Abstract
Cytomegaloviruses (CMVs) encode viral G-protein-coupled receptors (vGPCRs) that have diverged from their cellular homologues to perform new functions. Human cytomegalovirus (HCMV) encodes four vGPCRs: UL33, UL78, US27, and US28, which contribute to viral pathogenesis, cellular signalling, and latency. While the role of US28 in chemokine signalling and viral latency is well characterised, the functions of other vGPCRs remain incompletely understood. Rodent cytomegaloviruses only have homologues to UL33 and UL78, while primates have two to five additional GPCRs which are homologues of US27 and US28. Different CMVs appear to have evolved vGPCRs with functions specific to infection of their respective host. As non-human CMVs are used as model organisms to understand clinical cytomegalovirus disease and develop vaccines and antivirals, understanding the differences between these vGPCRs helps researchers understand critical differences between their models. This review aims to address the differences between CMV vGPCRs, and how these differences may affect models of CMV disease to facilitate future research.
1. Introduction
Cytomegaloviruses (CMVs) are betaherpesviruses which infect a diverse range of organisms in a species-specific manner [1,2]. This species specificity has arisen due to the divergent evolution of cytomegaloviruses with their respective hosts across millions of years [3]. During this time, cytomegaloviruses have acquired copies of genes from their host, thereby encoding homologues. These homologues include cytokines [4], ribonucleotide reductase [5], major histocompatibility (MHC) proteins [6], and G-protein-coupled receptors (GPCRs), the subject of this review.
GPCRs are a large superfamily of seven transmembrane domain proteins, which play roles in vision (such as the archetypical rhodopsin), taste, smell, and neurotransmission [7]. CMVs encode homologues of chemokine receptors which are type-A, rhodopsin-like GPCRs, and are characterised by a hallmark outward movement of transmembrane 6 during protein activation [8]. These receptors are found throughout the immune system and bind chemokines, which signal to induce chemotaxis and cellular differentiation [9]. Although not the main focus on this review, GPCRs are targets for 30–40% of current drugs [10], and the study of viral GPCRs could therefore lead to novel antiviral drugs.
GPCRs are coupled to a Gα/β/γ protein complex, and mediate signal transduction through the exchange of a GDP molecule with GTP on the coupled Gα protein (Figure 1). The Gα protein and Gβ/γ dimer then dissociate and diffuse laterally across the plasma membrane. Gα proteins canonically activate secondary messengers while Gβ/γ canonically inhibits Gα but can also signal too. There are multiple subfamilies of Gα proteins, categorised by sequence similarity, which include Gαi, Gαq, and Gαs, but host chemokine receptors signal via Gαi [11].
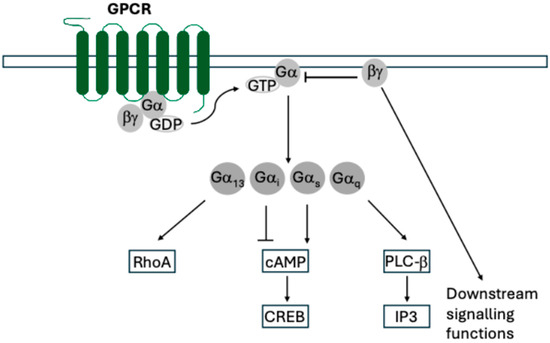
Figure 1.
General diagram of GPCR functions. Upon binding ligands, GPCRs exchange GDP for GTP on the associated Gα protein, leading to dissociation and lateral diffusion of Gα and Gβ/γ. Both complexes have downstream signalling functions with Gβ/γ also acting to sequester and inhibit Gα.
Despite being chemokine receptor homologues, the CMV-encoded GPCRs have diversified in function away from the host. CMV GPCRs tend to have signalling functions, but the importance of their signalling roles could still be better defined. Only one CMV GPCR, unique short (US)28, is known to bind extracellular chemokines, with others instead signalling constitutively, or potentially not at all. The roles of these CMV GPCRs have also diversified; some can still induce cellular chemotaxis but others play roles in viral replication, immune evasion, and latency/reactivation [12]. Additionally, some CMV GPCRs can bind multiple chemokine families, which is not seen by host chemokine receptors, and they can then signal via a range of Gα proteins [11]. Overall, this diversification of CMV GPCRs has created a class of proteins which modulate the host cell environment to favour viral replication and survival.
2. Conservation and Diversity of Cytomegalovirus GPCRs
Perhaps the most interesting aspect of the CMV GPCRs is that CMVs encode different numbers of GPCRs, and therefore, not all homologues are found in all CMVs and some CMVs encode multiple copies of some GPCRs. This raises the question of what functions these different CMV GPCRs serve in different hosts and will likely provide valuable insights into different host–virus interactions across the family of CMVs.
Human cytomegalovirus (HCMV) encodes four GPCRs: unique long (UL)33, UL78, US27, and US28 [13], which will act as the basis for this review. UL33 and UL78 are conserved among multiple mammalian CMVs, while US27 and US28 exist only in primate CMVs. This suggests a gene duplication event occurred in a common CMV ancestor, around the emergence of the primate order. This gene duplication could have occurred either from an ancestor of UL33/UL78 or from a host GPCR [12,13,14].
The HCMV-encoded GPCRs show remarkable differences between each other. In contrast to US28, which binds several human chemokines, the ligands for UL33, UL78, and US27 have not been identified [15,16]. Only UL33 and US28 have been shown to couple to Gα proteins and mediate Gα protein-dependent signalling [16,17,18], and while UL33 appears to only signal constitutively (ligand-independent), US28 can signal in both constitutive and ligand-dependent manners. US27 signals via Gβ/γ instead of Gα.
Many aspects of HCMV infections are difficult to replicate in vitro, including organ-level infection, immune interactions, and transmission in utero. Researchers therefore use animal models, infecting model organisms with their respective cytomegaloviruses, as HCMV is unable to replicate in these animals [19]. Mouse CMV (MCMV) has been used as a model for systemic CMV infection and uncovered several viral mechanisms involved in immunomodulation and viral dissemination [19,20,21,22]. Acute CMV in immunocompetent mice is characterised by robust viral replication in internal organs (such as the liver and spleen) within 2 to 3 days postinfection, followed by secondary virus dissemination to the salivary gland. It is presumed that the virus produced in the spleen is the virus that ultimately reaches the secondary sites such as salivary glands [19,23,24]. The virus can be detected in the saliva of both humans and mice for prolonged periods of time, and therefore, the salivary glands are likely important sites for horizontal transmission [19,25,26,27]. MCMV can persist in the salivary glands for months before eventually being cleared by a cluster of differentiation (CD)4+T cell responses [19,28,29]. Guinea pig CMV (GpCMV) and rhesus macaque CMV (RhCMV) are both used largely to study maternal–foetal CMV transmission, towards developing effective HCMV vaccines [30].
Understanding the differences between CMVs and their interactions with their hosts can inform which aspects of animal models are accurate and where limitations lie. As the viral GPCRs show major variability between CMV species, understanding these differences can guide our understanding of host–CMV interactions and the models we use.
3. Human Cytomegalovirus UL33 and Its Homologues
UL33 shows the closest homology to CC-chemokine receptor (CCR)3 and CCR10 [31] and currently appears to play two separate roles in CMV biology, which have not yet been linked. Firstly, UL33 and its homologues facilitate viral spread in specific cell types, which has been observed in in-vitro and in-rodent models. Additionally, UL33 has ligand-independent signalling functions, which vary somewhat between HCMV-encoded UL33, R33 and M33.
3.1. UL33 and Its Homologues Facilitate the Infection of Specific Cell Types and Organs, Which Vary Between Model Organisms
UL33 is expressed with true late kinetics [32] and plays a role in cell–cell spread in fibroblasts, but only in the Merlin strain of HCMV [33] and not in TB40E [34] or AD169 [35]. This difference may be due to Merlin preferentially spreading via the cell–cell route and. As Merlin likely better reflects clinical strains of HCMV [36], this may therefore reflect a physiologically relevant role for UL33 for viral spread in vivo. The mechanism for facilitating cell spread remains unclear, but UL33 gene deletion did not affect the expression of other HCMV proteins such as immediate early 1 (IE1), phosphoprotein (pp)28, glycoprotein B, or US28 [33], suggesting UL33 is not required to drive viral lytic infection. Merlin-encoded UL33 has a range of amino acid point mutations compared to AD169 and TB40/E, which may also change protein functions between viral strains. Identifying these differences may help explain why these strains show different phenotypes. In TB40/E, UL33 is required for replication in epithelial cells [37].
The HCMV UL33 gene is conserved among all betaherpesviruses. The murine cytomegalovirus (MCMV) and rat cytomegalovirus (RCMV) UL33 orthologs (M33 and R33, respectively) are both essential for viral replication within the host’s salivary glands and contribute to pathogenesis in vivo [19,38,39,40,41,42]. M33 promotes MCMV infection in heart tissue [43]. However, both M33 and R33 proteins are dispensable for virus replication in fibroblasts [38,39,40,41,42]. The GpCMV homologue GP33 was not required for viral pathogenesis in normal guinea pigs, but did contribute to viral load in guinea pig dams (pregnant female guinea pigs) in the spleen, liver, and placenta [44]. GP33 may also contribute to viral dissemination via the salivary glands as with MCMV and RCMV but as the bacterial artificial chromosome (BAC)-derived GpCMV used does not spread to the salivary glands, this has not yet been shown [44].
These results are similar to the role of UL33 in the viral spread of Merlin in fibroblasts [33] and TB40/E in epithelial cells [37]. The similarities in the phenotype here suggest that the UL33/M33/R33 family have maintained a function supporting cell-type-specific replication and viral pathogenesis throughout CMV speciation. Further support for this hypothesis comes from the sequencing of clinical HCMV samples, which showed extensive mutations in the extracellular loops but not the intracellular loops [45]. This is consistent with a vital signalling role without specific cytokine binding.
3.2. UL33 and Its Homologues Also Signal Constitutively
UL33 and its homologues also signal constitutively, in a ligand-independent manner. UL33 couples to Gαq/Gαi and Gαs to produce inositol phosphate (InsP) and activate cyclic AMP response element binding protein (CREB), respectively [17,18]. This constitutive activation of CREB plays a role in HCMV reactivation from latency by driving major immediate early promoter (MIEP) expression in THP-1 and Kasumi-3 models of latency [34]. As UL33 signals constitutively, how this activity is controlled to prevent MIEP activation during latency remains unclear. Possibilities include a currently unidentified agonist or antagonist ligand, increases in pUL33 expression, or interaction with another HCMV protein. pUS28 does interact with pUL33, showing reduced pUS28-mediated activation of nuclear factor kappa light-chain enhancer of activated B cells (NF-κB) [46]. Whether similar interactions occur during latency remains unclear. Apart from reactivation from latency, UL33 constitutive signalling enhances tumour growth via constitutive activation of the signal transducer and activator of the transcription (STAT)3 proangiogenic pathway to CREB, NF-κB, and serum response factor (SRF) [47].
