Abstract
Crohn’s disease (CD) is a multifactorial polygenic inflammatory bowel disease linked to aberrant immune response. Mycobacterium paratuberculosis (MAP) has been associated with CD; however, detecting MAP in CD tissues remains highly challenging. Recently, Human Endogenous Retroviruses (HERVs) differential gene expression has been reported in CD, but little is known about the involvement of MAP and HERVs in CD pathology. This study aimed to characterize the humoral response against HERV-K, HERV-W, and MAP antigens using an indirect ELISA in plasma samples from CD patients and age- and gender-matched healthy controls (HCs). We observed a significant antibody response against HERV-K and HERV-W epitopes in CD patients in comparison to MAP epitopes, as well as a higher overall antibody response in patients compared to HCs. This study is the first to report the presence of humoral immune response against HERVs antigens in CD. Considering the pro-inflammatory nature of CD, HERVs may contribute to the development or progression of disease in genetically predisposed individuals. However, further research is needed to better understand the complex role of HERVs in CD.
1. Introduction
Crohn’s disease (CD) is an inflammatory disease of the digestive tract characterized by diarrhea, abdominal cramps, and in some cases, due to persistent inflammation, it may cause complicated symptoms like fistulation, stricture, and intestinal perforation [1]. CD is a multifactorial polygenic condition [2] often linked to aberrant immune response to gut microbial flora in genetically predisposed individuals [3].
Numerous studies have explored the cause of CD over the past 40 years. Some conclusive studies linked Mycobacterium avium subspecies paratuberculosis (MAP) with CD after identifying insertional sequences IS900 unique to MAP through PCR [4]. In addition to that, antibodies against MAP antigen hsp65 have also been detected in CD patient serum [5]. Additionally, Significant attention has been given to the association between MAP and CD in genetically predisposed individuals due to CD’s similar pathophysiology to Johne’s disease, a chronic gastroenteritis condition in ruminants [6]. While these studies are compelling, the role of MAP in CD remains debated due to challenges in isolating it from patient samples [7].
Human Endogenous Retroviruses (HERVs) are retrovirus-derived DNA sequences that accumulated in the genome over the past 100 million years through multiple integration events. When retroviruses infect germ cells, the HERV provirus integrates into the host genome and is subsequently transmitted across generations in a Mendelian inheritance pattern [8,9]. Over time, HERVs lost the ability to produce infectious progeny due to accumulated mutations. However, their retroviral architecture remains preserved, consisting of three key genes, Gag (group-associated antigens), Pol (polymerase), and Env (envelope), surrounded by 5′ and 3′ regulatory LTRs (long terminal repeats) [10].
HERVs play a critical role in physiological processes like stem cell pluripotency and placenta formation [11,12,13]. However, aberrant HERV expression has been implicated in a range of diseases, including neurological disorders, autoimmune diseases, cancers, and COVID-19 infection [14,15,16]. For instance, HERVs involvement has been linked to multiple sclerosis, amyotrophic lateral sclerosis, autism, Parkinson’s disease, and schizophrenia [15,17,18,19,20,21,22]. HERVs reactivation has also been observed in autoimmune conditions such as rheumatoid arthritis, type 1 diabetes, and celiac disease [14,23,24]. Additionally, HERV differential gene expression has been detected in leukemia, prostate cancer, breast cancer, and germ cell tumors, suggesting a role in tumor progression [25,26,27].
Factors like viral and bacterial infections, caffeine, smoking, immune factors, and gene mutations play a critical role in HERV activation [24,28,29,30,31,32,33,34,35] and can contribute to the etiology of various diseases. One particular example is Herpes Simplex Virus 1’s involvement in Parkinson’s disease, in which HERV might play a protective role [36,37]. A recent study also suggested that stress, such as stimulated microgravity, may also contribute to aberrant HERV expression [38,39]. HERV-related activities can contribute to the variable expression of HERV RNA, synthesis of retroviral-like components, novel promoters, and oncogene activation [40,41].
Studies have reported the possible link between MAP and CD. However, a definitive answer remains elusive, underscoring the need for further investigation. Likewise, limited information on the potential role of HERVs in the onset or progression of CD, as well as their possible link to MAP in Crohn’s pathology, is available. Therefore, this study explored the role of HERVs and MAP in CD by analyzing the serological levels of HERVs and MAP peptides to elucidate their potential contribution to CD pathogenesis in comparison to the general population.
2. Materials and Methods
2.1. Study Population
Crohn’s patients were diagnosed based on European guidelines for the diagnosis and management of CD [42]. For each CD patient, an age- and gender-matched healthy control (HC) was included in this study. Variable numbers of samples were tested for each HERV and MAP peptide due to differences in sample availability throughout this study (Table 1). Written informed consent was obtained from all participants after full disclosure of information in compliance with regional and national regulations. CD patients with comorbidities of autoimmune diseases and active infections were excluded from the studied cohort. All CD patients and HCs were residents of Sardinia, Italy.

Table 1.
Demographics of Crohn’s disease patients and healthy controls tested against each peptide.
