Pediococcus pentosaceus MZF16 Probiotic Strain Prevents In Vitro Cytotoxic Effects of Pseudomonas aeruginosa H103 and Prolongs the Lifespan of Caenorhabditis elegans
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
2.2. P. aeruginosa H103-gfp and P. pentosaceus MZF16-mCherry Construction
2.3. Caco-2/TC7 Cell Culture and Infection
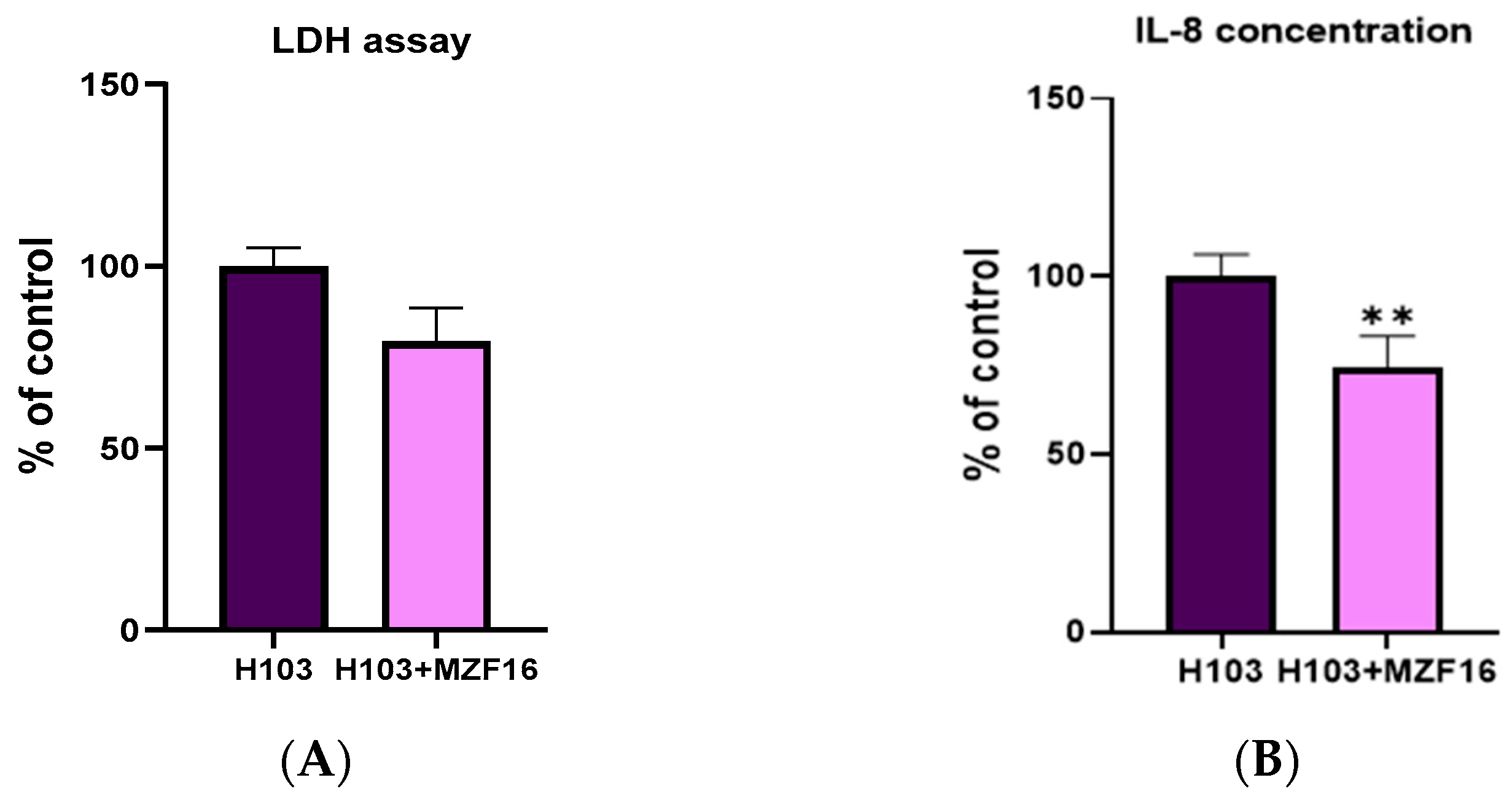
2.4. Cytotoxicity Assay and Interleukin-8 Quantification
2.5. In Vitro Adhesion and Invasion Assay
2.6. Bacterial Translocation and Transepithelial Electrical Resistance (TER) Measurement
2.7. Antimicrobial Activity Test
2.8. Bacterial Aggregation Assay
2.9. Pyoverdine Production Measurement
2.10. Extraction and Quantification of AHL and HAQ Molecules
2.11. In Vivo Caenorhabditis elegans Killing Assay
2.12. Statistical Analysis
3. Results
3.1. Cytotoxicity Assay and Interleukin-8 Quantification
3.2. Adhesion and Invasion
3.3. Transepithelial Electric Resistance (TER) and Translocation
3.4. Autoaggregation and Coaggregation
3.5. Antimicrobial Activity Test
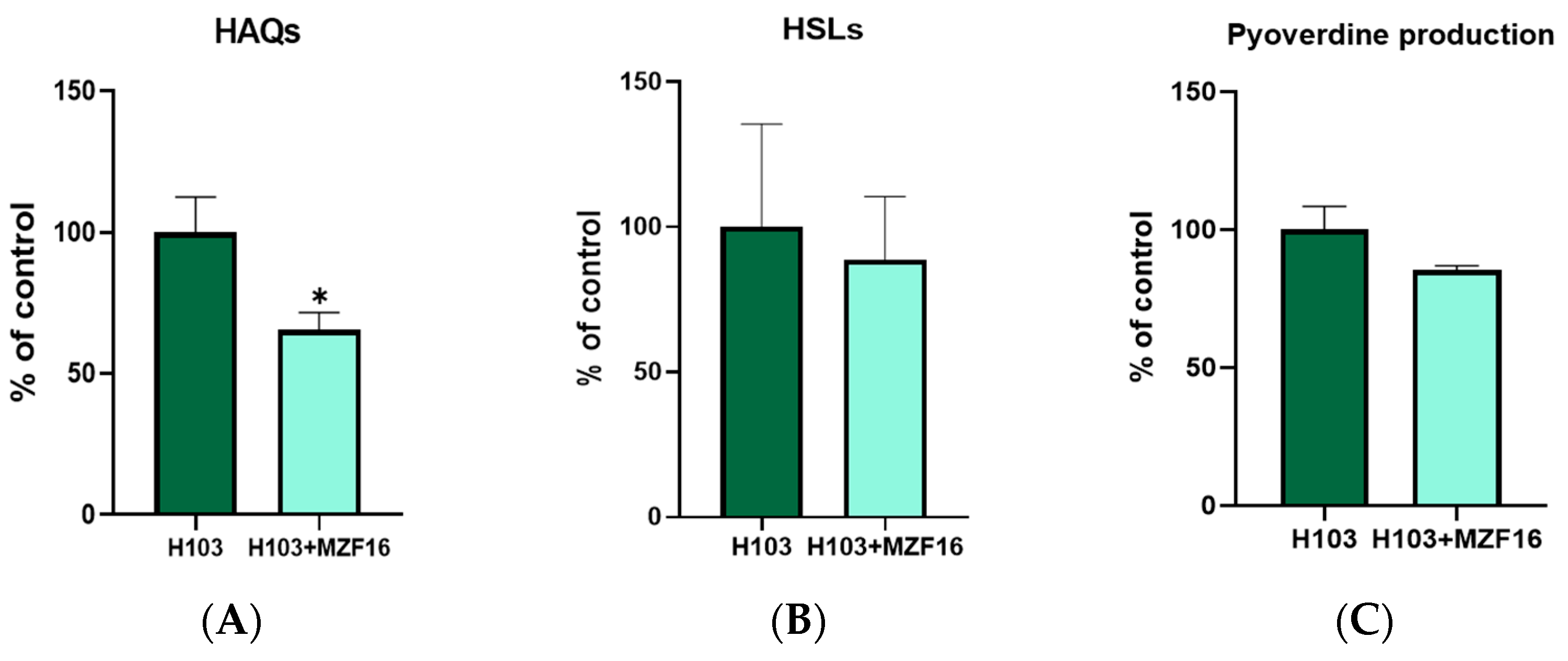
3.6. Quorum Sensing and Pyoverdine Production
3.7. In Vivo Virulence Test on C. elegans
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dimidi, E.; Cox, S.R.; Rossi, M.; Whelan, K. Fermented Foods: Definitions and Characteristics, Impact on the Gut Microbiota and Effects on Gastrointestinal Health and Disease. Nutrients 2019, 11, 1806. [Google Scholar] [CrossRef]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Gänzle, M.; Kort, R.; Pasin, G.; Pihlanto, A.; et al. Health Benefits of Fermented Foods: Microbiota and Beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef]
- Stiemsma, L.T.; Nakamura, R.E.; Nguyen, J.G.; Michels, K.B. Does Consumption of Fermented Foods Modify the Human Gut Microbiota? J. Nutr. 2020, 150, 1680–1692. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A Taxonomic Note on the Genus Lactobacillus: Description of 23 Novel Genera, Emended Description of the Genus Lactobacillus Beijerinck 1901, and Union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Díaz, M.A.; González, S.N.; Alberto, M.R.; Arena, M.E. Human Probiotic Bacteria Attenuate Pseudomonas aeruginosa Biofilm and Virulence by Quorum-Sensing Inhibition. Biofouling 2020, 36, 597–609. [Google Scholar] [CrossRef]