M33 appears to have similar signalling capabilities to UL33: activating Gαq/Gα11 phospholipase C (PLC-β), protein kinase C (PKC), NF-κB, nuclear factor of activated T cells (NF-AT), and CREB transcription factors [19,48,49]. This activation happens in the absence of ligands, i.e., constitutively [18,19]. Interestingly, although M33 is more similar in sequence to UL33 compared to US28 [18], US28 is sometimes described as a functional homologue of M33, as US28 and M33 both activate NF-κB and CREB in Cos-7 cells, while UL33 only activates CREB [18]. GP33 behaves similarly to M33, activating CREB and NF-AT [44].
R33 appears to signal quite differently to its viral homologues. Although signalling constitutively [42], R33 couples to the Gαq class of G proteins and activates PLC [42]. In addition, R33 partially activates Gαi, leading to a constitutive inhibition of CREB [17,42]. In that manner, R33 differs from UL33, M33, and GP33, which enhance CREB [17]. RCMV R33 is also a potent activator of Gαq/11, while HCMV UL33 was a weak activator of Gαq/11 in some studies [17,18].
3.3. UL33 and Its Homologues—Questions Yet to Be Answered
Case et al. showed in 2008 that M33 is required for replication in NIH3T3 cells, mouse embryonic fibroblasts (MEFs), and the salivary gland of BALB/C mice, but that the signalling functions of M33 are not [49]. This raises the question of why these proteins have retained signalling function, and are not instead signalling-dead—as is thought for UL78. One possibility is that R33/M33/GP33 signal to facilitate reactivation from latency, like UL33. As M33 and GP33 both activate CREB, which activates the MIEP and drives HCMV reactivation, this appears plausible. However, R33 appears to serve the exact opposite function. It could be that R33 signals differently in different cell types, as has been shown for US28 [50], or it could be that RCMV reactivates using different signalling pathways from other rodent (and human) CMVs.
The RhCMV and GMCMV homologues of UL33 have no known functions.
4. Human Cytomegalovirus UL78 and Its Homologues
UL78 is expressed with early kinetics [51], and transcripts have been detected in some [52] but not all [53,54] latency models. UL78 has reduced sequence similarity to other GPCRs, leading to its later identification as an HCMV-encoded GPCR [12,55,56]. UL78 is classified as an orphan receptor [33,57], and consistent with this, cryo-electron microscopy (EM) analysis revealed that the chemokine binding pocket is occluded by an inward-folded extracellular loop 2 [56]. The same paper revealed a unique extracellular conformation for UL78, where extracellular architecture is more compact and lacks the participation of extracellular loop-3 (ECL3) or interaction with the N-terminus.
UL78 is dispensable for replication in fibroblasts or an ex vivo renal artery organ culture [51] but required for viral entry in endothelial and epithelial cells [58]. The precise mechanism by which UL78 contributes to viral entry is not yet known but UL78 protein is present in the infectious virion [58]. UL78 has no described signalling activity and consistent with this, intracellular loop (ICL) 2 and the extensive C terminal domain of the receptor cover the Gαi binding site on the intracellular face of the GPCR [56]. In addition to this, UL78 does not have the classic DRY motif in its active site [59] but instead has DLR, which may reduce or ablate Gα protein activation.
The structure of UL78 was solved by cryo-EM as a trimer [56], and consistent with this, UL78 interacts in the membrane with other GPCRs. The pUS28:pUL78 dimer reduces pUS28-mediated activation of NF-κB [46]. UL78 forms heteromers with CCR5 and CXC-chemokine receptor (CXCR)4, which predominantly negatively affect a plethora of functions like cell surface expression, ligand-induced internalisation, and signal transduction [60,61].
The UL78 gene is highly conserved, both among clinical isolates of HCMV [51], and among other CMVs, including all rodent and primate CMVs [62], suggesting that it plays a less species-specific role than US27 or US28. Consistent with this, the deletion of M78 from MCMV resulted in the attenuation of replication in mouse fibroblast and macrophage cell lines in vitro [62], while the deletion of R78 from rCMV attenuated in vitro replication in rat embryo fibroblasts, and reduced viral pathogenesis and replication in the spleen [38,39]. Although not showing an identical phenotype to HCMV UL78, which is not required for replication in fibroblasts, the M78 and R78 homologues clearly play a role in cell spread and viral dissemination, making them critical for the viral life cycle. GP78 does not yet have a defined function [44,63], while Rh107, the RhCMV homologue, is non-essential and can be removed for the development of CMV-based vaccines [64,65].
How UL78 and its homologues achieve this at the molecular level is less well explained. M78 undergoes constitutive endocytosis [41,66], a phenomenon that is mainly dependent on the C terminus of the receptor [67]. M78 also contributes to MHC Class II degradation in RAW-C2TA mouse macrophage cells [68]. R78 prevents the formation of syncytia in fibroblasts, but why this occurs remains unclear. The presence of these viral GPCRs in the virion [58,62] and their role supporting immediate early gene expression or viral entry [58,62], but without signalling capacity, suggest perhaps that they contribute to the virion structure in a way that facilitates entry.
5. Human Cytomegalovirus US27 and Its Homologues
US27 is expressed during lytic infection with early–late [69] kinetics, and is the only HCMV-encoded GPCR with no evidence of expression during latent infection [52]. Indeed, US27 mRNA levels are expressed at much lower levels than US28 in Kasumi-3 and CD34+ in vitro latency models [70]. As US27 and US28 are expressed during lytic infection as a polycistronic transcript [71,72], it is unclear how the US28 transcript is expressed alone during latency, or why US28 protein expression shows phosphonoformic acid (PFA)-independent early kinetics, while US27 shows PFA-dependent true late kinetics of expression [32]. Either alternative splicing, separate monocistronic mRNAs, or translation-level control must be at play.
US27 encodes a heavily glycosylated [69] orphan receptor [60,73], which shows about 30% homology to chemokine receptors [12,56,74]. Cryo-EM structures show that US27 has an occluded ligand-binding pocket and so cannot bind ligands and captures a guanosine diphosphate-bound inactive Gαi, suggesting that the receptor is constitutively unable to signal [12,56,57].
US27, like UL33, contributes to the lytic infection of fibroblasts but is not essential for growth [75], facilitating viral extracellular spread, not cell–cell spread [76]. This appears to be the case for the clinical isolates FIX and TB40/E [76], but not the more laboratory-passaged isolates AD169 or Towne [77].
US27 modulates the expression and subsequent activity of CXCR4 [73,75,78,79]. CXCR4 is a human-encoded chemokine receptor which binds CXCL12 and signals via both Gαi-Akt-MEK-ERK (extracellular signal-regulated kinase) and PI3K-IP3/DAG. Consistent with structural analysis, suggesting US27 binds inactive Gαi, the protein signals via Gβ/γ. CXCR4 plays roles in cell growth [80], chemotaxis [81], and stem cell homeostasis [82]. CXCR4 mRNA is induced by US27 as early as 3 h post-infection, indicating that US27 carried by the virion mediates this effect, US27 upregulates transcription via constitutive activation of the transcription factor nuclear respiratory factor 1 (NRF-1), via Gβ/γ activation of phosphoinositide 3-kinase (PI3K) rather than Gα [75]. Additionally, US27 colocalises with and enhances endocytosis of CXCR4 upon CXCL12 binding, which slows the recycling of CXCR4 back to the cell surface [78]. Sequencing analysis of US27 from clinical HCMV samples shows occasional deletion of the N terminal region, suggesting that US27 may not require cytokine binding for HCMV replication [83].
These effects on CXCR4 were proposed by the authors to direct infected cells to areas with high CXCL12 secretion, including bone marrow stromal tissue, which would contribute to viral persistence and immune evasion through reseeding latent infection of stem cells [75]. This hypothesis remains to be validated. Additionally, as NRF-1 activates many other genes, the more global effects of US27 on host cell transcription remain to be elucidated. Additionally, whether the modulation of CXCR4 contributes to viral extracellular spread remains to be shown.
The rodent CMVs do not encode a US27 homologue. Despite there being five US28 homologues, RhCMV (AY186194.1) does not have a clear US27 homologue either: a clustal omega search of the RhCMV genome suggests rh220 has the closest homology to US27, which only has 30% sequence identity according to Expasy SIM. ChimpCMV (NC_003521.1), however, encodes chCMVgp157, which has 52.8% sequence identity and is likely a genuine US27 homologue. This raises the question of why US27 evolved in HCMV and CCMV. Potentially, US27 may have arisen during the speciation of great apes from other primates and plays a role specific to these hosts.
6. Human Cytomegalovirus US28 and Its Homologues
Compared to the other CMV GPCRs, HCMV US28 has been studied extensively and has a myriad of established and putative roles in HCMV infection. This in itself raises a question: do all CMV GPCRs have multiple roles, which have not been discovered, or is US28 really the “Swiss army knife” as it was once described, or have these myriad roles led to more research interest than in other GPCRs?
GPCRs signal by adopting an open conformation, exposing the intracellular face of the protein and allowing G protein binding and downstream signal transduction. The structure of US28 reveals that the protein sits in the closed conformation but can spontaneously adopt the open conformation, allowing for both ligand-induced and constitutive signalling [84].
During lytic infection, both ligand-induced and constitutive signalling occur, activating multiple different signalling pathways through different Gα proteins in a cell-type- and ligand-dependent manner (Table 1). It has been noted multiple times that US28 signals differently in different cell types [85].

Table 1.
Summary of US28 signalling properties during lytic infection, adapted from [86]. Abbreviations: PLC (phospholipase C), NF-κB (nuclear factor kappa-B), SRF (serum response factor), CREB (cyclic adenosine monophosphate response element binding protein), NFAT (nuclear factor of activated T cells), VEGF (vascular endothelial growth factor), MAPK (mitogen-activated protein kinase), COX2 (cyclooxygenase-2), IL-6 (interleukin 6), JAK (janus kinase), Akt (protein kinase B), PKM2 (pyruvate kinase M2), STAT3 (signal transducer and activator of transcription 3), ERK (extracellular signal-regulated kinase), FAK (focal adhesion kinase), eNOS (endothelial nitric oxide synthase).
US28 signals during both the lytic and latent phases of HCMV infections. This signalling induces binding of β-arrestin proteins to the C terminus of the US28 and internalisation from the plasma membrane, which stops signalling. Differences in US28 functions can be delineated using protein mutations. The R129A point mutation ablates GPCR signalling through Gα protein binding [18], while Y16F ablates cytokine binding [106]. Truncation of the US28 N terminus also eliminates US28 signalling, as well as decreasing trafficking to the plasma membrane [106], while truncation of the C terminus prevents β-arrestin binding and maintains US28 on the cell surface for longer [107]. Ideally, all functions of US28 should be confirmed in comparison with the relevant US28 mutant, as this controls for the effects of protein overexpression [50]. Importantly, US28 signalling in the cell occurs in the context of whole viral infection. This means that although it is useful to study the US28 protein in isolation, the most relevant information will be corroborated in HCMV infection models controlled with relevant US28 mutant viruses.