2.2. Blood Samples
5 mL peripheral venous blood sample was collected in an EDTA vial from each subject, and plasma was separated within 12 h of collection using Histopaque®-1077 (Sigma-Aldrich, St. Louis, MO, USA). 500 µL plasma aliquots were stored at −20 °C for short-term (<6 months) and at −80 °C for long-term (>6 months) storage.
2.3. Peptides
Epitopes derived from HERV (HERV-K(19–37) and HERV-W(248–262)) and MAP (MAP 38-65c(125–138), MAP 1,4-α-gbp(157–173), and MAP 2404c(70–85)) were designed using Epitope Database (Bebipred Linear Epitope Prediction 2.0) and Analysis Resource (https://www.iedb.org) software (Table 2). These epitopes were selected based on bioinformatics predictions, prioritizing regions of high antigenicity to ensure optimal immune recognition. All peptides were synthesized at >95% purity (LifeTein, South Plainfield, NJ, USA) and later quantified using HPLC. These peptides were then re-suspended at 10 mM in DMSO and stored at −80 °C.

Table 2.
Epitopes identified in MAP and HERV.
2.4. ELISA Assays
Indirect enzyme-linked immunosorbent assay was used to identify specific plasma antibodies (Abs) against selected antigens HERV-K(19–37), HERV-W(248–262), MAP 38-65c(125–138), MAP 1,4-α-gbp(157–173), and MAP 2404c(70–85). First, 96-well Nunc-Immuno Plates (Nalgen Nunc International, Rochester, NY, USA) were coated with 10 µg/mL of each peptide dissolved in 0.05 M carbonate–bicarbonate buffer, pH 9.5 (Sigma, St. Louis, MO, USA), and incubated overnight at 4 °C. Plates were then washed with 0.05% Tween-20 Tris Buffered Saline (TBS-T). Plates were blocked for 1 h with 1% skim milk in 1X-TBS and later washed twice with 0.05% Tween-20 washing buffer. Subsequently, 5 µL of plasma sample was added to 95 µL of blocking buffer, incubated for 2 h at room temperature, then washed four times with TBS-T, followed by the addition of 100 µL of secondary Ab IgG for 1 h. The secondary Ab, alkaline phosphatase-conjugated goat anti-human IgG polyclonal Ab, was added to the plate at a 1:1000 dilution (Sigma). ELISA plates were washed again four times in TBS-T buffer. The substrate, p-Nitrophenyl Phosphate (Sigma) tablets, was dissolved in 20 mL milli-Q water. Plates were incubated at room temperature with 200 µL of substrate for 15 min. The reaction was then stopped using 50 µL of 3N NaOH stop solution. Absorbance was recorded at the 405 nm wavelength using a SpectraMax Plus 384 microplate reader (Molecular Devices, Sunnyvale, CA, USA). Data were normalized to minimize experimental variation based on the reactivity of the positive control (absorbance reactivity set at 1.0 arbitrary unit) included in each plate. Immobilized peptide coated with IgG without a plasma sample was used as a negative control to minimize background noise.
2.5. Statistical Analysis
Data were analyzed with GraphPad Prism 8.0 (GraphPad Software Inc., La Jolla, CA, USA). The Shapiro–Wilk test was used to assess whether the sample distribution followed the Gaussian distribution. As samples were not normally distributed, the Mann–Whitney test was applied to analyze non-parametric data. ROC analysis was used to calculate the cut-off value for positivity in each dataset with specificity ≥90%, and sensitivity selected accordingly. Fisher’s exact test was used to determine statistically significant differences between CD patients and HCs groups. A p-value less than 0.05 was considered statistically significant. The Spearman test was used to determine the correlation between the tested HERV and MAP peptides.
3. Results
3.1. Antibody Response to HERV and MAP Immunogenic Epitopes in Crohn’s Disease Patients Versus Healthy Controls
Our study included CD patients and their age-matched HCs (Table 1). Abs were detected in the plasma samples of CD patients and HCs against HERV-K, HERV-W, MAP 38-65c, Map 1,4-α-gbp, and MAP 2404c (Figure 1). Our patient samples also showed increased levels of positivity against HERV peptides in comparison to MAP peptides.
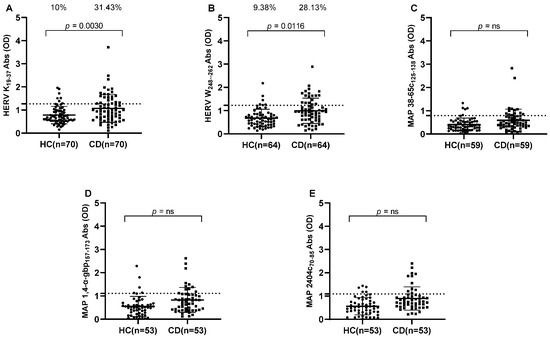
Figure 1.
ELISA-based antibodies reactivity analysis against HERV and MAP peptides in CD patients and HCs. Plasma samples from subjects were tested against HERV-K(19–37) (A), HERV-W(248–262) (B), MAP 38-65c(125–138) (C), MAP 1,4-α-gbp(157–173) (D), and MAP 2404c(70–85) (E). Dashed lines indicate thresholds for Abs calculated by ROC analysis, which were used to assess sample positivity. p-values derived from Fisher’s exact test and AUC are indicated in the graph, along with percentages of positive patients in the upper section of each graph. A p-value greater than 0.05 was considered statistically significant, while “ns” indicates non-significant results.