- Todorov, S.D.; Dioso, C.M.; Liong, M.-T.; Nero, L.A.; Khosravi-Darani, K.; Ivanova, I.V. Beneficial Features of Pediococcus: From Starter Cultures and Inhibitory Activities to Probiotic Benefits. World J. Microbiol. Biotechnol. 2022, 39, 4. [Google Scholar] [CrossRef]
- Papagianni, M.; Anastasiadou, S. Pediocins: The Bacteriocins of Pediococci. Sources, Production, Properties and Applications. Microb. Cell Factories 2009, 8, 3. [Google Scholar] [CrossRef]
- Fugaban, J.I.I.; Vazquez Bucheli, J.E.; Park, Y.J.; Suh, D.H.; Jung, E.S.; de Melo Franco, B.D.G.; Ivanova, I.V.; Holzapfel, W.H.; Todorov, S.D. Antimicrobial Properties of Pediococcus acidilactici and Pediococcus pentosaceus Isolated from Silage. J. Appl. Microbiol. 2022, 132, 311–330. [Google Scholar] [CrossRef]
- Chelliah, R.; Choi, J.-G.; Hwang, S.; Park, B.-J.; Daliri, E.B.-M.; Kim, S.-H.; Wei, S.; Ramakrishnan, S.R.; Oh, D.-H. In Vitro and in Vivo Defensive Effect of Probiotic LAB against Pseudomonas aeruginosa Using Caenorhabditis elegans Model. Virulence 2018, 9, 1489–1507. [Google Scholar] [CrossRef]
- Lee, D.-H.; Kim, B.S.; Kang, S.-S. Bacteriocin of Pediococcus acidilactici HW01 Inhibits Biofilm Formation and Virulence Factor Production by Pseudomonas aeruginosa. Probiotics Antimicrob. Proteins 2020, 12, 73–81. [Google Scholar] [CrossRef]
- Adnan, M.; Siddiqui, A.J.; Hamadou, W.S.; Ashraf, S.A.; Hassan, M.I.; Snoussi, M.; Badraoui, R.; Jamal, A.; Bardakci, F.; Awadelkareem, A.M.; et al. Functional and Structural Characterization of Pediococcus pentosaceus-Derived Biosurfactant and Its Biomedical Potential against Bacterial Adhesion, Quorum Sensing, and Biofilm Formation. Antibiotics 2021, 10, 1371. [Google Scholar] [CrossRef]
- Barigela, A.; Bhukya, B. Probiotic Pediococcus acidilactici Strain from Tomato Pickle Displays Anti-Cancer Activity and Alleviates Gut Inflammation in-Vitro. 3 Biotech 2021, 11, 23. [Google Scholar] [CrossRef]
- Keeratikunakorn, K.; Kaewchomphunuch, T.; Kaeoket, K.; Ngamwongsatit, N. Antimicrobial Activity of Cell Free Supernatants from Probiotics Inhibits against Pathogenic Bacteria Isolated from Fresh Boar Semen. Sci. Rep. 2023, 13, 5995. [Google Scholar] [CrossRef]