US28 signalling during lytic infection tends to support viral replication. This occurs through a range of putative mechanisms including MIEP activation, immune evasion, oncomodulation and cell migration, which have been reviewed extensively before [86]. Most recently, US28 was shown to modulate the proliferation of U251 cells via a Gαq/11-SK1-S1P-JAK-STAT3 signalling pathway [108].
Recently, US28 was found in secreted exosomes from HCMV-infected fibroblasts. This US28 protein can bind chemokines while in the exosome, thereby acting as a chemokine scavenger, which can have meaningful effects on chemokine-induced signalling in other cells [109].
7. US28 and Latent Infection
US28 also plays a major role in HCMV latent infection. US28 appears to be necessary for latent infection [50,70] or reactivation from latency [110]. As the same viral mutants have been used by different groups [111], the most likely explanation for these differences in phenotype could be between different model systems.
Since the initial observation that US28 is required for latent infection, studies have revealed multiple mechanisms that achieve this function. Perhaps central among these is the attenuation of the mitogen-activated protein kinase (MAPK) and NF-κB signalling pathways, reducing phospho-CREB, activator protein-1 (AP-1), Akt, and NF-κB occupancy of the major immediate early promotor (Figure 2). Additionally, US28 signalling via STAT3 activates inducible nitric oxide synthase (iNOS), pushing monocytes towards an immunosuppressive phenotype to maintain latency [112]. US28 signalling has downstream effects on cellular protein expression as well, downregulating the DNA sensors myeloid cell nuclear differentiation antigen (MNDA) and interferon-gamma-induced protein IFI16 (and subsequent interferon-β signalling triggered by these proteins) [113] as well as the repressive transcription factor CTCF [114], which represses MIEP activation through DNA looping [115]. Finally, upregulation of hematopoietic cell lineage-specific protein 1 by US28 increases monocyte cell motility during latency [116] and THP-1 cell adhesion via PLC-β signalling [117].
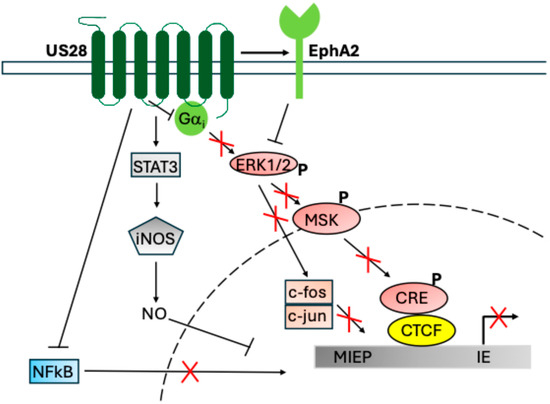
Figure 2.
US28 signalling functions during latency, adapted from Elder et al. [118]. US28 signals during latency to repress MIEP activation and downstream HCMV gene expression. Shown here are US28 activation of STAT3-iNOS-NO [112], attenuation of NF-κB [50], and activation of EphA2 [119], which leads to downstream attenuation of ERK-MSK-CREB [50] and fos [120]. NF-κB, p-CREB, and fos-jun (AP-1) directly bind and activate the MIEP in the absence of US28 [50,120]. The mechanism for NO and CTCF [115] suppression of the MIEP is less clear. Red crosses indicate reduced activity in the presence of US28.
Most fascinating about this mechanism is the fact that US28 is known to activate the MAPK/NF-κB pathways in lytic HCMV infection, while apparently having the opposite phenotype in latency. This appears to be a cell-type-dependent effect, where US28 signalling differs between monocytes (and monocytic cells) and the same cells after terminal differentiation into macrophages (or macrophage-like cells) and dendritic cells [50,120]. Indeed, earlier evidence already suggested that US28 signals in a cell-type-dependent manner [85]. The mechanisms for US28 attenuation of NF-κB and MAPK are currently unclear; however, two possible routes have been described. Firstly, US28 may interact directly with EphA2, a cell-surface integrin which activates to depress the MAPK pathway [119]; whether EphA2 also attenuates NF-κB is not known. Alternatively, recent structural analysis of the US28 protein suggests that it could bind and act as a Gαi protein “sink”, causing attenuation of signalling by sequestering the Gαi proteins that are needed for signalling [57]. As Gαi signalling could feed into multiple pathways, this may have global effects on cellular signalling such as is mediated by US28 in HCMV latency. Interestingly, this second mechanism notes that US27 ought to have similar functions to US28, although no link between US27 and latent infection currently exists [57]. These two mechanisms are not mutually exclusive and may both occur.
One very novel way to understand the importance of US28 functions in vivo is by deep sequencing US28 from clinical isolates [121]. This approach found key mutations in positions 18–25, N170, and a low rate of variability in positions 314–348 [121]. Molecular dynamics suggest that the N terminal mutations do affect cytokine binding, but importantly, the DRY signalling motif is highly conserved. This agrees with the observation that US28 signalling is required for latency, which is required for viral persistence. Intriguingly, the N170D mutation correlated with higher anti-IE1/2 antibodies and soluble IFN-a in the blood of patients with HIV, which hints at increased reactivation events. N170 is a largely uncharacterised residue in the US28 protein but is found in the third extracellular loop and might interact with binding cytokines.
US28 Homologues in Primate CMVs
Non-primate CMVs lack a direct US28 homologue, which raises an interesting question: if US28 is required for HCMV latency, how do these CMVs establish latency without US28?
M33, a US28 homologue in murine CMV, appears to play many similar roles to US28 in lytic infection [18], but has not been shown to attenuate signalling pathways in the same way as US28 during latency. However, while HCMV establishes latency in undifferentiated myeloid cells [122], MCMV latency in undifferentiated myeloid cells is controversial [123,124]. MCMV appears to establish latency in other cell types [125,126], so there may be no need for a US28 functional equivalent. M33 deletion in MCMV reduces viral load in the spleen [127], which could be interpreted either as a role in viral latency, or a role in viral tropism in cells that make up the spleen. R33, intriguingly, partially activates Gαi, leading to a constitutive inhibition of CREB [17,42], which is similar in function to US28 during latency [50]. This observation leads to a final possibility: perhaps M33/GP33/R33 attenuate signalling in relevant cell types to maintain latency?
US28 homologues are found in Old World monkey CMVs, including chimpanzee, golden macaque, and rhesus macaque CMVs, but not in closely related, non-primate CMVs, such as tree shrew CMVs or New World monkey CMVs, such as owl monkey CMVs [128] (Figure 3). This clearly indicates that US28 and its homologues emerged after the Old World/New World monkey split [128]. Chimpanzee CMV (CCMV) has both a US27 and a US28 homologue, like HCMV, while Rhesus CMV [129], golden macaque CMV [129], and cynomolgus macaque CMV [130] all have five. Rh220 can bind fractalkine [131], but little is known about the roles of these US28 homologues beyond this. It would be interesting to see which, if any, of these homologues can functionally replace US28 in an HCMV model latency system. It is likely that these US28 homologues have functions in Old World monkeys that are not required in great apes’ hosts.
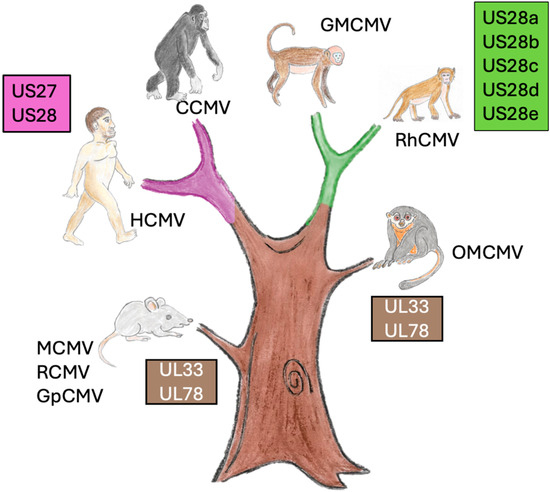
Figure 3.
Tree of life showing the evolution of vGPCRs. Every CMV, including those of rodents and New World monkeys, has a homologue of UL33 and UL78 (brown tree trunk). Great apes have US27 and US28 (red branch), while other Old World monkeys have 5 homologues of US28 (green branch).
8. Functions Derived from CMV-Encoded GPCRs in the Virion
Notably, all four HCMV GPCRs have been detected in the virion [35,62,69,120], which likely occurs as GPCRs in the plasma membrane of the viral assembly complex become part of the virion envelope. UL33 and UL78 play a direct role in viral entry [37,58], while US27 and US28 act at the immediate–early stage, before they are expressed [75,87].
If these GPCRs functioned only as virion-delivered proteins at early timepoints, one would expect their expression to be limited to the late stages of infection so that they are incorporated into the virion. This may be true for UL33, which has true late kinetics [35], but also has signalling functions that enhance MIEP activation [17].
US27’s signalling functions may be necessary throughout infection. Its early–late expression would therefore help maintain CXCR4 after virion-delivered protein is depleted. US28 plays key roles during latency, requiring both virion-delivered and expressed protein to establish and maintain latency [120]; however, why it needs to be expressed early during lytic infection is unclear. The kinetics of UL78, which is expressed early in infection [51] but is only known to play a role in viral entry [58], remain a mystery.
9. Inhibitors and Drugs Targeting GPCRs as Possible Therapeutic Options
Given their localisation to the cell surface and importance in the HCMV pathogenesis, HCMV-encoded GPCRs have been emerging as attractive targets to treat infections [50,132,133,134,135,136,137,138,139,140]. However, in order to design drugs to target GPCR activities, the mechanisms of action need to be known. The mechanism of action for UL78 is currently unknown, while UL33 appears to facilitate tropism in the absence of its signalling activity. This makes these two proteins currently difficult to target as therapies against HCMV.
US28, on the other hand, is much better characterised and both small and large molecule therapies have been developed against US28 (Table 2). These include both antagonists (which block chemokine-mediated signalling) and inverse agonists (which stop constitutive signalling as well) [50,134,140,141,142,143]. None of the small molecule inhibitors which target US28 have been refined for specific US28 binding, but VUF2274 can inhibit US28 sufficiently to reverse HCMV latency and allow targeting of latently infected cells using a “kick-and-kill” approach [50]. Alternatively, this kick-and-kill approach has also been achieved using US28 targeting inverse agonistic nanobodies [144] or nanobodies which degrade the US28 protein [145]. The US28 protein on the cell surface is already a target for host antibodies during latency [146], and so is an ideal target for nanobodies. As better-refined inverse agonists of US28 are developed, the possibility of kick and kill against US28 is becoming a reality.
Another option is F49A-FTP, a mutant of CX3CL1 with an F49A point mutation, which greatly biases binding to US28 over the host chemokine receptor CX3CR1 [147]. F49A is genetically conjugated to pseudomonas exotoxin, a protein that triggers cell death via translation inhibition [140]. This fusion toxin protein can target HCMV-infected cells in models of lytic [147] and latent [148] infection, as well as in ex vivo cadaverous lungs [149]. Further refinements to F49A-FTP show promising increases in affinity to US28 [150], suggesting possible clinical uses in the future.