HERV-K showed the highest immunogenicity, of 31.43% (n = 22) for CD patients and 10% (n = 7) for HCs (cut-off value = 1.27, AUC = 0.6553, p = 0.0030) (Figure 1A). Likewise, Abs against HERV-W displayed high plasma positivity, accounting for 28.13% (n = 18) in CD patients and 9.38% (n = 6) in HCs (cut-off value = 1.225, AUC = 0.6881, p = 0.0116) (Figure 1B).
All three MAP peptides, MAP 38-65c, MAP 1,4-α-gbp, and MAP 2404c, showed significantly different immunogenicity in CD patients and HCs (Mann–Whitney p-values = 0.0097, 0.0021, and 0.0002, respectively), but positive plasma reactivity at selected ≥ 90% sensitivity cut-offs among all MAP peptides tested in CD patients and HCs was not significant according to the Fisher test (Figure 1C–E).
We then stratified available data based on the clinical manifestation of CD into three distinct phenotypes: luminal CD, stricturing CD, and fistuling CD. This categorization allowed us to observe significant variations in the prevalence of plasma Abs against MAP and HERV peptides across different Crohn’s phenotypes. Results indicate an intriguing pattern of higher Abs against the HERV-K peptide in fistuling CD group that accounted for 81.82% (n = 9) in comparison to 9.09% (n = 1) in positive HCs (cut-off = 0.93, AUC= 0.8347, p = 0.0019) (Figure 2K), while no significant differences in HERV-K Abs levels were observed in luminal CD and stricturing CD groups (Figure 2A,F). Likewise, when HERV-W Abs were analyzed in distinct CD subgroups in comparison to HCs, higher Abs levels were found against fistuling CD in comparison to luminal CD and stricturing CD groups, in which no significant differences in Abs prevalence were found against healthy subjects. A 72.73% (n = 8) reactivity was determined against HERV-W in the fistuling CD group versus 9.09% (n = 1) in HCs (cut-off = 0.785, AUC = 0.8512, p = 0.0075) (Figure 2L).
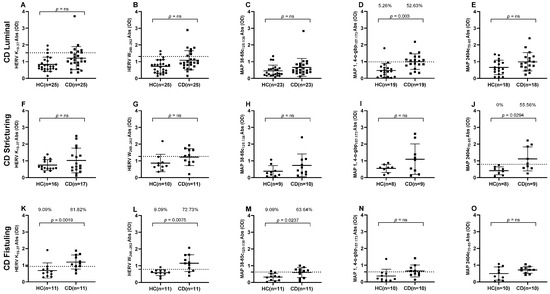
Figure 2.
ELISA based antibodies reactivity analysis against HERV and MAP peptides in CD patients and HCs, categorized by CD clinical symptoms. (A–E) Healthy and luminal CD subjects tested against HERV-K(19–37), HERV-W(248–262), MAP 38-65C(125–138), MAP 1,4-α-gbp(157–173), and MAP 2404c(70–85) peptides, respectively. (F–J) Healthy and stricturing CD subjects tested against HERV-K(19–37), HERV-W(248–262), MAP 38-65C(125–138), MAP 1,4-α-gbp(157–173), and MAP 2404c(70–85) peptides, respectively. (K–O) Healthy and fistuling CD subjects tested against HERV-K(19–37), HERV-W(248–262), MAP 38-65C(125–138), MAP 1,4-α-gbp(157–173), and MAP 2404c(70–85) peptides, respectively. Dashed lines indicate Abs thresholds calculated by ROC analysis, which were used to assess sample positivity. p-values derived from Fisher’s exact test along with percentages of positive patients are displayed in upper sections of graphs. A p-value greater than 0.05 was considered statistically significant, while “ns” indicates non-significant results.
Our analysis revealed particularly interesting results for MAP peptides, indicating that MAP 38-65c had higher significant immunogenicity, 63.64% (n = 7), in the fistuling CD group in comparison to 9% (n = 1) in healthy individuals (cut-off = 0.62, AUC = 0.77, p = 0.023) (Figure 2M). MAP 1,4-α-gbp was the only peptide that accounted for a significant Abs presence in the luminal CD group, 52.63% (n = 10), in comparison to 5.26% (n = 1) in HCs (cut-off = 0.985, AUC = 0.831, p = 0.003) (Figure 2D). Likewise, a higher Abs prevalence was reported against MAP 2404c in the stricturing CD group, which was 55.56% (n = 5) in comparison to 0% for HCs (cut-off = 0.795, AUC = 0.8472, p = 0.0294) (Figure 2J).
3.2. Correlation Analyses of HERV and MAP Antibodies
We performed correlation analysis of plasma Abs levels using the Spearman correlation test for non-parametric data to determine the association between the antigenic potential of tested HERV and MAP peptides. We found evidence of strong correlations in some tested peptides, but not in all, both in the collective cohort of CD samples and in subcategorized CD data based on CD phenotype. CD patient samples accounted for the highest r coefficient of 0.4 for the HERV-W and MAP 38-65c pairwise analysis (p = 0.002). No significant correlation was found in CD samples for other peptides pairwise analysis (Figure 3).