- Yi, E.-J.; Kim, A.-J. Antimicrobial and Antibiofilm Effect of Bacteriocin-Producing Pediococcus inopinatus K35 Isolated from Kimchi against Multidrug-Resistant Pseudomonas aeruginosa. Antibiotics 2023, 12, 676. [Google Scholar] [CrossRef]
- Marcelli, V.; Osimani, A.; Aquilanti, L. Research Progress in the Use of Lactic Acid Bacteria as Natural Biopreservatives against Pseudomonas spp. in Meat and Meat Products: A Review. Food Res. Int. 2024, 196, 115129. [Google Scholar] [CrossRef]
- Li, X.; Gu, N.; Huang, T.Y.; Zhong, F.; Peng, G. Pseudomonas aeruginosa: A Typical Biofilm Forming Pathogen and an Emerging but Underestimated Pathogen in Food Processing. Front. Microbiol. 2023, 13, 1114199. [Google Scholar] [CrossRef]
- Allydice-Francis, K.; Brown, P.D. Diversity of Antimicrobial Resistance and Virulence Determinants in Pseudomonas aeruginosa Associated with Fresh Vegetables. Int. J. Microbiol. 2012, 2012, 426241. [Google Scholar] [CrossRef]
- Ohara, T.; Itoh, K. Significance of Pseudomonas aeruginosa Colonization of the Gastrointestinal Tract. Intern. Med. 2003, 42, 1072–1076. [Google Scholar] [CrossRef]
- Markou, P.; Apidianakis, Y. Pathogenesis of Intestinal Pseudomonas aeruginosa Infection in Patients with Cancer. Front. Cell. Infect. Microbiol. 2014, 3, 115. [Google Scholar] [CrossRef]
- Rodríguez-Rojas, A.; Oliver, A.; Blázquez, J. Intrinsic and Environmental Mutagenesis Drive Diversification and Persistence of Pseudomonas aeruginosa in Chronic Lung Infections. J. Infect. Dis. 2012, 205, 121–127. [Google Scholar] [CrossRef]
- Gellatly, S.L.; Hancock, R.E. Pseudomonas aeruginosa: New Insights into Pathogenesis and Host Defenses. Pathog. Dis. 2013, 67, 159–173. [Google Scholar] [CrossRef]
- Chatterjee, M.; Anju, C.P.; Biswas, L.; Anil Kumar, V.; Gopi Mohan, C.; Biswas, R. Antibiotic Resistance in Pseudomonas aeruginosa and Alternative Therapeutic Options. Int. J. Med. Microbiol. 2016, 306, 48–58. [Google Scholar] [CrossRef]
- Zommiti, M.; Cambronel, M.; Maillot, O.; Barreau, M.; Sebei, K.; Feuilloley, M.; Ferchichi, M.; Connil, N. Evaluation of Probiotic Properties and Safety of Enterococcus faecium Isolated From Artisanal Tunisian Meat “Dried Ossban”. Front. Microbiol. 2018, 9, 1685. [Google Scholar] [CrossRef]