Table 2.
Targets against US28.
Table 2.
Targets against US28.
| Small Molecules Discovered to Act on US28 | ||||
| Type | Target | Mechanism | References | |
| VUF2274 | Antagonist and inverse agonist | CCR1 CCL5 | Inhibits the PLC-β signalling pathway by sterically blocking chemokine binding and lowering the US28 constitutive signalling activity | [17,136,151,152] |
| Methiothepin/octoclothepin | Antagonist or partial inverse agonist | CCL5 | Blocks CCL5 and inhibits US28 constitutive activity | [16,88,141,153,154] |
| Hydroisoquinoline-based | Antagonist | CX3CR1 | Inhibits US28 constitutive activity (p42/p44 mitogen-activated protein kinase (MAPK) and p38 MAPK-dependent pathways) | [153] |
| Flavonoids | Inverse agonist | --- | Inhibit US28 constitutive activity | [155] |
| Biphenyl amide | Inverse agonist | --- | Inhibit US28 constitutive activity | [156] |
| Macromolecules Designed to Target US28 (Human Cytomegalovirus) | ||||
| Type | Mechanism | References | ||
| F49A-FTP | Fusion toxin protein (FTP) | A modified CX3CL1 which binds US28 with high affinity and targets PE to kill infected cells | [140,148,149] | |
| Bivalent nanobody | Inverse agonist | Displaces CCL5 binding to US28 and prevents constitutive activation of NF-κB and inositol triphosphate (IP3) accumulation. Impairs US28-enhanced tumour growth in vitro and in vivo | [145,157] | |
PLC: phospholipase C, FTP: fusion toxin protein, PE: pseudomonas exotoxin A.
10. Conclusions
Cytomegalovirus-encoded G-protein-coupled receptors play crucial roles in viral pathogenesis, cell spread, cell signalling, and latency. While US28 is well characterised for its involvement in chemokine signalling and latency, the roles of other vGPCRs remain less understood. Indeed, many non-HCMV-encoded vGPCRs have no defined roles. The diversity of vGPCRs among different CMVs presumably reflects their adaptation to host-specific environments. Understanding these differences is necessary for understanding the relevance of animal models in CMV research. Therapeutic strategies targeting US28 are showing promise, and further studies into other GPCRs may elucidate useful future antiviral targets.
Author Contributions
Writing—original draft and editing, S.F.; writing—review and editing, B.A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by a Wellcome award (225023/Z/22/Z) to B.A.K.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were generated or analysed in support of this review.
Acknowledgments
The authors would like to thank Marianne Perera, John Sinclair, Matthew Murray, and Charlotte Houldcroft for the helpful discussions. Illustrations in Figure 3 were a generous artistic contribution produced by Tony Krishna.
Conflicts of Interest
Author Suzan Fares is employed by the company Occlutech Holding AG. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Jurak, I.; Brune, W. Induction of apoptosis limits cytomegalovirus cross-species infection. EMBO J. 2006, 25, 2634–2642. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Maul, G.G. Mouse cytomegalovirus crosses the species barrier with help from a few human cytomegalovirus proteins. J. Virol. 2006, 80, 7510–7521. [Google Scholar] [CrossRef] [PubMed]
- Burwitz, B.J.; Malouli, D.; Bimber, B.N.; Reed, J.S.; Ventura, A.B.; Hancock, M.H.; Uebelhoer, L.S.; Bhusari, A.; Hammond, K.B.; Espinosa Trethewy, R.G. Cross-species rhesus cytomegalovirus infection of cynomolgus macaques. PLoS Pathog. 2016, 12, e1006014. [Google Scholar] [CrossRef] [PubMed]
- McSharry, B.P.; Avdic, S.; Slobedman, B. Human cytomegalovirus encoded homologs of cytokines, chemokines and their receptors: Roles in immunomodulation. Viruses 2012, 4, 2448–2470. [Google Scholar] [CrossRef]
- Fanunza, E.; Cheng, A.Z.; Auerbach, A.A.; Stefanovska, B.; Moraes, S.N.; Lokensgard, J.R.; Biolatti, M.; Dell’Oste, V.; Bierle, C.J.; Bresnahan, W.A. Human cytomegalovirus mediates APOBEC3B relocalization early during infection through a ribonucleotide reductase-independent mechanism. J. Virol. 2023, 97, e0078123. [Google Scholar] [CrossRef]
- Reyburn, H.T.; Mandelboim, O.; Valés-Gómez, M.; Davis, D.M.; Pazmany, L.; Strominger, J.L. The class I MHC homologue of human cytomegalovirus inhibits attack by natural killer cells. Nature 1997, 386, 514–517. [Google Scholar] [CrossRef]
- Geppetti, P.; Veldhuis, N.A.; Lieu, T.; Bunnett, N.W. G protein-coupled receptors: Dynamic machines for signaling pain and itch. Neuron 2015, 88, 635–649. [Google Scholar] [CrossRef]
- Zhou, Q.; Yang, D.; Wu, M.; Guo, Y.; Guo, W.; Zhong, L.; Cai, X.; Dai, A.; Jang, W.; Shakhnovich, E.I. Common activation mechanism of class A GPCRs. eLife 2019, 8, e50279. [Google Scholar] [CrossRef]
- Bachelerie, F.; Graham, G.J.; Locati, M.; Mantovani, A.; Murphy, P.M.; Nibbs, R.; Rot, A.; Sozzani, S.; Thelen, M. New nomenclature for atypical chemokine receptors. Nat. Immunol. 2014, 15, 207–208. [Google Scholar] [CrossRef]
- Hauser, A.S.; Attwood, M.M.; Rask-Andersen, M.; Schiöth, H.B.; Gloriam, D.E. Trends in GPCR drug discovery: New agents, targets and indications. Nat. Rev. Drug Discov. 2017, 16, 829–842. [Google Scholar] [CrossRef]
- O’Connor, C.; Adams, J. 4.2 G-Protein-Coupled Receptors Play Many Different Roles in Eukaryotic Cell Signaling. In Essentials of Cell Biology; NPG Education: Cambridge, MA, USA, 2010. [Google Scholar]
- Rosenkilde, M.M.; Tsutsumi, N.; Knerr, J.M.; Kildedal, D.F.; Garcia, K.C. Viral G Protein–Coupled Receptors Encoded by β- and γ-Herpesviruses. Annu. Rev. Virol. 2022, 9, 329–351. [Google Scholar] [CrossRef] [PubMed]
- Chee, M.S.; Satchwell, S.C.; Preddie, E.; Weston, K.M.; Barrell, B.G. Human cytomegalovirus encodes three G protein-coupled receptor homologues. Nature 1990, 344, 774–777. [Google Scholar] [CrossRef]
- Holst, P.J.; Lüttichau, H.R.; Schwartz, T.W.; Rosenkilde, M.M. Virally encoded chemokines and chemokine receptors in the role of viral infections. Host Response Mech. Infect. Dis. 2003, 10, 232–252. [Google Scholar]
- Randolph-Habecker, J.; Rahill, B.; Torok-Storb, B.; Vieira, J.; Kolattukudy, P.E.; Rovin, B.H.; Sedmak, D.D. The expression of the cytomegalovirus chemokine receptor homolog US28 sequesters biologically active CC chemokines and alters IL-8 production. Cytokine 2002, 19, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Vischer, H.F.; Siderius, M.; Leurs, R.; Smit, M.J. Herpesvirus-encoded GPCRs: Neglected players in inflammatory and proliferative diseases? Nat. Rev. Drug Discov. 2014, 13, 123–139. [Google Scholar] [CrossRef]
- Casarosa, P.; Gruijthuijsen, Y.K.; Michel, D.; Beisser, P.S.; Holl, J.; Fitzsimons, C.P.; Verzijl, D.; Bruggeman, C.A.; Mertens, T.; Leurs, R.; et al. Constitutive signaling of the human cytomegalovirus-encoded receptor UL33 differs from that of its rat cytomegalovirus homolog R33 by promiscuous activation of G proteins of the Gq, Gi, and Gs classes. J. Biol. Chem. 2003, 278, 50010–50023. [Google Scholar] [CrossRef]
- Waldhoer, M.; Kledal, T.N.; Farrell, H.; Schwartz, T.W. Murine Cytomegalovirus (CMV) M33 and Human CMV US28 Receptors Exhibit Similar Constitutive Signaling Activities. J. Virol. 2002, 76, 8161–8168. [Google Scholar] [CrossRef]
- Bittencourt, F.M.; Wu, S.-E.; Bridges, J.P.; Miller, W.E. The M33 G protein-coupled receptor encoded by murine cytomegalovirus is dispensable for hematogenous dissemination but is required for growth within the salivary gland. J. Virol. 2014, 88, 11811–11824. [Google Scholar] [CrossRef]
- Cekinovic, D.; Lisnic, V.; Jonjic, S.; Yurochko, A.; Miller, W. Human Cytomegaloviruses Methods and Protocols; Springer Nature: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Scalzo, A.A.; Corbett, A.J.; Rawlinson, W.D.; Scott, G.M.; Degli-Esposti, M.A. The interplay between host and viral factors in shaping the outcome of cytomegalovirus infection. Immunol. Cell Biol. 2007, 85, 46–54. [Google Scholar] [CrossRef]
- Sacher, T.; Mohr, C.A.; Weyn, A.; Schlichting, C.; Koszinowski, U.H.; Ruzsics, Z. The role of cell types in cytomegalovirus infection in vivo. Eur. J. Cell Biol. 2012, 91, 70–77. [Google Scholar] [CrossRef]
- Bale Jr, J.F.; O’Neil, M.E. Detection of murine cytomegalovirus DNA in circulating leukocytes harvested during acute infection of mice. J. Virol. 1989, 63, 2667–2673. [Google Scholar] [CrossRef] [PubMed]
- Collins, T.M.; Quirk, M.R.; Jordan, M.C. Biphasic viremia and viral gene expression in leukocytes during acute cytomegalovirus infection of mice. J. Virol. 1994, 68, 6305–6311. [Google Scholar] [CrossRef] [PubMed]
- Henson, D.; Strano, A.J. Mouse cytomegalovirus: Necrosis of infected and morphologically normal submaxillary gland acinar cells during termination of chronic infection. Am. J. Pathol. 1972, 68, 183. [Google Scholar] [PubMed]