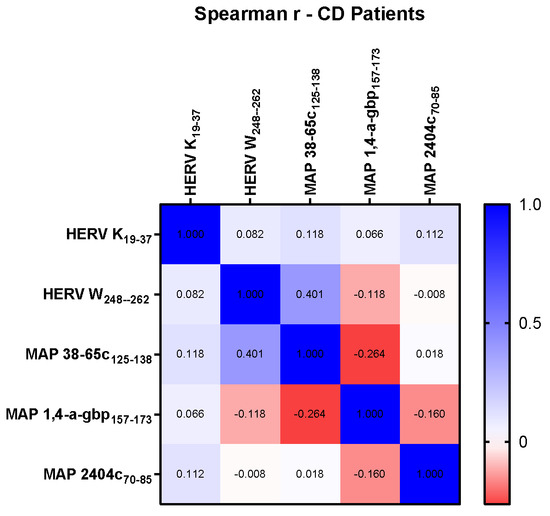
Figure 3.
Heat-map indicating r values computed using Spearman correlation test among peptides HERV-K(19–37), HERV-W(248–262), MAP 38-65C(125–138), MAP 1,4-α-gbp(157–173), and MAP 2404c(70–85).
However, when we analyzed association by categorizing data based on the distinct behavior of CD, our results revealed an intriguing pattern of HERV and MAP peptides suggesting a potential link for their expression in the manifestation of symptoms of CD. An r value equal to 0.75 was obtained in MAP 1,4-α-gbp/HERV-K pairwise analysis (p = 0.025) in the stricturing CD group (Figure 4B), indicating a strong connection between HERV-K and MAP 1,4-α-gbp contributing to developing strictures in Crohn’s patients.
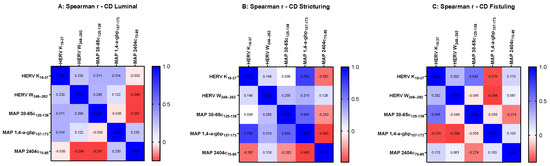
Figure 4.
Heat-map indicating r values computed using Spearman correlation analysis among peptides HERV-K(19–37), HERV-W(248–262), MAP 38-65C(125–138), MAP 1,4-α-gbp(157–173), and MAP 2404c(70–85) in Crohn’s disease cohort categorized based on disease behavior as luminal (A), stricturing (B), and fistuling (C).
4. Discussion
CD is an autoimmune inflammatory disorder of the gastrointestinal tract, particularly affecting the ileum and colon. CD cases are globally rising in adults and pediatrics associated with complex symptoms like abscessed strictures and fistulas [43]. Multiple factors, like genetics and environment, can contribute to the onset and progression of etiology. However, one definite causative agent for CD has not been defined, but some studies highlight MAP as a potential triggering factor for the onset of Crohn’s symptoms due to its similarities with Johne’s disease, which is an infectious pathology of the small intestine in ruminants caused by MAP [44,45].
Studies have reported higher transcriptional levels of various HERV genes like HERV-H pol and HERV-K pol in inflammatory bowel diseases (IBDs) like Crohn’s and ulcerative colitis [46]. HERV-W envelope protein (synctin 1) has been shown to upregulate C-reactive protein (CRP) expression, which is a well-recognized biomarker of inflammation in inflammatory bowel disease [47,48]. Differential expression of HERVs like HML, HERV-ERI, HERV-W, and HERV-H has also been observed in a tissue-dependent manner in the ileal and colon of CD patients. HERV envelope genes (synctin 1 and 2) have shown variable expression in inflamed and non-inflamed tissues in CD patients, suggesting that their expression might modify immune response in the gut barrier [49]. Our study supports the involvement of HERVs in the pathogenesis of CD.
A possible mechanism by which HERVs could contribute to CD might be through disrupting the immune homeostasis of TNF-α, the cGAS/STING/NF-Κb pathway, and M1 macrophages. Tumor necrosis factor (TNF-α) plays a crucial role in the immune-mediated response by promoting immune cell activation and wound healing. Its level can be upregulated by HERV-W env [50]. Anti-TNF therapies like infliximab and adalimumab are commonly used to treat CD by reducing inflammation [51,52,53]. The cGAS/STING/NF-Κb inflammatory cascade and M1 macrophages are involved in maintaining homeostasis in colon mucosa, which can be interfered with by HERVs. If retroviruses are inhibited with a retrovirus reverse transcriptase inhibitor azidothymidine, this can eliminate inflammatory bowel disease (colitis) symptoms in mice [54].
