The Overlooked Nucleocapsid Response: A Cohort Study of SARS-CoV-2 Vaccines in Brazil
Abstract
1. Introduction
2. Materials and Methods
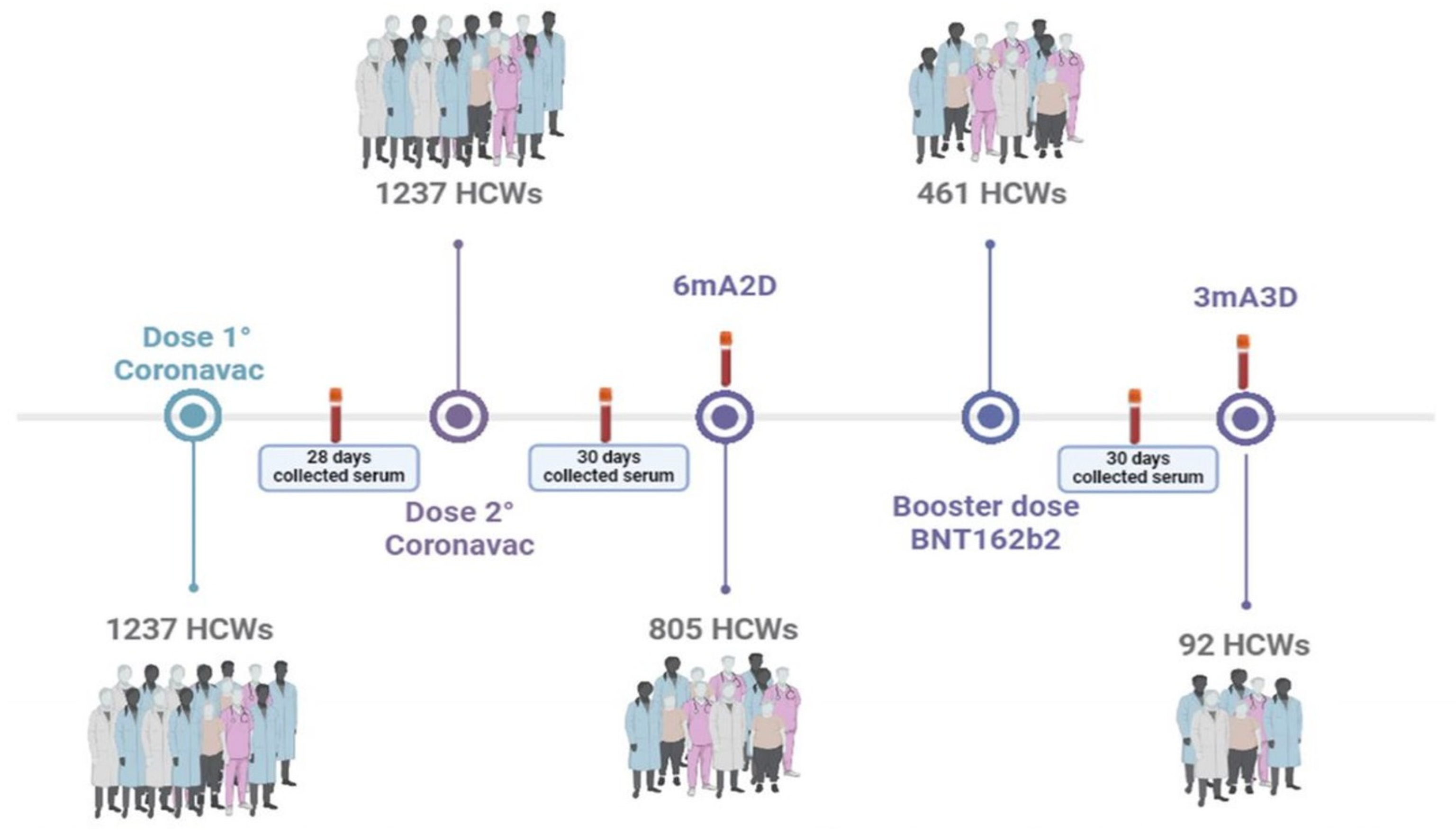
2.1. Participants and Study Design
2.2. Methods
2.3. Statistical Analyses
3. Results
3.1. Longitudnal Analysis of Anti-N Antibody Responses Following Vaccination with CoronaVac/BNT162b2 Booster in a Cohort of Healthcare Workers (HCWs)
3.2. Anti-S Antibody Responses Following mRNA BNT162b2 Booster in a Cohort of Healthcare Workers (HCWs)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| S | Spike |
| M | Membrane |
| E | Envelope |
| N | Nucleocapsid |
| HCWs | Health professionals |
| 1D | 28 days after the first dose |
| 2D | 30 days after the second dose |
| 6mA2D | 6 months after the second dose |
| 3D | 30 days after the booster dose |
| 3mA3D | 3 months after the booster dose |
| IgG | Immunoglobulin G |
| RBD | Receptor-binding domain |
| IQR | Interquartile range |
References
- Lai, C.-C.; Shih, T.-P.; Ko, W.-C.; Tang, H.-J.; Hsueh, P.-R. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and Coronavirus Disease-2019 (COVID-19): The Epidemic and the Challenges. Int. J. Antimicrob. Agents 2020, 55, 105924. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Flores, D.; Zepeda-Cervantes, J.; Cruz-Reséndiz, A.; Aguirre-Sampieri, S.; Sampieri, A.; Vaca, L. SARS-CoV-2 Vaccines Based on the Spike Glycoprotein and Implications of New Viral Variants. Front. Immunol. 2021, 12, 701501. [Google Scholar] [CrossRef]
- Dai, L.; Gao, G.F. Viral Targets for Vaccines against COVID-19. Nat. Rev. Immunol. 2021, 21, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Trougakos, I.P.; Terpos, E.; Alexopoulos, H.; Politou, M.; Paraskevis, D.; Scorilas, A.; Kastritis, E.; Andreakos, E.; Dimopoulos, M.A. Adverse Effects of COVID-19 MRNA Vaccines: The Spike Hypothesis. Trends Mol. Med. 2022, 28, 542–554. [Google Scholar] [CrossRef] [PubMed]
- Devaux, C.A.; Camoin-Jau, L. Molecular Mimicry of the Viral Spike in the SARS-CoV-2 Vaccine Possibly Triggers Transient Dysregulation of ACE2, Leading to Vascular and Coagulation Dysfunction Similar to SARS-CoV-2 Infection. Viruses 2023, 15, 1045. [Google Scholar] [CrossRef] [PubMed]
- Feikin, D.R.; Higdon, M.M.; Abu-Raddad, L.J.; Andrews, N.; Araos, R.; Goldberg, Y.; Groome, M.J.; Huppert, A.; O’Brien, K.L.; Smith, P.G.; et al. Duration of Effectiveness of Vaccines against SARS-CoV-2 Infection and COVID-19 Disease: Results of a Systematic Review and Meta-Regression. Lancet 2022, 399, 924–944. [Google Scholar] [CrossRef] [PubMed]
- Ikezaki, H.; Nomura, H.; Shimono, N. Dynamics of Anti-Spike IgG Antibody Level after the Second BNT162b2 COVID-19 Vaccination in Health Care Workers. J. Infect. Chemother. Off. J. Jpn. Soc. Chemother. 2022, 28, 802–805. [Google Scholar] [CrossRef] [PubMed]