- Humphreys, I.R.; De Trez, C.; Kinkade, A.; Benedict, C.A.; Croft, M.; Ware, C.F. Cytomegalovirus exploits IL-10–mediated immune regulation in the salivary glands. J. Exp. Med. 2007, 204, 1217–1225. [Google Scholar] [CrossRef]
- Campbell, A.E.; Cavanaugh, V.J.; Slater, J.S. The salivary glands as a privileged site of cytomegalovirus immune evasion and persistence. Med. Microbiol. Immunol. 2008, 197, 205–213. [Google Scholar] [CrossRef]
- Jonjić, S.; Pavić, I.; Lucin, P.; Rukavina, D.; Koszinowski, U.H. Efficacious control of cytomegalovirus infection after long-term depletion of CD8+ T lymphocytes. J. Virol. 1990, 64, 5457–5464. [Google Scholar] [CrossRef]
- Walton, S.M.; Mandaric, S.; Torti, N.; Zimmermann, A.; Hengel, H.; Oxenius, A. Absence of cross-presenting cells in the salivary gland and viral immune evasion confine cytomegalovirus immune control to effector CD4 T cells. PLoS Pathog. 2011, 7, e1002214. [Google Scholar] [CrossRef]
- Roark, H.K.; Jenks, J.A.; Permar, S.R.; Schleiss, M.R. Animal models of congenital cytomegalovirus transmission: Implications for vaccine development. J. Infect. Dis. 2020, 221, S60–S73. [Google Scholar] [CrossRef]
- Vink, C.; Beuken, E.; Bruggeman, C.A. Complete DNA sequence of the rat cytomegalovirus genome. J. Virol. 2000, 74, 7656–7665. [Google Scholar] [CrossRef]
- Weekes, M.P.; Tomasec, P.; Huttlin, E.L.; Fielding, C.A.; Nusinow, D.; Stanton, R.J.; Wang, E.C.; Aicheler, R.; Murrell, I.; Wilkinson, G.W. Quantitative temporal viromics: An approach to investigate host-pathogen interaction. Cell 2014, 157, 1460–1472. [Google Scholar] [CrossRef]
- van Senten, J.R.; Bebelman, M.P.; van Gasselt, P.; Bergkamp, N.D.; van den Bor, J.; Siderius, M.; Smit, M.J. Human Cytomegalovirus-Encoded G Protein-Coupled Receptor UL33 Facilitates Virus Dissemination via the Extracellular and Cell-to-Cell Route. Viruses 2020, 12, 594. [Google Scholar] [CrossRef] [PubMed]
- Krishna, B.A.; Wass, A.B.; Dooley, A.L.; O’Connor, C.M. CMV-encoded GPCR pUL33 activates CREB and facilitates its recruitment to the MIE locus for efficient viral reactivation. J. Cell Sci. 2021, 134, jcs254268. [Google Scholar] [CrossRef] [PubMed]
- Margulies, B.J.; Browne, H.; Gibson, W. Identification of the human cytomegalovirus G protein-coupled receptor homologue encoded by UL33 in infected cells and enveloped virus particles. Virology 1996, 225, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, G.W.; Davison, A.J.; Tomasec, P.; Fielding, C.A.; Aicheler, R.; Murrell, I.; Seirafian, S.; Wang, E.C.; Weekes, M.; Lehner, P.J. Human cytomegalovirus: Taking the strain. Med. Microbiol. Immunol. 2015, 204, 273–284. [Google Scholar] [CrossRef]
- Freeman, M.R.; Dooley, A.L.; Beucler, M.J.; Sanders, W.; Moorman, N.J.; O’Connor, C.M.; Miller, W.E. The Human Cytomegalovirus vGPCR UL33 is Essential for Efficient Lytic Replication in Epithelial Cells. bioRxiv 2024. [Google Scholar] [CrossRef]
- Beisser, P.S.; Vink, C.; Van Dam, J.G.; Grauls, G.; Vanherle, S.J.; Bruggeman, C.A. The R33 G protein-coupled receptor gene of rat cytomegalovirus plays an essential role in the pathogenesis of viral infection. J. Virol. 1998, 72, 2352–2363. [Google Scholar] [CrossRef]
- Kaptein, S.J.; Beisser, P.S.; Gruijthuijsen, Y.K.; Savelkouls, K.G.; van Cleef, K.W.; Beuken, E.; Grauls, G.E.; Bruggeman, C.A.; Vink, C. The rat cytomegalovirus R78 G protein-coupled receptor gene is required for production of infectious virus in the spleen. J. Gen. Virol. 2003, 84, 2517–2530. [Google Scholar] [CrossRef]
- Farrell, H.E.; Abraham, A.M.; Cardin, R.D.; Sparre-Ulrich, A.H.; Rosenkilde, M.M.; Spiess, K.; Jensen, T.H.; Kledal, T.N.; Davis-Poynter, N. Partial functional complementation between human and mouse cytomegalovirus chemokine receptor homologues. J. Virol. 2011, 85, 6091–6095. [Google Scholar] [CrossRef]
- Davis-Poynter, N.J.; Lynch, D.M.; Vally, H.; Shellam, G.R.; Rawlinson, W.D.; Barrell, B.G.; Farrell, H.E. Identification and characterization of a G protein-coupled receptor homolog encoded by murine cytomegalovirus. J. Virol. 1997, 71, 1521–1529. [Google Scholar] [CrossRef]
- Gruijthuijsen, Y.K.; Casarosa, P.; Kaptein, S.J.; Broers, J.L.; Leurs, R.; Bruggeman, C.A.; Smit, M.J.; Vink, C. The rat cytomegalovirus R33-encoded G protein-coupled receptor signals in a constitutive fashion. J. Virol. 2002, 76, 1328–1338. [Google Scholar] [CrossRef]
- Bonavita, C.M.; White, T.M.; Francis, J.; Farrell, H.E.; Davis-Poynter, N.J.; Cardin, R.D. The viral G-protein-coupled receptor homologs M33 and US28 promote cardiac dysfunction during murine cytomegalovirus infection. Viruses 2023, 15, 711. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Watanabe, S.; Katano, H.; Noguchi, K.; Sato, Y.; Kojima, S.; Miura, T.; Majima, R.; Yamada, S.; Inoue, N. Roles of GP33, a guinea pig cytomegalovirus-encoded G protein-coupled receptor homolog, in cellular signaling, viral growth and inflammation in vitro and in vivo. PLoS Pathog. 2018, 14, e1007487. [Google Scholar] [CrossRef] [PubMed]
- Deckers, M.; Hofmann, J.; Kreuzer, K.-A.; Reinhard, H.; Edubio, A.; Hengel, H.; Voigt, S.; Ehlers, B. High genotypic diversity and a novel variant of human cytomegalovirus revealed by combined UL33/UL55 genotyping with broad-range PCR. Virol. J. 2009, 6, 210. [Google Scholar] [CrossRef] [PubMed]
- Tschische, P.; Tadagaki, K.; Kamal, M.; Jockers, R.; Waldhoer, M. Heteromerization of human cytomegalovirus encoded chemokine receptors. Biochem. Pharmacol. 2011, 82, 610–619. [Google Scholar] [CrossRef]
- Van Senten, J.R.; Bebelman, M.P.; Fan, T.S.; Heukers, R.; Bergkamp, N.D.; Van Gasselt, P.; Langemeijer, E.V.; Slinger, E.; Lagerweij, T.; Rahbar, A. The human cytomegalovirus-encoded G protein–coupled receptor UL33 exhibits oncomodulatory properties. J. Biol. Chem. 2019, 294, 16297–16308. [Google Scholar] [CrossRef]
- Sherrill, J.D.; Stropes, M.P.; Schneider, O.D.; Koch, D.E.; Bittencourt, F.M.; Miller, J.L.; Miller, W.E. Activation of intracellular signaling pathways by the murine cytomegalovirus G protein-coupled receptor M33 occurs via PLC-β/PKC-dependent and-independent mechanisms. J. Virol. 2009, 83, 8141–8152. [Google Scholar] [CrossRef]
- Case, R.; Sharp, E.; Benned-Jensen, T.; Rosenkilde, M.M.; Davis-Poynter, N.; Farrell, H.E. Functional analysis of the murine cytomegalovirus chemokine receptor homologue M33: Ablation of constitutive signaling is associated with an attenuated phenotype in vivo. J. Virol. 2008, 82, 1884–1898. [Google Scholar] [CrossRef]
- Krishna, B.A.; Poole, E.L.; Jackson, S.E.; Smit, M.J.; Wills, M.R.; Sinclair, J.H. Latency-Associated Expression of Human Cytomegalovirus US28 Attenuates Cell Signaling Pathways To Maintain Latent Infection. mBio 2017, 8, e01754-17. [Google Scholar] [CrossRef]
- Michel, D.; Milotić, I.; Wagner, M.; Vaida, B.; Holl, J.; Ansorge, R.; Mertens, T. The human cytomegalovirus UL78 gene is highly conserved among clinical isolates, but is dispensable for replication in fibroblasts and a renal artery organ-culture system. J. Gen. Virol. 2005, 86, 297–306. [Google Scholar] [CrossRef]
- Cheng, S.; Caviness, K.; Buehler, J.; Smithey, M.; Nikolich-Zugich, J.; Goodrum, F. Transcriptome-wide characterization of human cytomegalovirus in natural infection and experimental latency. Proc. Natl. Acad. Sci. USA 2017, 114, E10586–E10595. [Google Scholar] [CrossRef]
- Rossetto, C.C.; Tarrant-Elorza, M.; Pari, G.S. Cis and trans acting factors involved in human cytomegalovirus experimental and natural latent infection of CD14 (+) monocytes and CD34 (+) cells. PLoS Pathog. 2013, 9, e1003366. [Google Scholar] [CrossRef] [PubMed]
- Goodrum, F.D.; Jordan, C.T.; High, K.; Shenk, T. Human cytomegalovirus gene expression during infection of primary hematopoietic progenitor cells: A model for latency. Proc. Natl. Acad. Sci. USA 2002, 99, 16255–16260. [Google Scholar] [CrossRef] [PubMed]
- Gompels, U.; Nicholas, J.; Lawrence, G.; Jones, M.; Thomson, B.; Martin, M.; Efstathiou, S.; Craxton, M.; Macaulay, H. The DNA sequence of human herpesvirus-6: Structure, coding content, and genome evolution. Virology 1995, 209, 29–51. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, Y.; Zhou, Q.; Cong, Z.; Lin, S.; Yan, J.; Chen, X.; Yang, D.; Ying, T.; Wang, M.-W. A homotrimeric GPCR architecture of the human cytomegalovirus revealed by cryo-EM. Cell Discov. 2024, 10, 52. [Google Scholar] [CrossRef]
- Tsutsumi, N.; Maeda, S.; Qu, Q.; Vögele, M.; Jude, K.M.; Suomivuori, C.-M.; Panova, O.; Waghray, D.; Kato, H.E.; Velasco, A. Atypical structural snapshots of human cytomegalovirus GPCR interactions with host G proteins. Sci. Adv. 2022, 8, eabl5442. [Google Scholar] [CrossRef]