Unfortunately, we did not find a statistically significant reactivity difference between patients and HCs with the Fisher’s test against MAP antigens (many were closer to significance), but similar results have been previously reported as well [7,55]. However, when we analyzed these stratified data based on CD’s distinct behavior, particularly focusing on luminal, stricturing, and fistuling CD phenotypes, an interesting observation was made regarding the prevalence of MAP and HERVs peptides across different disease behaviors. These results revealed intriguing patterns, indicating the presence of Abs against various MAP antigens in different phenotypes of CD, and as the severity of disease progressed from luminal and strictures to fistulation we observed a significantly higher seropositivity rate for HERVs antigens in CD patients in comparison to HCs. We initially hypothesized the involvement of MAP in transactivation of HERVs, but we could not observe any strong correlation between HERVs and MAP peptides; interestingly we identified a strong association between MAP 1,4-α-gbp and HERV-K in the stricturing CD group, suggesting a potential link between microbial or viral peptide expression and the manifestation of particular CD symptoms.
The assumption of MAP involvement in the transactivation of HERVs and its possible role in triggering the pathophysiology of CD are supported by elevated CD68 expression in macrophages-activated granulomatous lesions in infected goats. However, the humoral response of CD patients indicates that the involvement of MAP in the pathogenesis of CD is a more complex cascade than we anticipated [56,57].
Despite valuable insights, our study had some limitations. The sample sizes of CD patients tested for each peptide varied because some samples were exhausted and no longer available. The sample size of stratified subgroups under investigation was relatively small, and therefore prompts the need for the validation of our results with a larger cohort. Although our study highlights the potential involvement of HERVs in the immune response against CD, the current experimental design does not identify the molecular pathways involved in HERV activation and its involvement in the pathophysiology of CD, leaving room for further investigation. Similarly, our experimental strategy needs to be refined to determine whether MAP contributes to CD and, if so, how, to elucidate its mechanism of action. However, to our knowledge this is the first study that reports the presence of an immune response against HERV antigens in CD patients.
In conclusion, while our findings suggest a potential role of HERVs in the development of CD, further research is needed to fully elucidate the mechanisms of HERV activation and their potential as biomarkers for CD. To address these questions, our future studies will integrate gene expression profiling and validation using qPCR to provide a comprehensive understanding of the contribution of HERVs in CD pathogenesis. These efforts will be critical in advancing our knowledge and could ultimately lead to improved diagnostic and therapeutic strategies for CD.
Author Contributions
L.A.S. conceived and supervised this study and reviewed the manuscript. A.F. designed and performed experiments, conducted data analysis, drafted the initial manuscript, and made revisions. L.C. facilitated patient recruitment. M.N., S.J. and E.R.S. contributed to sample collection and processing. E.R.S. also supervised experimental procedures, evaluated analyzed data, and reviewed the manuscript draft. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the European Union—e.INS Ecosystem of Innovation for Next Generation Sardinia to S.J., Regione Autonoma Sardegna grant: legge regionale 12 22 December 2022 n. 22 to L.A.S., PRIN 2022 n: 2022BP837R to L.A.S., and Ministero della Salute PNRR-MCNT1-2023-12376993.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board Ethical Committee ASL1 Sassari (prot 2149/CE), approved on 16 June 2015.
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mintz, M.J.; Lukin, D.J. Mycobacterium avium subspecies paratuberculosis (MAP) and Crohn’s disease: The debate continues. Transl. Gastroenterol. Hepatol. 2023, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Louis, E.; Collard, A.; Oger, A.; Degroote, E.; El Yafi, F.A.N.; Belaiche, J. Behaviour of Crohn’s disease according to the Vienna classification: Changing pattern over the course of the disease. Gut 2001, 49, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Choung, R.; Princen, F.; Stockfisch, T.; Torres, J.; Maue, A.; Porter, C.; Leon, F.; De Vroey, B.; Singh, S.; Riddle, M. Serologic microbial associated markers can predict Crohn’s disease behaviour years before disease diagnosis. Aliment. Pharmacol. Ther. 2016, 43, 1300–1310. [Google Scholar] [CrossRef] [PubMed]