- Zommiti, M.; Boukerb, A.M.; Feuilloley, M.G.J.; Ferchichi, M.; Connil, N. Draft Genome Sequence of Pediococcus pentosaceus MZF16, a Bacteriocinogenic Probiotic Strain Isolated from Dried Ossban in Tunisia. Microbiol. Resour. Announc. 2019, 8, e00285-19. [Google Scholar] [CrossRef]
- Hancock, R.E.; Carey, A.M. Outer Membrane of Pseudomonas aeruginosa: Heat-2-Mercaptoethanol-Modifiable Proteins. J. Bacteriol. 1979, 140, 902–910. [Google Scholar] [CrossRef]
- Zommiti, M.; Bouffartigues, E.; Maillot, O.; Barreau, M.; Szunerits, S.; Sebei, K.; Feuilloley, M.; Connil, N.; Ferchichi, M. In Vitro Assessment of the Probiotic Properties and Bacteriocinogenic Potential of Pediococcus pentosaceus MZF16 Isolated From Artisanal Tunisian Meat “Dried Ossban”. Front. Microbiol. 2018, 9, 2607. [Google Scholar] [CrossRef]
- Cheng, H.P.; Walker, G.C. Succinoglycan Is Required for Initiation and Elongation of Infection Threads during Nodulation of Alfalfa by Rhizobium meliloti. J. Bacteriol. 1998, 180, 5183–5191. [Google Scholar] [CrossRef]
- Pérez-Ramos, A.; Mohedano, M.L.; Pardo, M.Á.; López, P. β-Glucan-Producing Pediococcus parvulus 2.6: Test of Probiotic and Immunomodulatory Properties in Zebrafish Models. Front. Microbiol. 2018, 9, 1684. [Google Scholar] [CrossRef]
- Chantret, I.; Rodolosse, A.; Barbat, A.; Dussaulx, E.; Brot-Laroche, E.; Zweibaum, A.; Rousset, M. Differential Expression of Sucrase-Isomaltase in Clones Isolated from Early and Late Passages of the Cell Line Caco-2: Evidence for Glucose-Dependent Negative Regulation. J. Cell Sci. 1994, 107, 213–225. [Google Scholar] [CrossRef]
- Salvini, S.; Charbonnier, M.; Defoort, C.; Alquier, C.; Lairon, D. Functional Characterization of Three Clones of the Human Intestinal Caco-2 Cell Line for Dietary Lipid Processing. Br. J. Nutr. 2002, 87, 211–217. [Google Scholar] [CrossRef]
- Madi, A.; Lakhdari, O.; Blottière, H.M.; Guyard-Nicodème, M.; Le Roux, K.; Groboillot, A.; Svinareff, P.; Doré, J.; Orange, N.; Feuilloley, M.G.; et al. The Clinical Pseudomonas fluorescens MFN1032 Strain Exerts a Cytotoxic Effect on Epithelial Intestinal Cells and Induces Interleukin-8 via the AP-1 Signaling Pathway. BMC Microbiol. 2010, 10, 215. [Google Scholar] [CrossRef]