- O’Connor, C.M.; Shenk, T. Human cytomegalovirus pUL78 G protein-coupled receptor homologue is required for timely cell entry in epithelial cells but not fibroblasts. J. Virol. 2012, 86, 11425–11433. [Google Scholar] [CrossRef]
- Rovati, G.E.; Capra, V.; Neubig, R.R. The highly conserved DRY motif of class AG protein-coupled receptors: Beyond the ground state. Mol. Pharmacol. 2007, 71, 959–964. [Google Scholar] [CrossRef]
- Tadagaki, K.; Tudor, D.; Gbahou, F.; Tschische, P.; Waldhoer, M.; Bomsel, M.; Jockers, R.; Kamal, M. Human cytomegalovirus-encoded UL33 and UL78 heteromerize with host CCR5 and CXCR4 impairing their HIV coreceptor activity. Blood J. Am. Soc. Hematol. 2012, 119, 4908–4918. [Google Scholar] [CrossRef]
- Wagner, S.; Arnold, F.; Wu, Z.; Schubert, A.; Walliser, C.; Tadagaki, K.; Jockers, R.; Mertens, T.; Michel, D. The 7-transmembrane protein homologue UL78 of the human cytomegalovirus forms oligomers and traffics between the plasma membrane and different intracellular compartments. Arch. Virol. 2012, 157, 935–949. [Google Scholar] [CrossRef]
- Oliveira, S.A.; Shenk, T.E. Murine cytomegalovirus M78 protein, a G protein-coupled receptor homologue, is a constituent of the virion and facilitates accumulation of immediate-early viral mRNA. Proc. Natl. Acad. Sci. USA 2001, 98, 3237–3242. [Google Scholar] [CrossRef]
- Schleiss, M.R.; McGregor, A.; Choi, K.Y.; Date, S.V.; Cui, X.; McVoy, M.A. Analysis of the nucleotide sequence of the guinea pig cytomegalovirus (GPCMV) genome. Virol. J. 2008, 5, 139. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.G.; Hancock, M.H.; Malouli, D.; Marshall, E.E.; Hughes, C.M.; Randall, K.T.; Morrow, D.; Ford, J.C.; Gilbride, R.M.; Selseth, A.N.; et al. Myeloid cell tropism enables MHC-E–restricted CD8+ T cell priming and vaccine efficacy by the RhCMV/SIV vaccine. Sci. Immunol. 2022, 7, eabn9301. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.G.; Zak, D.E.; Xu, G.; Ford, J.C.; Marshall, E.E.; Malouli, D.; Gilbride, R.M.; Hughes, C.M.; Ventura, A.B.; Ainslie, E.; et al. Prevention of tuberculosis in rhesus macaques by a cytomegalovirus-based vaccine. Nat. Med. 2018, 24, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Sharp, E.; Davis-Poynter, N.; Farrell, H. Analysis of the subcellular trafficking properties of murine cytomegalovirus M78, a 7 transmembrane receptor homologue. J. Gen. Virol. 2009, 90, 59–68. [Google Scholar] [CrossRef]
- Davis-Poynter, N.; Yunis, J.; Farrell, H.E. The cytoplasmic C-tail of the mouse cytomegalovirus 7 transmembrane receptor homologue, M78, regulates endocytosis of the receptor and modulates virus replication in different cell types. PLoS ONE 2016, 11, e0165066. [Google Scholar] [CrossRef]
- Yunis, J.; Farrell, H.E.; Bruce, K.; Lawler, C.; Sidenius, S.; Wyer, O.; Davis-Poynter, N.; Stevenson, P.G. Murine cytomegalovirus degrades MHC class II to colonize the salivary glands. PLoS Pathog. 2018, 14, e1006905. [Google Scholar] [CrossRef]
- Margulies, B.J.; Gibson, W. The chemokine receptor homologue encoded by US27 of human cytomegalovirus is heavily glycosylated and is present in infected human foreskin fibroblasts and enveloped virus particles. Virus Res. 2007, 123, 57–71. [Google Scholar] [CrossRef]
- Humby, M.S.; O’Connor, C.M. Human Cytomegalovirus US28 Is Important for Latent Infection of Hematopoietic Progenitor Cells. J. Virol. 2015, 90, 2959–2970. [Google Scholar] [CrossRef]
- Welch, A.R.; McGregor, L.M.; Gibson, W. Cytomegalovirus homologs of cellular G protein-coupled receptor genes are transcribed. J. Virol. 1991, 65, 3915–3918. [Google Scholar] [CrossRef]
- Balázs, Z.; Tombácz, D.; Szűcs, A.; Csabai, Z.; Megyeri, K.; Petrov, A.N.; Snyder, M.; Boldogkői, Z. Long-Read Sequencing of Human Cytomegalovirus Transcriptome Reveals RNA Isoforms Carrying Distinct Coding Potentials. Sci. Rep. 2017, 7, 15989. [Google Scholar] [CrossRef]
- Arnolds, K.L.; Lares, A.P.; Spencer, J.V. The US27 gene product of human cytomegalovirus enhances signaling of host chemokine receptor CXCR4. Virology 2013, 439, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Stegman, J.R.; Margulies, B.J. The human cytomegalovirus chemokine receptor homolog encoded by US27. Virus Genes 2017, 53, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Boeck, J.M.; Stowell, G.A.; O’Connor, C.M.; Spencer, J.V. The human cytomegalovirus US27 gene product constitutively activates antioxidant response element-mediated transcription through Gβγ, phosphoinositide 3-kinase, and nuclear respiratory factor 1. J. Virol. 2018, 92, e02185-18. [Google Scholar] [CrossRef]
- O’Connor, C.M.; Shenk, T. Human cytomegalovirus pUS27 G protein-coupled receptor homologue is required for efficient spread by the extracellular route but not for direct cell-to-cell spread. J. Virol. 2011, 85, 3700–3707. [Google Scholar] [CrossRef] [PubMed]
- Dunn, W.; Chou, C.; Li, H.; Hai, R.; Patterson, D.; Stolc, V.; Zhu, H.; Liu, F. Functional profiling of a human cytomegalovirus genome. Proc. Natl. Acad. Sci. USA 2003, 100, 14223–14228. [Google Scholar] [CrossRef]
- Boeck, J.M.; Spencer, J.V. Effect of human cytomegalovirus (HCMV) US27 on CXCR4 receptor internalization measured by fluorogen-activating protein (FAP) biosensors. PLoS ONE 2017, 12, e0172042. [Google Scholar] [CrossRef]
- Frank, T.; Reichel, A.; Larsen, O.; Stilp, A.-C.; Rosenkilde, M.M.; Stamminger, T.; Ozawa, T.; Tschammer, N. Attenuation of chemokine receptor function and surface expression as an immunomodulatory strategy employed by human cytomegalovirus is linked to vGPCR US28. Cell Commun. Signal. 2016, 14, 31. [Google Scholar] [CrossRef]
- Bianchi, M.E.; Mezzapelle, R. The chemokine receptor CXCR4 in cell proliferation and tissue regeneration. Front. Immunol. 2020, 11, 2109. [Google Scholar] [CrossRef]
- Gao, A.; Lin, Y.; Chai, Y.; Han, J.; Wu, L.; Ye, J. CXCL12/CXCR4 Axis promotes the chemotaxis and phagocytosis of B cells through the PI3K-AKT signaling pathway in an early vertebrate. J. Immunol. 2024, 213, 1676–1690. [Google Scholar] [CrossRef]
- Singh, P.; Mohammad, K.S.; Pelus, L.M. CXCR4 expression in the bone marrow microenvironment is required for hematopoietic stem and progenitor cell maintenance and early hematopoietic regeneration after myeloablation. Stem Cells 2020, 38, 849–859. [Google Scholar] [CrossRef]
- Sijmons, S.; Thys, K.; Mbong Ngwese, M.; Van Damme, E.; Dvorak, J.; Van Loock, M.; Li, G.; Tachezy, R.; Busson, L.; Aerssens, J. High-throughput analysis of human cytomegalovirus genome diversity highlights the widespread occurrence of gene-disrupting mutations and pervasive recombination. J. Virol. 2015, 89, 7673–7695. [Google Scholar] [CrossRef] [PubMed]
- Burg, J.S.; Ingram, J.R.; Venkatakrishnan, A.; Jude, K.M.; Dukkipati, A.; Feinberg, E.N.; Angelini, A.; Waghray, D.; Dror, R.O.; Ploegh, H.L. Structural basis for chemokine recognition and activation of a viral G protein–coupled receptor. Science 2015, 347, 1113–1117. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.E.; Zagorski, W.A.; Brenneman, J.D.; Avery, D.; Miller, J.L.; O’Connor, C.M. US28 is a potent activator of phospholipase C during HCMV infection of clinically relevant target cells. PLoS ONE 2012, 7, e50524. [Google Scholar] [CrossRef] [PubMed]
- Krishna, B.A.; Miller, W.E.; O’Connor, C.M. US28: HCMV’s Swiss Army knife. Viruses 2018, 10, 445. [Google Scholar] [CrossRef]
- Casarosa, P.; Bakker, R.A.; Verzijl, D.; Navis, M.; Timmerman, H.; Leurs, R.; Smit, M.J. Constitutive signaling of the human cytomegalovirus-encoded chemokine receptor US28. J. Biol. Chem. 2001, 276, 1133–1137. [Google Scholar] [CrossRef]
- Fraile-Ramos, A.; Kledal, T.N.; Pelchen-Matthews, A.; Bowers, K.; Schwartz, T.W.; Marsh, M. The human cytomegalovirus US28 protein is located in endocytic vesicles and undergoes constitutive endocytosis and recycling. Mol. Biol. Cell 2001, 12, 1737–1749. [Google Scholar] [CrossRef]
- Bakker, R.A.; Casarosa, P.; Timmerman, H.; Smit, M.J.; Leurs, R. Constitutively active Gq/11-coupled receptors enable signaling by co-expressed Gi/o-coupled receptors. J. Biol. Chem. 2004, 279, 5152–5161. [Google Scholar] [CrossRef]
- Moepps, B.; Tulone, C.; Kern, C.; Minisini, R.; Michels, G.; Vatter, P.; Wieland, T.; Gierschik, P. Constitutive serum response factor activation by the viral chemokine receptor homologue pUS28 is differentially regulated by Gαq/11 and Gα16. Cell. Signal. 2008, 20, 1528–1537. [Google Scholar] [CrossRef]