- Sechi, L.A.; Scanu, A.M.; Molicotti, P.; Cannas, S.; Mura, M.; Dettori, G.; Fadda, G.; Zanetti, S. Detection and isolation of Mycobacterium avium subspecies paratuberculosis from intestinal mucosal biopsies of patients with and without Crohn’s disease in Sardinia. Am. J. Gastroenterol. 2005, 100, 1529–1536. [Google Scholar] [CrossRef]
- Zhang, P.; Minardi, L.; Kuenstner, J.; Zhang, S.; Zekan, S.; Kruzelock, R. Serological testing for Mycobacterial heat shock protein HSP65 antibody in health and diseases. Microorganisms 2019, 8, 47. [Google Scholar] [CrossRef]
- Aitken, J.M.; Phan, K.; Bodman, S.E.; Sharma, S.; Watt, A.; George, P.M.; Agrawal, G.; Tie, A.B. A Mycobacterium species for Crohn’s disease? Pathology 2021, 53, 818–823. [Google Scholar] [CrossRef]
- Bernstein, C.N.; Blanchard, J.F.; Rawsthorne, P.; Collins, M.T. Population-based case control study of seroprevalence of Mycobacterium paratuberculosis in patients with Crohn’s disease and ulcerative colitis. J. Clin. Microbiol. 2004, 42, 1129–1135. [Google Scholar] [CrossRef]
- Dupressoir, A.; Lavialle, C.; Heidmann, T. From ancestral infectious retroviruses to bona fide cellular genes: Role of the captured syncytins in placentation. Placenta 2012, 33, 663–671. [Google Scholar] [CrossRef]
- Stein, R.A.; DePaola, R.V. Human endogenous retroviruses: Our genomic fossils and companions. Physiol. Genom. 2023, 55, 249–258. [Google Scholar] [CrossRef]
- Johnson, W.E. Origins and evolutionary consequences of ancient endogenous retroviruses. Nat. Rev. Microbiol. 2019, 17, 355–370. [Google Scholar] [CrossRef]
- Santoni, F.A.; Guerra, J.; Luban, J. HERV-H RNA is abundant in human embryonic stem cells and a precise marker for pluripotency. Retrovirology 2012, 9, 111. [Google Scholar] [CrossRef] [PubMed]
- Soygur, B.; Sati, L. The role of syncytins in human reproduction and reproductive organ cancers. Reproduction 2016, 152, R167–R178. [Google Scholar] [CrossRef] [PubMed]
- Durnaoglu, S.; Lee, S.-K.; Ahnn, J. Syncytin, envelope protein of human endogenous retrovirus (HERV): No longer ‘fossil’in human genome. Anim. Cells Syst. 2021, 25, 358–368. [Google Scholar] [CrossRef]
- Noli, M.; Meloni, G.; Ruberto, S.; Jasemi, S.; Simula, E.R.; Cossu, D.; Bo, M.; Palermo, M.; Sechi, L.A. HERV-K envelope protein induces long-lasting production of autoantibodies in T1DM patients at onset in comparison to ZNT8 autoantibodies. Pathogens 2022, 11, 1188. [Google Scholar] [CrossRef]
- Garcia-Montojo, M.; Fathi, S.; Rastegar, C.; Simula, E.R.; Doucet-O’Hare, T.; Cheng, Y.H.; Abrams, R.P.; Pasternack, N.; Malik, N.; Bachani, M. TDP-43 proteinopathy in ALS is triggered by loss of ASRGL1 and associated with HML-2 expression. Nat. Commun. 2024, 15, 4163. [Google Scholar] [CrossRef] [PubMed]
- Balestrieri, E.; Minutolo, A.; Petrone, V.; Fanelli, M.; Iannetta, M.; Malagnino, V.; Zordan, M.; Vitale, P.; Charvet, B.; Horvat, B. Evidence of the pathogenic HERV-W envelope expression in T lymphocytes in association with the respiratory outcome of COVID-19 patients. EBioMedicine 2021, 66, 103341. [Google Scholar] [CrossRef]
- Li, W.; Xue, X.; Li, X.; Wu, X.; Zhou, P.; Xia, Y.; Zhang, J.; Zhang, M.; Zhu, F. Ancestral retrovirus envelope protein ERVWE1 upregulates circ_0001810, a potential biomarker for schizophrenia, and induces neuronal mitochondrial dysfunction via activating AK2. Cell Biosci. 2024, 14, 138. [Google Scholar] [CrossRef] [PubMed]
- Bo, M.; Carta, A.; Cipriani, C.; Cavassa, V.; Simula, E.R.; Huyen, N.T.; Phan, G.T.H.; Noli, M.; Matteucci, C.; Sotgiu, S. HERVs Endophenotype in Autism Spectrum Disorder: Human Endogenous Retroviruses, Specific Immunoreactivity, and Disease Association in Different Family Members. Microorganisms 2024, 13, 9. [Google Scholar] [CrossRef]
- Ruberto, S.; Domınguez-Mozo, M.I.; Garcıa-Martınez, M.A.; Cossu, D.; Sechi, L.A.; Alvarez-Lafuente, R. Immune response profiling of HERV-W envelope proteins in multiple sclerosis: Potential biomarkers for disease progression. Front. Immunol. 2025, 15, 1505239. [Google Scholar] [CrossRef]
- Simula, E.R.; Jasemi, S.; Paulus, K.; Sechi, L.A. Upregulation of microRNAs correlates with downregulation of HERV-K expression in Parkinson’s disease. J. Neurovirol. 2024, 30, 550–555. [Google Scholar] [CrossRef]
