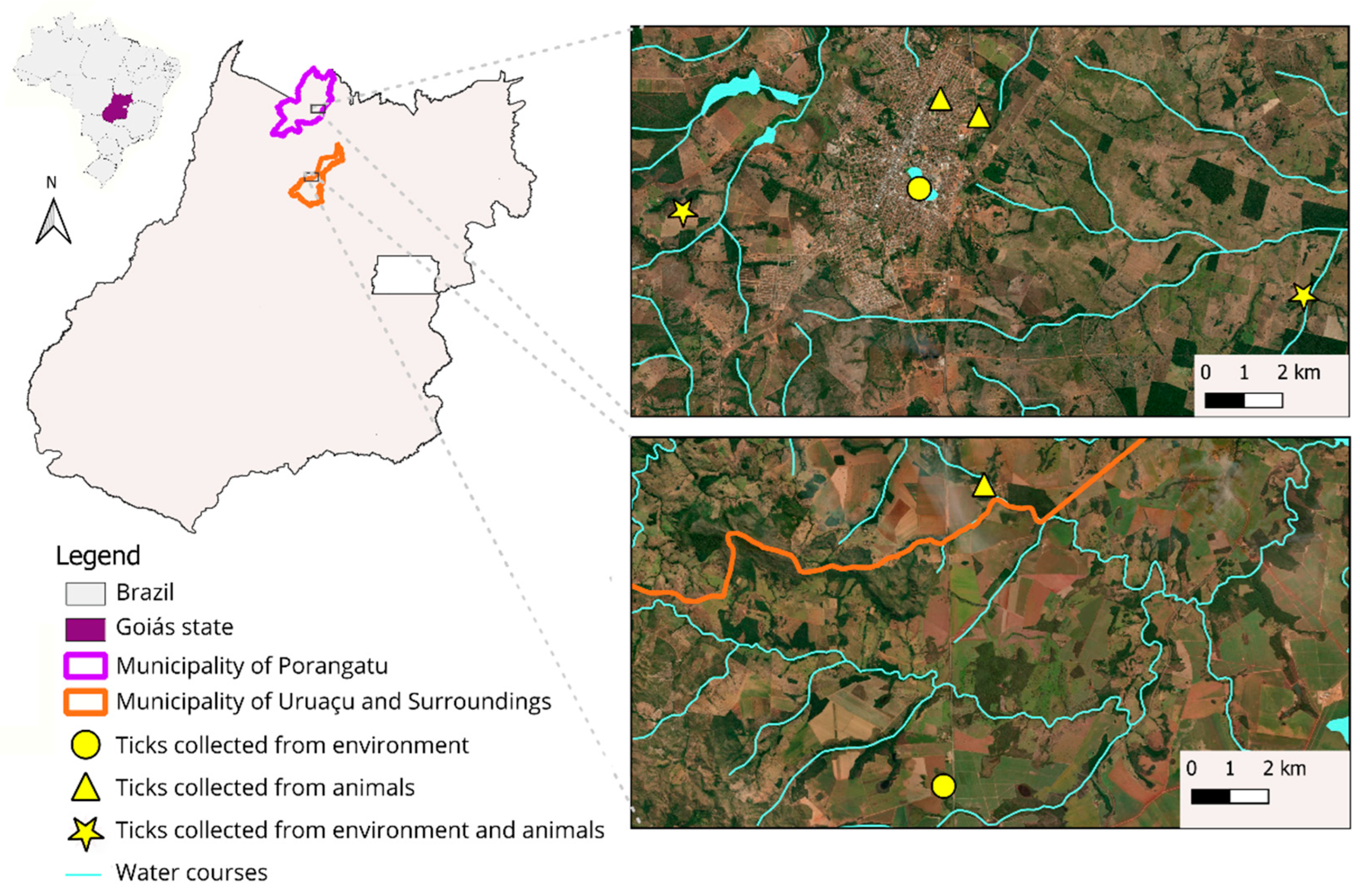
| Municipality | Location | Collection in Equids | Collection in Environment | Presence of Capybaras |
|---|---|---|---|---|
| Uruaçu | 14°21′54.50″ S and 49°09′05.80″ W | X | ||
| Uruaçu | 14°26′05.80″ S and 49°09′37.00″ W | X | X | |
| Porangatu | 13°25′54.80″ S and 49°07′46.20″ W | X | ||
| Porangatu | 13°25′40.79″ S and 49°08′14.60″ W | X | ||
| Porangatu | 13°26′35.96″ S and 49°08′32.23″ W | X | X | |
| Porangatu | 13°27′03.59″ S and 49°11′22.74″ W | X | X | X |
| Porangatu | 13°28′00.66″ S and 49°03′54.76″ W | X | X |
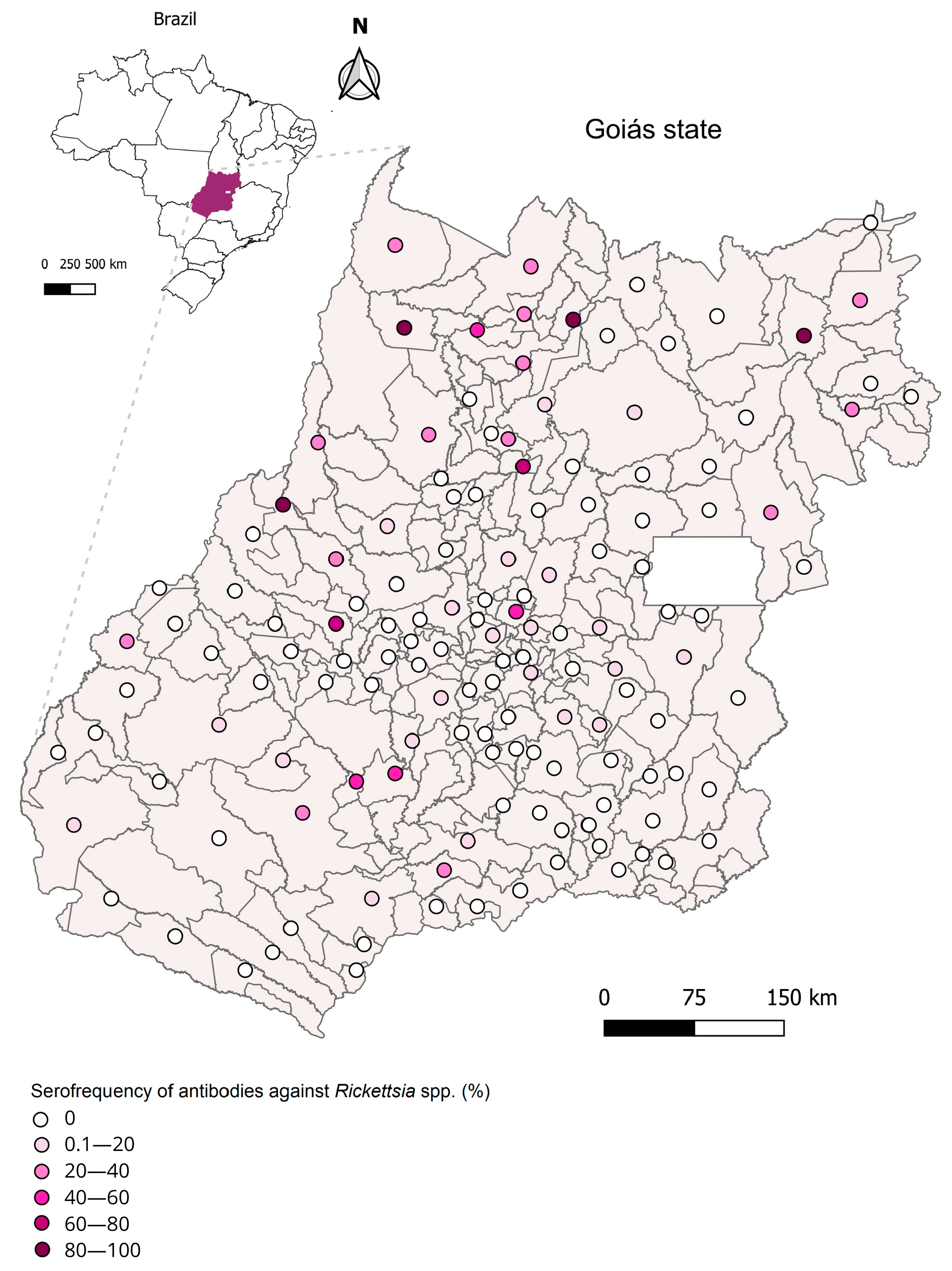
| Municipalities with Positive Samples | No. of Seroreactive Animals to Each Rickettsia Species (No. of Positives/No. of Animals Tested; % Seroreactivity) | No. Animals with PAIHR a | |||
|---|---|---|---|---|---|
| R. rickettsii | R. parkeri | R. amblyommatis | R. bellii | ||
| Abadiânia | 0 (0) | 0 (0) | 0 (0) | 1 (1/7; 14.3) | 1 R.be b |
| Acreúna | 2 (2/6; 33.3) | 4 (4/6; 66.7) | 2 (2/6; 33.3) | 0 (0) | |
| Amaralina | 1 (1/12; 8.3) | 1 (1/12; 8.3) | 5 (5/12; 41.7) | 0 (0) | 2 R.am c |
| Aruanã | 0 (0) | 0 (0) | 1 (1/3; 33.3) | 0 (0) | 1 R.am |
| Baliza | 0 (0) | 1 (1/8; 12.5) | 2 (2/8; 25) | 0 (0) | |
| Bela Vista de Goiás | 2 (2/6; 33.3) | 0 (0) | 0 (0) | 0 (0) | |
| Bom Jesus de Goiás | 1 (1/3; 33.3) | 1 (1/3; 33.3) | 0 (0) | 1 (1/3; 33.3) | |
| Britânia | 1 (1/1; 100) | 1 (1/1; 100) | 1 (1/1; 100) | 1 (1/1; 100) | |
| Caiapônia | 1 (1/2; 50) | 0 (0) | 0 (0) | 0 (0) | |
| Campo Limpo de Goiás | 0 (0) | 0 (0) | 1 (1/2; 50) | 0 (0) | |