- Xu, H.; Jeong, H.S.; Lee, H.Y.; Ahn, J. Assessment of Cell Surface Properties and Adhesion Potential of Selected Probiotic Strains. Lett. Appl. Microbiol. 2009, 49, 434–442. [Google Scholar] [CrossRef]
- Madi, A.; Svinareff, P.; Orange, N.; Feuilloley, M.G.; Connil, N. Pseudomonas fluorescens Alters Epithelial Permeability and Translocates across Caco-2/TC7 Intestinal Cells. Gut Pathog. 2010, 2, 16. [Google Scholar] [CrossRef]
- Collado, M.C.; Meriluoto, J.; Salminen, S. Adhesion and Aggregation Properties of Probiotic and Pathogen Strains. Eur. Food Res. Technol. 2008, 226, 1065–1073. [Google Scholar] [CrossRef]
- Hoegy, F.; Mislin, G.L.A.; Schalk, I.J. Pyoverdine and Pyochelin Measurements. Methods Mol. Biol. 2014, 1149, 293–301. [Google Scholar] [CrossRef]
- Fletcher, M.P.; Diggle, S.P.; Cámara, M.; Williams, P. Biosensor-Based Assays for PQS, HHQ and Related 2-Alkyl-4-Quinolone Quorum sensing Signal Molecules. Nat Protoc 2007, 2, 1254–1262. [Google Scholar] [CrossRef]
- Tan, M.W.; Mahajan-Miklos, S.; Ausubel, F.M. Killing of Caenorhabditis elegans by Pseudomonas aeruginosa Used to Model Mammalian Bacterial Pathogenesis. Proc. Natl. Acad. Sci. USA 1999, 96, 715–720. [Google Scholar] [CrossRef]
- Blier, A.-S.; Veron, W.; Bazire, A.; Gerault, E.; Taupin, L.; Vieillard, J.; Rehel, K.; Dufour, A.; Le Derf, F.; Orange, N.; et al. C-Type Natriuretic Peptide Modulates Quorum sensing Molecule and Toxin Production in Pseudomonas aeruginosa. Microbiology 2011, 157, 1929–1944. [Google Scholar] [CrossRef]
- Pachori, P.; Gothalwal, R.; Gandhi, P. Emergence of Antibiotic Resistance Pseudomonas aeruginosa in Intensive Care Unit; a Critical Review. Genes Dis. 2019, 6, 109–119. [Google Scholar] [CrossRef]
- Choi, M.K.; Choi, S. In Vitro and in Vivo Anti-Clostridial Activity of Newly Isolated Pediococcus acidilactici SPM138 against Clostridium difficile. Anaerobe 2020, 61, 102146. [Google Scholar] [CrossRef]
- Vasiee, A.; Falah, F.; Behbahani, B.A.; Tabatabaee-yazdi, F. Probiotic Characterization of Pediococcus Strains Isolated from Iranian Cereal-Dairy Fermented Product: Interaction with Pathogenic Bacteria and the Enteric Cell Line Caco-2. J. Biosci. Bioeng. 2020, 130, 471–479. [Google Scholar] [CrossRef]