- Kitro, A.; Sirikul, W.; Thongkum, W.; Soponpong, S.; Yasamut, U.; Kiratipaisarl, W.; Kosai, A.; Kasinrerk, W.; Tayapiwatana, C.; Srithanaviboonchai, K. Dynamic of Anti-Spike Receptor Binding Domain (RBD) Levels and Short-Term Adverse Events Following a Heterologous Booster Dose of BNT162b2 after Two Doses of CoronaVac in Thai Health Care Workers. Vaccine 2022, 40, 2915–2924. [Google Scholar] [CrossRef] [PubMed]
- Van Elslande, J.; Weemaes, M.; Godderis, L.; Van Pottelbergh, G.; Bossuyt, X.; Vermeersch, P. IgG Anti-Spike Antibody Levels in Healthcare Workers with and without Prior COVID-19 up to 3 Months after BNT162b2 Vaccination. Diagn. Microbiol. Infect. Dis. 2022, 102, 115638. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, M.H.G.; de Souza, T.D.F.G.; de Carvalho Araújo, F.M.; de Andrade, L.O.M. Dynamics of Antibody Response to CoronaVac Vaccine. J. Med. Virol. 2022, 94, 2139–2148. [Google Scholar] [CrossRef] [PubMed]
- Bryan, A.; Pepper, G.; Wener, M.H.; Fink, S.L.; Morishima, C.; Chaudhary, A.; Jerome, K.R.; Mathias, P.C.; Greninger, A.L. Performance Characteristics of the Abbott Architect SARS-CoV-2 IgG Assay and Seroprevalence in Boise, Idaho. J. Clin. Microbiol. 2020, 58, e00941-20. [Google Scholar] [CrossRef] [PubMed]
- Naaber, P.; Tserel, L.; Kangro, K.; Sepp, E.; Jürjenson, V.; Adamson, A.; Haljasmägi, L.; Rumm, A.P.; Maruste, R.; Kärner, J.; et al. Dynamics of Antibody Response to BNT162b2 Vaccine after Six Months: A Longitudinal Prospective Study. Lancet Reg. Heal. Eur. 2021, 10, 100208. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, M.H.G.; Pinto, A.C.M.D.; Silva, M.F.S.; de Melo, A.C.L.; Vasconcelos, G.S.; dos Santos, E.R.; de Carvalho Araújo, F.M.; de Andrade, L.O.M. Dynamics of SARS-CoV-2 Antibody Response to CoronaVac Followed by Booster Dose of BNT162b2 Vaccine. Emerg. Infect. Dis. 2022, 28, 1237–1240. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Coley, I.; Cervantes-Ceballos, L.; Tejeda-Benítez, L.; Sierra-Márquez, L.; Cabarcas-Montalvo, M.; García-Espiñeira, M.; Coronell-Rodríguez, W.; Arroyo-Salgado, B. Vaccines Platforms and COVID-19: What You Need to Know. Trop. Dis. Travel Med. Vaccines 2022, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Rosa Duque, J.S.; Wang, X.; Leung, D.; Cheng, S.M.; Cohen, C.A.; Mu, X.; Hachim, A.; Zhang, Y.; Chan, S.M.; Chaothai, S.; et al. Immunogenicity and reactogenicity of SARS-CoV-2 vaccines BNT162b2 and CoronaVac in healthy adolescents. Nat. Commun. 2022, 13, 3700. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.F.S.; Pinto, A.C.M.D.; de Oliveira, F.d.C.E.; Caetano, L.F.; de Carvalho Araújo, F.M.; Fonseca, M.H.G. Antibody Response 6 Months after the Booster Dose of Pfizer in Previous Recipients of CoronaVac. J. Med. Virol. 2023, 95, e28169. [Google Scholar] [CrossRef] [PubMed]