| Crixás | 3 (3/15; 20) | 0 (0) | 5 (5/15; 33.3) | 0 (0) | 1 R.ri d; 3 R.am |
| Faina | 0 (0) | 0 (0) | 1 (1/9; 11.1) | 0 (0) | |
| Fazenda Nova | 0 (0) | 0 (0) | 2 (2/3; 66.7) | 1 (1/3; 33.3) | |
| Formosa | 7 (7/11; 63.6) | 6 (6/11; 54.5) | 3 (3/11; 27.3) | 1 (1/11; 9.1) | 1 R.ri |
| Formoso | 2 (2/2; 100) | 1 (1/2; 50) | 2 (2/2; 100) | 0 (0) | 1 R.am |
| Goiânia | 1 (1/4; 25) | 0 (0) | 0 (0) | 1 (1/4; 25) | |
| Goiatuba | 1 (1/9; 11.1) | 1 (1/9; 11.1) | 1 (1/9; 11.1) | 1 (1/9; 11.1) | |
| Hidrolina | 0 (0) | 0 (0) | 1 (1/4; 25) | 0 (0) | |
| Inhumas | 2 (2/9; 22.2) | 2 (2/9; 22.2) | 0 (0) | 0 (0) | |
| Itaberaí | 0 (0) | 0 (0) | 0 (0) | 1 (1/9; 11.1) | |
| Itapirapuã | 1 (1/3; 33.3) | 1 (1/3; 33.3) | 1 (1/3; 33.3) | 0 (0) | |
| Jandaia | 0 (0) | 0 (0) | 1 (1/9; 11.1) | 0 (0) | |
| Jaraguá | 0 (0) | 0 (0) | 0 (0) | 1 (1/2; 50) | 1 R.be |
| Luziânia | 0 (0) | 0 (0) | 0 (0) | 1 (1/4; 25) | |
| Mara Rosa | 0 (0) | 0 (0) | 1 (1/2; 50) | 0 (0) | |
| Mineiros | 1 (1/6; 16.6) | 0 (0) | 1 (1/6; 16.6) | 0 (0) | |
| Montividiu do Norte | 0 (0) | 0 (0) | 1 (1/9; 11.1) | 0 (0) | |
| Mundo Novo | 0 (0) | 0 (0) | 2 (2/2; 100) | 0 (0) | 2 R.am |
| Mutunópolis | 3 (3/6; 50) | 2 (2/6; 33.3) | 2 (2/6; 33.3) | 0 (0) | |
| Niquelândia | 0 (0) | 0 (0) | 1 (1/5; 20) | 0 (0) | |
| Nova Roma | 0 (0) | 0 (0) | 1 (1/1; 100) | 0 (0) | 1 R.am |
| Palmeiras de Goiás | 1 (1/7; 14.3) | 0 (0) | 2 (2/7; 28.6) | 0 (0) | |
| Pirenópolis | 0 (0) | 0 (0) | 1 (1/7; 14.3) | 0 (0) | |
| Porangatu | 3 (3/37; 8.1) | 0 (0) | 19 (19/37; 51.3) | 0 (0) | 9 R.am |