- Simula, E.R.; Arru, G.; Zarbo, I.R.; Solla, P.; Sechi, L.A. TDP-43 and HERV-K envelope-specific immunogenic epitopes are recognized in ALS patients. Viruses 2021, 13, 2301. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Montojo, M.; Simula, E.R.; Fathi, S.; McMahan, C.; Ghosal, A.; Berry, J.D.; Cudkowicz, M.; Elkahloun, A.; Johnson, K.; Norato, G. Antibody response to HML-2 may be protective in amyotrophic lateral sclerosis. Ann. Neurol. 2022, 92, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Levet, S.; Charvet, B.; Bertin, A.; Deschaumes, A.; Perron, H.; Hober, D. Human endogenous retroviruses and type 1 diabetes. Curr. Diabetes Rev. 2019, 19, 141. [Google Scholar] [CrossRef] [PubMed]
- Dow, C.T.; Pierce, E.S.; Sechi, L.A. Mycobacterium paratuberculosis: A HERV Turn-On for Autoimmunity, Neurodegeneration, and Cancer? Microorganisms 2024, 12, 1890. [Google Scholar] [CrossRef]
- Gao, Y.; Yu, X.-F.; Chen, T. Human endogenous retroviruses in cancer: Expression, regulation and function. Oncol. Lett. 2021, 21, 121. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Wen, X.; Xing, X.; Fozza, C.; Sechi, L.A. Endogenous retroviruses Suppressyn and Syncytin-2 as innovative prognostic biomarkers in Acute Myeloid Leukemia. Front. Cell. Infect. Microbiol. 2024, 13, 1339673. [Google Scholar] [CrossRef]
- Steiner, M.C.; Marston, J.L.; Iñiguez, L.P.; Bendall, M.L.; Chiappinelli, K.B.; Nixon, D.F.; Crandall, K.A. Locus-specific characterization of human endogenous retrovirus expression in prostate, breast, and colon cancers. Cancer Res. J. 2021, 81, 3449–3460. [Google Scholar] [CrossRef]
- Turcanova, V.L.; Bundgaard, B.; Höllsberg, P. Human herpesvirus-6B induces expression of the human endogenous retrovirus K18-encoded superantigen. J. Clin. Virol. 2009, 46, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Kwun, H.J.; Kim, H.S.; Jang, K.L. Activation of the human endogenous retrovirus W long terminal repeat by herpes simplex virus type 1 immediate early protein 1. Mol. Cells 2003, 15, 75–80. [Google Scholar] [CrossRef]
- Liu, C.; Liu, L.; Wang, X.; Liu, Y.; Wang, M.; Zhu, F. HBV X Protein induces overexpression of HERV-W env through NF-κB in HepG2 cells. Virus Genes 2017, 53, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chen, Y.; Li, S.; Yu, H.; Zeng, J.; Wang, X.; Zhu, F. Activation of elements in HERV-W family by caffeine and aspirin. Virus Genes 2013, 47, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, U.; Steidler, A.; Trojan, L.; Michel, M.S.; Seifarth, W.; Fabarius, A. Smoking increases transcription of human endogenous retroviruses in a newly established in vitro cell model and in normal urothelium. AIDS Res. Hum. Retrovir. 2010, 26, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Evans, E.F.; Saraph, A.; Tokuyama, M. Transactivation of Human Endogenous Retroviruses by Viruses. Viruses 2024, 16, 1649. [Google Scholar] [CrossRef] [PubMed]
- Nellåker, C.; Yao, Y.; Jones-Brando, L.; Mallet, F.; Yolken, R.H.; Karlsson, H. Transactivation of elements in the human endogenous retrovirus W family by viral infection. Retrovirology 2006, 3, 44. [Google Scholar] [CrossRef]
- Yu, H.; Liu, T.; Zhao, Z.; Chen, Y.; Zeng, J.; Liu, S.; Zhu, F. Mutations in 3′-long terminal repeat of HERV-W family in chromosome 7 upregulate syncytin-1 expression in urothelial cell carcinoma of the bladder through interacting with c-Myb. Oncogene 2014, 33, 3947–3958. [Google Scholar] [CrossRef]
- Jasemi, S.; Paulus, K.; Noli, M.; Simula, E.R.; Ruberto, S.; Sechi, L.A. Antibodies against HSV-1 and curli show the highest correlation in Parkinson’s disease patients in comparison to healthy controls. Int. J. Mol. Sci. 2022, 23, 14816. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Adler, G.L.; Youssef, P.; Phan, K.; Halliday, G.M.; Dzamko, N.; Kim, W.S. Human Endogenous Retrovirus K in Astrocytes Is Altered in Parkinson’s Disease. Mov. Disord. 2025. [Google Scholar] [CrossRef]
- Jasemi, S.; Simula, E.R.; Pantaleo, A.; Sechi, L.A. Transcriptional upregulation of HERV-env genes under simulated microgravity. Viruses 2025, 17, 306. [Google Scholar] [CrossRef]
- Jasemi, S.; Simula, E.R.; Yasushi, K.; Sechi, L.A. Unveiling the impact of simulated microgravity on HSV-1 infection, neuroinflammation, and endogenous retroviral activation in SH-SY5Y cells. J. Neurovirol. 2025, 1–9. [Google Scholar] [CrossRef]
- Hosseiniporgham, S.; Sechi, L.A. Anti-HERV-K drugs and vaccines, possible therapies against tumors. Vaccines 2023, 11, 751. [Google Scholar] [CrossRef]