- Minisini, R.; Tulone, C.; Lüske, A.; Michel, D.; Mertens, T.; Gierschik, P.; Moepps, B. Constitutive inositol phosphate formation in cytomegalovirus-infected human fibroblasts is due to expression of the chemokine receptor homologue pUS28. J. Virol. 2003, 77, 4489–4501. [Google Scholar] [CrossRef]
- Neote, K.; DiGregorio, D.; Mak, J.Y.; Horuk, R.; Schall, T.J. Molecular cloning, functional expression, and signaling characteristics of a CC chemokine receptor. Cell 1993, 72, 415–425. [Google Scholar] [CrossRef]
- Maussang, D.; Verzijl, D.; Van Walsum, M.; Leurs, R.; Holl, J.; Pleskoff, O.; Michel, D.; Van Dongen, G.A.; Smit, M.J. Human cytomegalovirus-encoded chemokine receptor US28 promotes tumorigenesis. Proc. Natl. Acad. Sci. USA 2006, 103, 13068–13073. [Google Scholar] [CrossRef] [PubMed]
- Maussang, D.; Langemeijer, E.; Fitzsimons, C.P.; Stigter-van Walsum, M.; Dijkman, R.; Borg, M.K.; Slinger, E.; Schreiber, A.; Michel, D.; Tensen, C.P. The human cytomegalovirus–encoded chemokine receptor US28 promotes angiogenesis and tumor formation via cyclooxygenase-2. Cancer Res. 2009, 69, 2861–2869. [Google Scholar] [CrossRef] [PubMed]
- Slinger, E.; Maussang, D.; Schreiber, A.; Siderius, M.; Rahbar, A.; Fraile-Ramos, A.; Lira, S.A.; Söderberg-Nauclér, C.; Smit, M.J. HCMV-encoded chemokine receptor US28 mediates proliferative signaling through the IL-6–STAT3 axis. Sci. Signal. 2010, 3, ra58. [Google Scholar] [CrossRef] [PubMed]
- Langemeijer, E.V.; Slinger, E.; de Munnik, S.; Schreiber, A.; Maussang, D.; Vischer, H.; Verkaar, F.; Leurs, R.; Siderius, M.; Smit, M.J. Constitutive ss-catenin signaling by the viral chemokine receptor US28. PLoS ONE 2012, 7, e48935. [Google Scholar] [CrossRef]
- de Wit, R.H.; Mujić-Delić, A.; van Senten, J.R.; Fraile-Ramos, A.; Siderius, M.; Smit, M.J. Human cytomegalovirus encoded chemokine receptor US28 activates the HIF-1α/PKM2 axis in glioblastoma cells. Oncotarget 2016, 7, 67966. [Google Scholar] [CrossRef]
- Gao, J.-L.; Murphy, P.M. Human cytomegalovirus open reading frame US28 encodes a functional beta chemokine receptor. J. Biol. Chem. 1994, 269, 28539–28542. [Google Scholar] [CrossRef]
- Billstrom, M.A.; Johnson, G.L.; Avdi, N.J.; Worthen, G.S. Intracellular signaling by the chemokine receptor US28 during human cytomegalovirus infection. J. Virol. 1998, 72, 5535–5544. [Google Scholar] [CrossRef]
- Vieira, J.; Schall, T.J.; Corey, L.; Geballe, A.P. Functional analysis of the human cytomegalovirus US28 gene by insertion mutagenesis with the green fluorescent protein gene. J. Virol. 1998, 72, 8158–8165. [Google Scholar] [CrossRef]
- Melnychuk, R.M.; Streblow, D.N.; Smith, P.P.; Hirsch, A.J.; Pancheva, D.; Nelson, J.A. Human cytomegalovirus-encoded G protein-coupled receptor US28 mediates smooth muscle cell migration through Gα12. J. Virol. 2004, 78, 8382–8391. [Google Scholar] [CrossRef]
- Streblow, D.N.; Vomaske, J.; Smith, P.; Melnychuk, R.; Hall, L.; Pancheva, D.; Smit, M.; Casarosa, P.; Schlaepfer, D.D.; Nelson, J.A. Human cytomegalovirus chemokine receptor US28-induced smooth muscle cell migration is mediated by focal adhesion kinase and Src. J. Biol. Chem. 2003, 278, 50456–50465. [Google Scholar] [CrossRef]
- Vomaske, J.; Melnychuk, R.M.; Smith, P.P.; Powell, J.; Hall, L.; DeFilippis, V.; Früh, K.; Smit, M.; Schlaepfer, D.D.; Nelson, J.A. Differential ligand binding to a human cytomegalovirus chemokine receptor determines cell type–specific motility. PLoS Pathog. 2009, 5, e1000304. [Google Scholar] [CrossRef] [PubMed]
- Streblow, D.N.; Soderberg-Naucler, C.; Vieira, J.; Smith, P.; Wakabayashi, E.; Ruchti, F.; Mattison, K.; Altschuler, Y.; Nelson, J.A. The human cytomegalovirus chemokine receptor US28 mediates vascular smooth muscle cell migration. Cell 1999, 99, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Soroceanu, L.; Matlaf, L.; Bezrookove, V.; Harkins, L.; Martinez, R.; Greene, M.; Soteropoulos, P.; Cobbs, C.S. Human cytomegalovirus US28 found in glioblastoma promotes an invasive and angiogenic phenotype. Cancer Res. 2011, 71, 6643–6653. [Google Scholar] [CrossRef] [PubMed]
- Casarosa, P.; Waldhoer, M.; LiWang, P.J.; Vischer, H.F.; Kledal, T.; Timmerman, H.; Schwartz, T.W.; Smit, M.J.; Leurs, R. CC and CX3C chemokines differentially interact with the N terminus of the human cytomegalovirus-encoded US28 receptor. J. Biol. Chem. 2005, 280, 3275–3285. [Google Scholar] [CrossRef]
- Miller, W.E.; Houtz, D.A.; Nelson, C.D.; Kolattukudy, P.; Lefkowitz, R.J. G-protein-coupled receptor (GPCR) kinase phosphorylation and β-arrestin recruitment regulate the constitutive signaling activity of the human cytomegalovirus US28 GPCR. J. Biol. Chem. 2003, 278, 21663–21671. [Google Scholar] [CrossRef]
- Bergkamp, N.D.; van Senten, J.R.; Brink, H.J.; Bebelman, M.P.; van den Bor, J.; Çobanoğlu, T.S.; Dinkla, K.; Köster, J.; Klau, G.; Siderius, M. A virally encoded GPCR drives glioblastoma through feed-forward activation of the SK1-S1P1 signaling axis. Sci. Signal. 2023, 16, eade6737. [Google Scholar] [CrossRef]
- Bebelman, M.P.; Setiawan, I.M.; Bergkamp, N.D.; van Senten, J.R.; Crudden, C.; Bebelman, J.P.M.; Verweij, F.J.; van Niel, G.; Siderius, M.; Pegtel, D.M. Exosomal release of the virus-encoded chemokine receptor US28 contributes to chemokine scavenging. iScience 2023, 26, 107412. [Google Scholar] [CrossRef]
- Crawford, L.B.; Caposio, P.; Kreklywich, C.; Pham, A.H.; Hancock, M.H.; Jones, T.A.; Smith, P.P.; Yurochko, A.D.; Nelson, J.A.; Streblow, D.N. Human cytomegalovirus US28 ligand binding activity is required for latency in CD34+ hematopoietic progenitor cells and humanized NSG mice. mBio 2019, 10, 10-1128. [Google Scholar] [CrossRef]
- Krishna, B.A.; Wass, A.B.; Sridharan, R.; O’Connor, C.M. The Requirement for US28 During Cytomegalovirus Latency Is Independent of US27 and US29 Gene Expression. Front. Cell. Infect. Microbiol. 2020, 10, 186. [Google Scholar] [CrossRef]
- Zhu, D.; Pan, C.; Sheng, J.; Liang, H.; Bian, Z.; Liu, Y.; Trang, P.; Wu, J.; Liu, F.; Zhang, C.-Y.; et al. Human cytomegalovirus reprogrammes haematopoietic progenitor cells into immunosuppressive monocytes to achieve latency. Nat. Microbiol. 2018, 3, 503–513. [Google Scholar] [CrossRef]
- Elder, E.G.; Krishna, B.A.; Williamson, J.; Lim, E.Y.; Poole, E.; Sedikides, G.X.; Wills, M.; O’Connor, C.M.; Lehner, P.J.; Sinclair, J. Interferon-Responsive Genes Are Targeted during the Establishment of Human Cytomegalovirus Latency. mBio 2019, 10, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Elder, E.G.; Krishna, B.A.; Poole, E.; Perera, M.; Sinclair, J. Regulation of host and viral promoters during human cytomegalovirus latency via US28 and CTCF. J. Gen. Virol. 2021, 102, 001609. [Google Scholar] [CrossRef] [PubMed]
- Groves, I.J.; Matthews, S.M.; O’Connor, C.M. Host-encoded CTCF regulates human cytomegalovirus latency via chromatin looping. Proc. Natl. Acad. Sci. USA 2024, 121, e2315860121. [Google Scholar] [CrossRef] [PubMed]
- Aslam, Y.; Williamson, J.; Romashova, V.; Elder, E.; Krishna, B.; Wills, M.; Lehner, P.; Sinclair, J.; Poole, E. Human cytomegalovirus upregulates expression of HCLS1 resulting in increased cell motility and transendothelial migration during latency. iScience 2019, 20, 60–72. [Google Scholar] [CrossRef]
- Wu, S.-e.; Miller, W.E. The HCMV US28 vGPCR induces potent Gαq/PLC-β signaling in monocytes leading to increased adhesion to endothelial cells. Virology 2016, 497, 233–243. [Google Scholar] [CrossRef]
- Elder, E.; Sinclair, J. HCMV latency: What regulates the regulators? Med. Microbiol. Immunol. 2019, 208, 431–438. [Google Scholar] [CrossRef]
- Wass, A.B.; Krishna, B.A.; Herring, L.E.; Gilbert, T.S.; Nukui, M.; Groves, I.J.; Dooley, A.L.; Kulp, K.H.; Matthews, S.M.; Rotroff, D.M. Cytomegalovirus US28 regulates cellular EphA2 to maintain viral latency. Sci. Adv. 2022, 8, eadd1168. [Google Scholar] [CrossRef]
- Krishna, B.A.; Humby, M.S.; Miller, W.E.; O’Connor, C.M. Human cytomegalovirus G protein-coupled receptor US28 promotes latency by attenuating c-fos. Proc. Natl. Acad. Sci. USA 2019, 116, 1755–1764. [Google Scholar] [CrossRef]
- Waters, S.; Agostino, M.; Lee, S.; Ariyanto, I.; Kresoje, N.; Leary, S.; Munyard, K.; Gaudieri, S.; Gaff, J.; Irish, A. Sequencing directly from clinical specimens reveals genetic variations in HCMV-encoded chemokine receptor US28 that may influence antibody levels and interactions with human chemokines. Microbiol. Spectr. 2021, 9, e00020–e00021. [Google Scholar] [CrossRef]
- Sinclair, J.; Sissons, P. Latency and reactivation of human cytomegalovirus. J. Gen. Virol. 2006, 87, 1763–1779. [Google Scholar] [CrossRef]