| Quirinópolis | 1 (1/2; 50) | 0 (0) | 0 (0) | 0 (0) | |
| Rio Verde | 3 (3/14; 21.4) | 0 (0) | 0 (0) | 2 (2/14; 14.3) | 1 R.ri |
| Santo Antônio da Barra | 0 (0) | 0 (0) | 0 (0) | 1 (1/2; 50) | 1 R.be |
| São Domingos | 0 (0) | 0 (0) | 2 (2/5; 40) | 1 (1/5; 20) | 1 R.am |
| São Luíz do Norte | 1 (1/7; 14.3) | 0 (0) | 5 (5/7; 71.4) | 0 (0) | 2 R.am |
| São Miguel do Araguaia | 0 (0) | 0 (0) | 6 (6/19; 31.6) | 0 (0) | 3 R.am |
| São Mg do Passa Quatro | 0 (0) | 0 (0) | 1 (1/8; 12.5) | 0 (0) | |
| Silvânia | 0 (0) | 0 (0) | 1 (1/9; 11.1) | 0 (0) | 1 R.am |
| Simolândia | 1 (1/6; 16.7) | 0 (0) | 1 (1/6; 16.7) | 1 (1/6; 16.7) | |
| Taquaral de Goiás | 0 (0) | 0 (0) | 0 (0) | 1 (1/5; 20) | 1 R.be |
| Turvelândia | 0 (0) | 0 (0) | 1 (1/6; 16.7) | 0 (0) | |
| Uruaçu | 1 (1/9; 11.1) | 0 (0) | 0 (0) | 0 (0) | 1 R.ri |
| TOTAL (317) | 40 (40/317; 12.6) | 21 (21/317; 6.6) | 77 (77/317; 24.3) | 16 (16/317; 5.04) | |
| Tick Host (No. of Individuals) | Location | D. nitens | A. sculptum | A. cajennense s.l | A. nodosum | R. sp. | R. microplus | |||
|---|---|---|---|---|---|---|---|---|---|---|
| L | N | A | N | A | A | A | L | A | ||
| Equine (10) | Uruaçu | 85 | 38 | 92 | 37 | |||||
| Environment | Uruaçu | 1 | 19 | |||||||
| Equine (17) | Porangatu | 7 | 59 | 161 | 67 | 118 | 1 | 6 | ||
| Mule (1) | Porangatu | 7 | 2 | 3 | ||||||
| Environment | Porangatu | 1 | 111 | 122 | 1 | |||||