- Yin, H.; Ye, P.; Lei, Q.; Cheng, Y.; Yu, H.; Du, J.; Pan, H.; Cao, Z. In Vitro Probiotic Properties of Pediococcus pentosaceus L1 and Its Effects on Enterotoxigenic Escherichia coli-Induced Inflammatory Responses in Porcine Intestinal Epithelial Cells. Microb. Pathog. 2020, 144, 104163. [Google Scholar] [CrossRef]
- Wang, P.; Chen, S.; Liao, C.; Jia, Y.; Li, J.; Shang, K.; Chen, J.; Cao, P.; Li, W.; Li, Y.; et al. Probiotic Properties of Chicken-Derived Highly Adherent Lactic Acid Bacteria and Inhibition of Enteropathogenic Bacteria in Caco-2 Cells. Microorganisms 2022, 10, 2515. [Google Scholar] [CrossRef]
- Li, J.; Chen, X.; Xie, Z.; Liang, L.; Li, A.; Zhao, C.; Wen, Y.; Lou, Z. Screening and Metabolomic Analysis of Lactic Acid Bacteria-Antagonizing Pseudomonas aeruginosa. Foods 2023, 12, 2799. [Google Scholar] [CrossRef]
- Kompramool, S.; Singkhamanan, K.; Pomwised, R.; Chaichana, N.; Suwannasin, S.; Wonglapsuwan, M.; Jitpakdee, J.; Kantachote, D.; Yaikhan, T.; Surachat, K. Genomic Insights into Pediococcus pentosaceus ENM104: A Probiotic with Potential Antimicrobial and Cholesterol-Reducing Properties. Antibiotics 2024, 13, 813. [Google Scholar] [CrossRef]
- Barnett, A.M.; Roy, N.C.; Cookson, A.L.; McNabb, W.C. Metabolism of Caprine Milk Carbohydrates by Probiotic Bacteria and Caco-2:HT29−MTX Epithelial Co-Cultures and Their Impact on Intestinal Barrier Integrity. Nutrients 2018, 10, 949. [Google Scholar] [CrossRef]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.-J.; Cheng, Z. Antibiotic Resistance in Pseudomonas aeruginosa: Mechanisms and Alternative Therapeutic Strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef]
- Eickhoff, M.J.; Bassler, B.L. SnapShot: Bacterial Quorum sensing. Cell 2018, 174, 1328-1328.e1. [Google Scholar] [CrossRef]
- Wade, D.S.; Calfee, M.W.; Rocha, E.R.; Ling, E.A.; Engstrom, E.; Coleman, J.P.; Pesci, E.C. Regulation of Pseudomonas Quinolone Signal Synthesis in Pseudomonas aeruginosa. J. Bacteriol. 2005, 187, 4372–4380. [Google Scholar] [CrossRef]
- Díaz-Pérez, S.P.; Solis, C.S.; López-Bucio, J.S.; Valdez Alarcón, J.J.; Villegas, J.; Reyes-De la Cruz, H.; Campos-Garcia, J. Pathogenesis in Pseudomonas aeruginosa PAO1 Biofilm-Associated Is Dependent on the Pyoverdine and Pyocyanin Siderophores by Quorum Sensing Modulation. Microb. Ecol. 2023, 86, 727–741. [Google Scholar] [CrossRef]