- Tavakolian, S.; Goudarzi, H.; Faghihloo, E. Evaluating the expression level of HERV-K env, np9, rec and gag in breast tissue. Infect. Agents Cancer 2019, 14, 42. [Google Scholar] [CrossRef]
- Gomollón, F.; Dignass, A.; Annese, V.; Tilg, H.; Van Assche, G.; Lindsay, J.O.; Peyrin-Biroulet, L.; Cullen, G.J.; Daperno, M.; Kucharzik, T. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: Part 1: Diagnosis and medical management. J. Crohns Colitis 2017, 11, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Dolinger, M.; Torres, J.; Vermeire, S. Crohn’s disease. Lancet 2024, 403, 1177–1191. [Google Scholar] [CrossRef]
- Field, N.L.; McAloon, C.G.; Gavey, L.; Mee, J.F. Mycobacterium avium subspecies paratuberculosis infection in cattle–a review in the context of seasonal pasture-based dairy herds. Ir. Vet. J. 2022, 75, 12. [Google Scholar] [CrossRef] [PubMed]
- O′Connell, L.M.; Coffey, A.; O′Mahony, J.M. Alternatives to antibiotics in veterinary medicine: Considerations for the management of Johne’s disease. Anim. Health Res. Rev. 2023, 24, 12–27. [Google Scholar] [CrossRef]
- Tovo, P.-A.; Ribaldone, D.G.; Galliano, I.; Caviglia, G.P.; Dini, M.; Veglio, V.; Calvi, C.; Montanari, P.; Pitoni, D.; Frara, S. Enhanced transcription of human endogenous retroviruses and TRIM28 downregulation in patients with inflammatory bowel disease. Viruses 2024, 16, 1570. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Z.; Wang, P.; Li, S.; Zeng, J.; Tu, X.; Yan, Q.; Xiao, Z.; Pan, M.; Zhu, F. Syncytin-1, an endogenous retroviral protein, triggers the activation of CRP via TLR3 signal cascade in glial cells. Brain Behavior. Immun. 2018, 67, 324–334. [Google Scholar] [CrossRef]
- Chamouard, P.; Richert, Z.; Meyer, N.; Rahmi, G.; Baumann, R. Diagnostic value of C-reactive protein for predicting activity level of Crohn’s disease. Clin. Gastroenterol. Hepatol. 2006, 4, 882–887. [Google Scholar] [CrossRef]
- Klag, T.; Courth, L.; Ostaff, M.J.; Ott, G.; Stange, E.F.; Malek, N.P.; Seifarth, W.; Wehkamp, J. Human endogenous retroviruses: Residues of ancient times are differentially expressed in Crohn’s disease. Inflamm. Intest. Dis. 2019, 3, 125–137. [Google Scholar] [CrossRef]
- Wang, X.; Wu, X.; Huang, J.; Li, H.; Yan, Q.; Zhu, F. Human endogenous retrovirus W family envelope protein (HERV-W env) facilitates the production of TNF-α and IL-10 by inhibiting MyD88s in glial cells. Arch. Virol. 2021, 166, 1035–1045. [Google Scholar] [CrossRef]
- Dotlacil, V.; Coufal, S.; Lerchova, T.; Zarubova, K.; Kucerova, B.; Tlaskalova-Hogenova, H.; Kverka, M.; Skaba, R.; Bronsky, J.; Hradsky, O. Intestinal tissue levels of anti-TNF alpha, antibodies, and cytokines in paediatric Crohn disease. Sci. Rep. 2025, 15, 1138. [Google Scholar] [CrossRef]
- Derkx, B.; Taminiau, J.; Radema, S.; Stronkhorst, A.; Wortel, C.; Tytgat, G.; van Deventer, S. Tumour-necrosis-factor antibody treatment in Crohn’s disease. Lancet 1993, 342, 173–174. [Google Scholar] [CrossRef] [PubMed]
- Spoettl, T.; Hausmann, M.; Klebl, F.; Dirmeier, A.; Klump, B.; Hoffmann, J.; Herfarth, H.; Timmer, A.; Rogler, G. Serum soluble TNF receptor I and II levels correlate with disease activity in IBD patients. Inflamm. Bowel Dis. 2007, 13, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Liu, Y.; Ma, X.; Liu, L.; Li, S.; Li, R.; Wang, T.; Song, H.; Niu, D. Disrupting endogenous retroelements with a reverse transcriptase inhibitor alleviates DSS-induced colitis in mice. Mucosal Immunol. 2024, 17, 54–66. [Google Scholar] [CrossRef]
- Collins, M.T.; Lisby, G.; Moser, C.; Chicks, D.; Christensen, S.; Reichelderfer, M.; Høiby, N.; Harms, B.A.; Thomsen, O.Ø.; Skibsted, U. Results of multiple diagnostic tests for Mycobacterium avium subsp. paratuberculosis in patients with inflammatory bowel disease and in controls. J. Clin. Microbiol. 2000, 38, 4373–4381. [Google Scholar] [CrossRef] [PubMed]
- Valheim, M.; Sigurðardóttir, Ó.; Storset, A.; Aune, L.; Press, C.M. Characterization of macrophages and occurrence of T cells in intestinal lesions of subclinical paratuberculosis in goats. J. Comp. Pathol. 2004, 131, 221–232. [Google Scholar] [CrossRef]
- Niegowska, M.; Wajda-Cuszlag, M.; Stępień-Ptak, G.; Trojanek, J.; Michałkiewicz, J.; Szalecki, M.; Sechi, L.A. Anti-HERV-WEnv antibodies are correlated with seroreactivity against Mycobacterium avium subsp. paratuberculosis in children and youths at T1D risk. Sci. Rep. 2019, 9, 6282. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).