- Silva Vasconcelos, G.; da Conceição Rodrigues Fernandes, M.; Cardoso Matsui, T.; Claudia dos Santos Luciano, M.; Leite Costa, C.; Perdigão Mello Ferraz, C.; Braga Stehling Dias, F.; Miyajima, F.; Montenegro de Carvalho Araújo, F.; Helena Gambim Fonseca, M. Persistent SARS-COV-2 Infection in Vaccinated Individual with Three Doses of COVID-19 Vaccine. Vaccine 2023, 41, 1778–1782. [Google Scholar] [CrossRef] [PubMed]

| Phases | S IgGs | p-Value | N IgG | p-Value |
|---|---|---|---|---|
| 1D | 723.4 (109.6–1 875) | 0.87 (0.07–2.485) | ||
| 2D | 1208 (706.1–2 236) | 1.97 (0.85–3.425) | <0.0001 | |
| 6mA2D | 453.1 (190.9–1 166) | 0.69 (0.215–1.735) | <0.0001 | |
| 3D | 31394 (19 255–46 155) | - | 0.45 (0.100–1.050) | <0.0001 |
| 3mA3D | 15 107 (8 580–24 427) | <0.0001 | 1.765 (0.1825–6.945) | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira, F.d.C.E.; Dinelly Pinto, A.C.M.; Silva, M.F.S.; Lizano Garcia, M.M.; Rodrigues Fernandes, M.d.C.; Damasceno, G.A.; Lima de Melo, A.C.; Matsui, T.C.; Goebel de Souza, T.d.F.; Severino, F.G.; et al. The Overlooked Nucleocapsid Response: A Cohort Study of SARS-CoV-2 Vaccines in Brazil. Pathogens 2025, 14, 445. https://doi.org/10.3390/pathogens14050445
de Oliveira FdCE, Dinelly Pinto ACM, Silva MFS, Lizano Garcia MM, Rodrigues Fernandes MdC, Damasceno GA, Lima de Melo AC, Matsui TC, Goebel de Souza TdF, Severino FG, et al. The Overlooked Nucleocapsid Response: A Cohort Study of SARS-CoV-2 Vaccines in Brazil. Pathogens. 2025; 14(5):445. https://doi.org/10.3390/pathogens14050445
Chicago/Turabian Stylede Oliveira, Fatima de Cássia Evangelista, Ana Carolina Matias Dinelly Pinto, Maria Francilene Souza Silva, Max Moreira Lizano Garcia, Maria da Conceição Rodrigues Fernandes, Gabriela Alexandria Damasceno, Amanda Campelo Lima de Melo, Tamires Cardoso Matsui, Tamiris de Fátima Goebel de Souza, Fernanda Gadelha Severino, and et al. 2025. "The Overlooked Nucleocapsid Response: A Cohort Study of SARS-CoV-2 Vaccines in Brazil" Pathogens 14, no. 5: 445. https://doi.org/10.3390/pathogens14050445
APA Stylede Oliveira, F. d. C. E., Dinelly Pinto, A. C. M., Silva, M. F. S., Lizano Garcia, M. M., Rodrigues Fernandes, M. d. C., Damasceno, G. A., Lima de Melo, A. C., Matsui, T. C., Goebel de Souza, T. d. F., Severino, F. G., Silveira Reis, V. A., Passaes, C., de Carvalho Araújo, F. M., Monteiro de Andrade, L. O., & Gambim Fonseca, M. H. (2025). The Overlooked Nucleocapsid Response: A Cohort Study of SARS-CoV-2 Vaccines in Brazil. Pathogens, 14(5), 445. https://doi.org/10.3390/pathogens14050445