- Ghanei-Motlagh, R.; Mohammadian, T.; Gharibi, D.; Menanteau-Ledouble, S.; Mahmoudi, E.; Khosravi, M.; Zarea, M.; El-Matbouli, M. Quorum quenching Properties and Probiotic Potentials of Intestinal Associated Bacteria in Asian Sea Bass Lates Calcarifer. Mar. Drugs 2020, 18, 23. [Google Scholar] [CrossRef] [PubMed]
- Devi, S.; Chhibber, S.; Harjai, K. Optimization of Cultural Conditions for Enhancement of Anti-Quorum Sensing Potential in the Probiotic Strain Lactobacillus rhamnosus GG against Pseudomonas aeruginosa. 3 Biotech 2022, 12, 133. [Google Scholar] [CrossRef] [PubMed]
- Kiymaci, M.E.; Altanlar, N.; Gumustas, M.; Ozkan, S.A.; Akin, A. Quorum Sensing Signals and Related Virulence Inhibition of Pseudomonas aeruginosa by a Potential Probiotic Strain’s Organic Acid. Microb. Pathog. 2018, 121, 190–197. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boujnane, M.; Zommiti, M.; Lesouhaitier, O.; Ferchichi, M.; Tahrioui, A.; Boukerb, A.M.; Connil, N. Pediococcus pentosaceus MZF16 Probiotic Strain Prevents In Vitro Cytotoxic Effects of Pseudomonas aeruginosa H103 and Prolongs the Lifespan of Caenorhabditis elegans. Pathogens 2025, 14, 244. https://doi.org/10.3390/pathogens14030244
Boujnane M, Zommiti M, Lesouhaitier O, Ferchichi M, Tahrioui A, Boukerb AM, Connil N. Pediococcus pentosaceus MZF16 Probiotic Strain Prevents In Vitro Cytotoxic Effects of Pseudomonas aeruginosa H103 and Prolongs the Lifespan of Caenorhabditis elegans. Pathogens. 2025; 14(3):244. https://doi.org/10.3390/pathogens14030244
Chicago/Turabian StyleBoujnane, Meryem, Mohamed Zommiti, Olivier Lesouhaitier, Mounir Ferchichi, Ali Tahrioui, Amine M. Boukerb, and Nathalie Connil. 2025. "Pediococcus pentosaceus MZF16 Probiotic Strain Prevents In Vitro Cytotoxic Effects of Pseudomonas aeruginosa H103 and Prolongs the Lifespan of Caenorhabditis elegans" Pathogens 14, no. 3: 244. https://doi.org/10.3390/pathogens14030244
APA StyleBoujnane, M., Zommiti, M., Lesouhaitier, O., Ferchichi, M., Tahrioui, A., Boukerb, A. M., & Connil, N. (2025). Pediococcus pentosaceus MZF16 Probiotic Strain Prevents In Vitro Cytotoxic Effects of Pseudomonas aeruginosa H103 and Prolongs the Lifespan of Caenorhabditis elegans. Pathogens, 14(3), 244. https://doi.org/10.3390/pathogens14030244