| TOTAL | 449 | 238 | 243 | 1 | 1 | 6 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pádua, G.T.; Tavares, M.A.; de Lima, N.J.; Paula, W.V.d.F.; dos Santos, G.C.; Neves, L.C.; Bittencourt, R.B.M.; Paludo, R.L.d.R.; Cardoso, E.R.N.; da Silva, B.B.F.; et al. Spatial Distribution of Equid Exposure to Rickettsia spp. in Goiás State, Midwestern Brazil. Pathogens 2025, 14, 449. https://doi.org/10.3390/pathogens14050449
Pádua GT, Tavares MA, de Lima NJ, Paula WVdF, dos Santos GC, Neves LC, Bittencourt RBM, Paludo RLdR, Cardoso ERN, da Silva BBF, et al. Spatial Distribution of Equid Exposure to Rickettsia spp. in Goiás State, Midwestern Brazil. Pathogens. 2025; 14(5):449. https://doi.org/10.3390/pathogens14050449
Chicago/Turabian StylePádua, Gracielle Teles, Mariana Avelar Tavares, Nicolas Jalowitzki de Lima, Warley Vieira de Freitas Paula, Gabriel Cândido dos Santos, Lucianne Cardoso Neves, Raphaela Bueno Mendes Bittencourt, Raquel Loren dos Reis Paludo, Ennya Rafaella Neves Cardoso, Bianca Barbara Fonseca da Silva, and et al. 2025. "Spatial Distribution of Equid Exposure to Rickettsia spp. in Goiás State, Midwestern Brazil" Pathogens 14, no. 5: 449. https://doi.org/10.3390/pathogens14050449
APA StylePádua, G. T., Tavares, M. A., de Lima, N. J., Paula, W. V. d. F., dos Santos, G. C., Neves, L. C., Bittencourt, R. B. M., Paludo, R. L. d. R., Cardoso, E. R. N., da Silva, B. B. F., Pádua, B. R. d., Borsanelli, A. C., Dantas-Torres, F., Polo, G. P., & Krawczak, F. d. S. (2025). Spatial Distribution of Equid Exposure to Rickettsia spp. in Goiás State, Midwestern Brazil. Pathogens, 14(5), 449. https://doi.org/10.3390/pathogens14050449