- Liu, X.-f.; Swaminathan, S.; Yan, S.; Engelmann, F.; Abbott, D.A.; VanOsdol, L.A.; Heald-Sargent, T.; Qiu, L.; Chen, Q.; Iovane, A. A novel murine model of differentiation-mediated cytomegalovirus reactivation from latently infected bone marrow haematopoietic cells. J. Gen. Virol. 2019, 100, 1680–1694. [Google Scholar] [CrossRef] [PubMed]
- Seckert, C.K.; Renzaho, A.; Tervo, H.-M.; Krause, C.; Deegen, P.; Kühnapfel, B.; Reddehase, M.J.; Grzimek, N.K. Liver sinusoidal endothelial cells are a site of murine cytomegalovirus latency and reactivation. J. Virol. 2009, 83, 8869–8884. [Google Scholar] [CrossRef] [PubMed]
- Pollock, J.L.; Presti, R.M.; Paetzold, S.; IVth, H.W.V. Latent murine cytomegalovirus infection in macrophages. Virology 1997, 227, 168–179. [Google Scholar] [CrossRef]
- Brautigam, A.R.; Dutko, F.J.; Olding, L.B.; Oldstone, M.B. Pathogenesis of murine cytomegalovirus infection: The macrophage as a permissive cell for cytomegalovirus infection, replication and latency. J. Gen. Virol. 1979, 44, 349–359. [Google Scholar] [CrossRef]
- Cardin, R.D.; Schaefer, G.C.; Allen, J.R.; Davis-Poynter, N.J.; Farrell, H.E. The M33 chemokine receptor homolog of murine cytomegalovirus exhibits a differential tissue-specific role during in vivo replication and latency. J. Virol. 2009, 83, 7590–7601. [Google Scholar] [CrossRef] [PubMed]
- Alcendor, D.J.; Zong, J.; Dolan, A.; Gatherer, D.; Davison, A.J.; Hayward, G.S. Patterns of divergence in the vCXCL and vGPCR gene clusters in primate cytomegalovirus genomes. Virology 2009, 395, 21–32. [Google Scholar] [CrossRef]
- Hansen, S.G.; Strelow, L.I.; Franchi, D.C.; Anders, D.G.; Wong, S.W. Complete sequence and genomic analysis of rhesus cytomegalovirus. J. Virol. 2003, 77, 6620–6636. [Google Scholar] [CrossRef]
- Marsh, A.K.; Willer, D.O.; Ambagala, A.P.; Dzamba, M.; Chan, J.K.; Pilon, R.; Fournier, J.; Sandstrom, P.; Brudno, M.; MacDonald, K.S. Genomic sequencing and characterization of cynomolgus macaque cytomegalovirus. J. Virol. 2011, 85, 12995–13009. [Google Scholar] [CrossRef]
- Penfold, M.; Schmidt, T.; Dairaghi, D.; Barry, P.; Schall, T. Characterization of the rhesus cytomegalovirus US28 locus. J. Virol. 2003, 77, 10404–10413. [Google Scholar] [CrossRef]
- Arfelt, K.N.; Fares, S.; Rosenkilde, M.M. EBV, the human host, and the 7TM receptors: Defense or offense? Prog. Mol. Biol. Transl. Sci. 2015, 129, 395–427. [Google Scholar]
- Berg, C.; Spiess, K.; Lüttichau, H.R.; Rosenkilde, M.M. Biased small-molecule ligands for selective inhibition of HIV-1 cell entry via CCR5. Pharmacol. Res. Perspect. 2016, 4, e00262. [Google Scholar] [CrossRef] [PubMed]
- Fares, S.; Spiess, K.; Olesen, E.T.; Zuo, J.; Jackson, S.; Kledal, T.N.; Wills, M.R.; Rosenkilde, M.M. Distinct roles of extracellular domains in the epstein-barr virus-encoded BILF1 receptor for signaling and major histocompatibility complex class I downregulation. mBio 2019, 10, e01707-18. [Google Scholar] [CrossRef] [PubMed]
- Krishna, B.A.; Wills, M.R.; Sinclair, J.H. Advances in the treatment of cytomegalovirus. Br. Med. Bull. 2019, 131, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Chung, Y.H.; Lee, C. US28, a virally-encoded GPCR as an antiviral target for human cytomegalovirus infection. Biomol. Ther. 2017, 25, 69. [Google Scholar] [CrossRef]
- Lüttichau, H.; Schwartz, T. Validation of chemokine receptors as drug targets. Curr. Opin. Drug Discov. Dev. 2000, 3, 610–623. [Google Scholar]
- Rosenkilde, M.M.; Kledal, T.N. Targeting herpesvirus reliance of the chemokine system. Curr. Drug Targets 2006, 7, 103–118. [Google Scholar] [CrossRef]
- Smit, J.; Vink, C.; Verzijl, D.; Casarosa, P.; Bruggeman, A.; Leurs, R. Virally encoded G protein-coupled receptors: Targets for potentially innovative anti-viral drug development. Curr. Drug Targets 2003, 4, 431–441. [Google Scholar] [CrossRef]
- Spiess, K.; Jeppesen, M.G.; Malmgaard-Clausen, M.; Krzywkowski, K.; Kledal, T.N.; Rosenkilde, M.M. Novel Chemokine-Based Immunotoxins for Potent and Selective Targeting of Cytomegalovirus Infected Cells. J. Immunol. Res. 2017, 2017, 4069260. [Google Scholar] [CrossRef]
- Hulshof, J.W.; Vischer, H.F.; Verheij, M.H.; Fratantoni, S.A.; Smit, M.J.; de Esch, I.J.; Leurs, R. Synthesis and pharmacological characterization of novel inverse agonists acting on the viral-encoded chemokine receptor US28. Bioorg. Med. Chem. 2006, 14, 7213–7230. [Google Scholar] [CrossRef]
- Hassing, H.A.; Fares, S.; Larsen, O.; Pad, H.; Hauge, M.; Jones, R.M.; Schwartz, T.W.; Hansen, H.S.; Rosenkilde, M.M. Biased signaling of lipids and allosteric actions of synthetic molecules for GPR119. Biochem. Pharmacol. 2016, 119, 66–75. [Google Scholar] [CrossRef]
- Steen, A.; Larsen, O.; Thiele, S.; Rosenkilde, M.M. Biased and g protein-independent signaling of chemokine receptors. Front. Immunol. 2014, 5, 277. [Google Scholar] [CrossRef] [PubMed]
- De Groof, T.W.; Elder, E.G.; Lim, E.Y.; Heukers, R.; Bergkamp, N.D.; Groves, I.J.; Wills, M.; Sinclair, J.H.; Smit, M.J. Targeting the latent human cytomegalovirus reservoir for T-cell-mediated killing with virus-specific nanobodies. Nat. Commun. 2021, 12, 4436. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, J.; Poole, E.; Groves, I.; Owen, D.J.; Graham, S.C.; Sinclair, J.; Kelly, B.T. Repurposing an endogenous degradation domain for antibody-mediated disposal of cell-surface proteins. EMBO Rep. 2024, 25, 951–970. [Google Scholar] [CrossRef] [PubMed]
- Elder, E.; Krishna, B.; Williamson, J.; Aslam, Y.; Farahi, N.; Wood, A.; Romashova, V.; Roche, K.; Murphy, E.; Chilvers, E. Monocytes latently infected with human cytomegalovirus evade neutrophil killing. iScience 2019, 12, 13–26. [Google Scholar] [CrossRef]
- Spiess, K.; Jeppesen, M.G.; Malmgaard-Clausen, M.; Krzywkowski, K.; Dulal, K.; Cheng, T.; Hjortø, G.M.; Larsen, O.; Burg, J.S.; Jarvis, M.A. Rationally designed chemokine-based toxin targeting the viral G protein-coupled receptor US28 potently inhibits cytomegalovirus infection in vivo. Proc. Natl. Acad. Sci. USA 2015, 112, 8427–8432. [Google Scholar] [CrossRef]
- Krishna, B.A.; Spiess, K.; Poole, E.L.; Lau, B.; Voigt, S.; Kledal, T.N.; Rosenkilde, M.M.; Sinclair, J.H. Targeting the latent cytomegalovirus reservoir with an antiviral fusion toxin protein. Nat. Commun. 2017, 8, 14321. [Google Scholar] [CrossRef]
- Ribeiro, R.V.; Ku, T.; Wang, A.; Pires, L.; Ferreira, V.H.; Michaelsen, V.; Ali, A.; Galasso, M.; Moshkelgosha, S.; Gazzalle, A. Ex vivo treatment of cytomegalovirus in human donor lungs using a novel chemokine-based immunotoxin. J. Heart Lung Transplant. 2022, 41, 287–297. [Google Scholar] [CrossRef]
- Elder, E.G. Understanding and Exploiting Viral Protein US28 During Human Cytomegalovirus Latency. Doctoral Dissertation, University of Cambridge, Cambridge, UK, 2020. [Google Scholar]
- Hesselgesser, J.; Ng, H.P.; Liang, M.; Zheng, W.; May, K.; Bauman, J.G.; Monahan, S.; Islam, I.; Wei, G.P.; Ghannam, A. Identification and characterization of small molecule functional antagonists of the CCR1 chemokine receptor. J. Biol. Chem. 1998, 273, 15687–15692. [Google Scholar] [CrossRef]
- Tschammer, N. Allosteric modulation of the G protein-coupled US28 receptor of human cytomegalovirus: Are the small-weight inverse agonist of US28 ‘camouflaged’ agonists? Bioorg. Med. Chem. Lett. 2014, 24, 3744–3747. [Google Scholar] [CrossRef]
- Kralj, A.; Wetzel, A.; Mahmoudian, S.; Stamminger, T.; Tschammer, N.; Heinrich, M.R. Identification of novel allosteric modulators for the G-protein coupled US28 receptor of human cytomegalovirus. Bioorg. Med. Chem. Lett. 2011, 21, 5446–5450. [Google Scholar] [CrossRef]
- Vischer, H.F.; Hulshof, J.W.; Hulscher, S.; Fratantoni, S.A.; Verheij, M.H.; Victorina, J.; Smit, M.J.; de Esch, I.J.; Leurs, R. Identification of novel allosteric nonpeptidergic inhibitors of the human cytomegalovirus-encoded chemokine receptor US28. Bioorg. Med. Chem. 2010, 18, 675–688. [Google Scholar] [CrossRef] [PubMed]
- Kralj, A.; Nguyen, M.-T.; Tschammer, N.; Ocampo, N.; Gesiotto, Q.; Heinrich, M.R.; Phanstiel IV, O. Development of flavonoid-based inverse agonists of the key signaling receptor US28 of human cytomegalovirus. J. Med. Chem. 2013, 56, 5019–5032. [Google Scholar] [CrossRef] [PubMed]
- Kralj, A.; Kurt, E.; Tschammer, N.; Heinrich, M.R. Synthesis and biological evaluation of biphenyl amides that modulate the US28 receptor. ChemMedChem 2014, 9, 151–168. [Google Scholar] [CrossRef] [PubMed]
- Heukers, R.; Fan, T.S.; de Wit, R.H.; van Senten, J.R.; De Groof, T.W.; Bebelman, M.P.; Lagerweij, T.; Vieira, J.; de Munnik, S.M.; Smits-de Vries, L. The constitutive activity of the virally encoded chemokine receptor US28 accelerates glioblastoma growth. Oncogene 2018, 37, 4110–4121. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).