Viral Vaccines as an Alternative to Antimicrobials: A Perspective from Swine Veterinarians on Challenges, Opportunities, and Future Directions
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
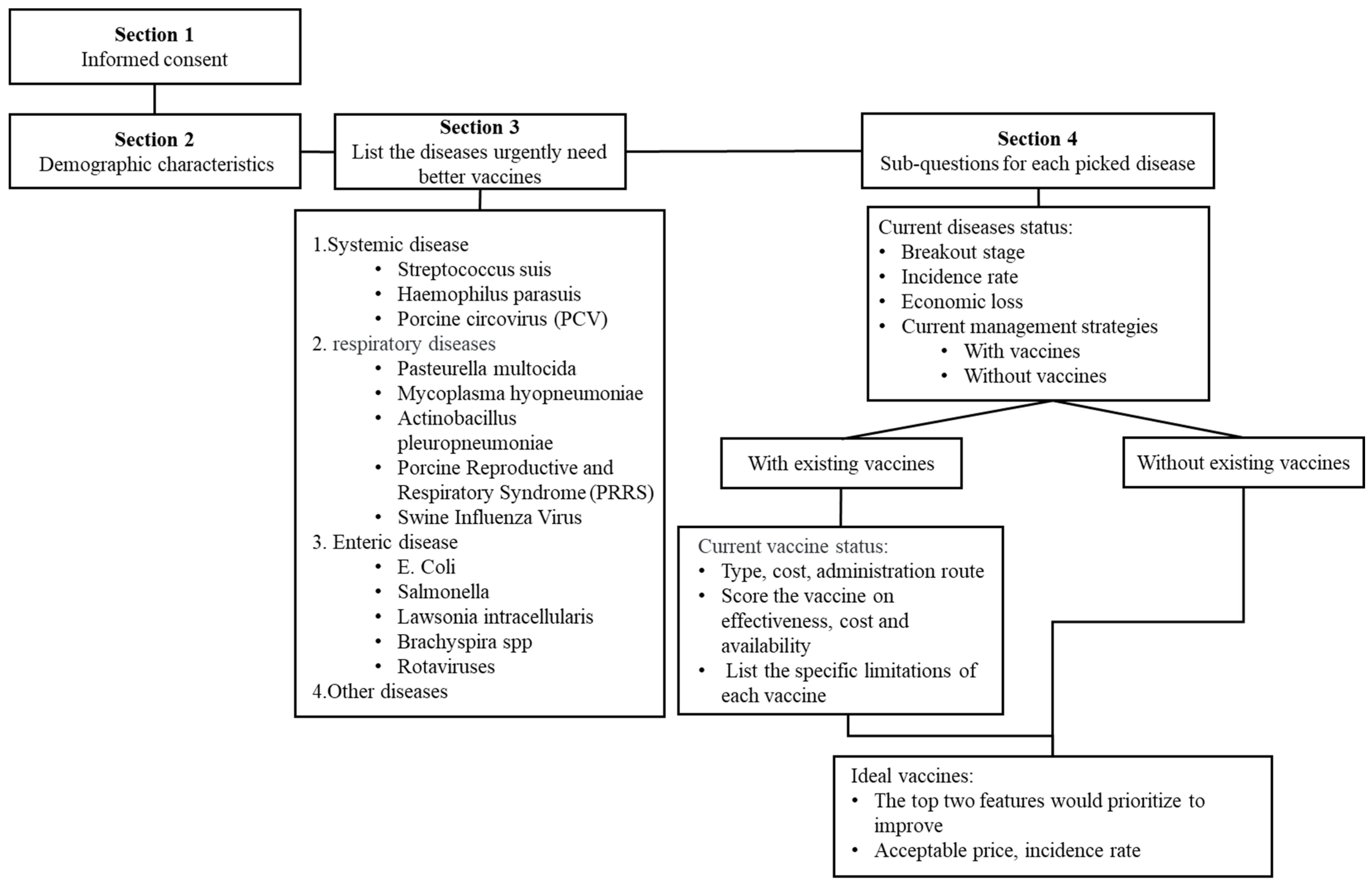
2.2. Survey Design and Dissemination
2.3. Interview
2.4. Data and Statistical Analysis
3. Results
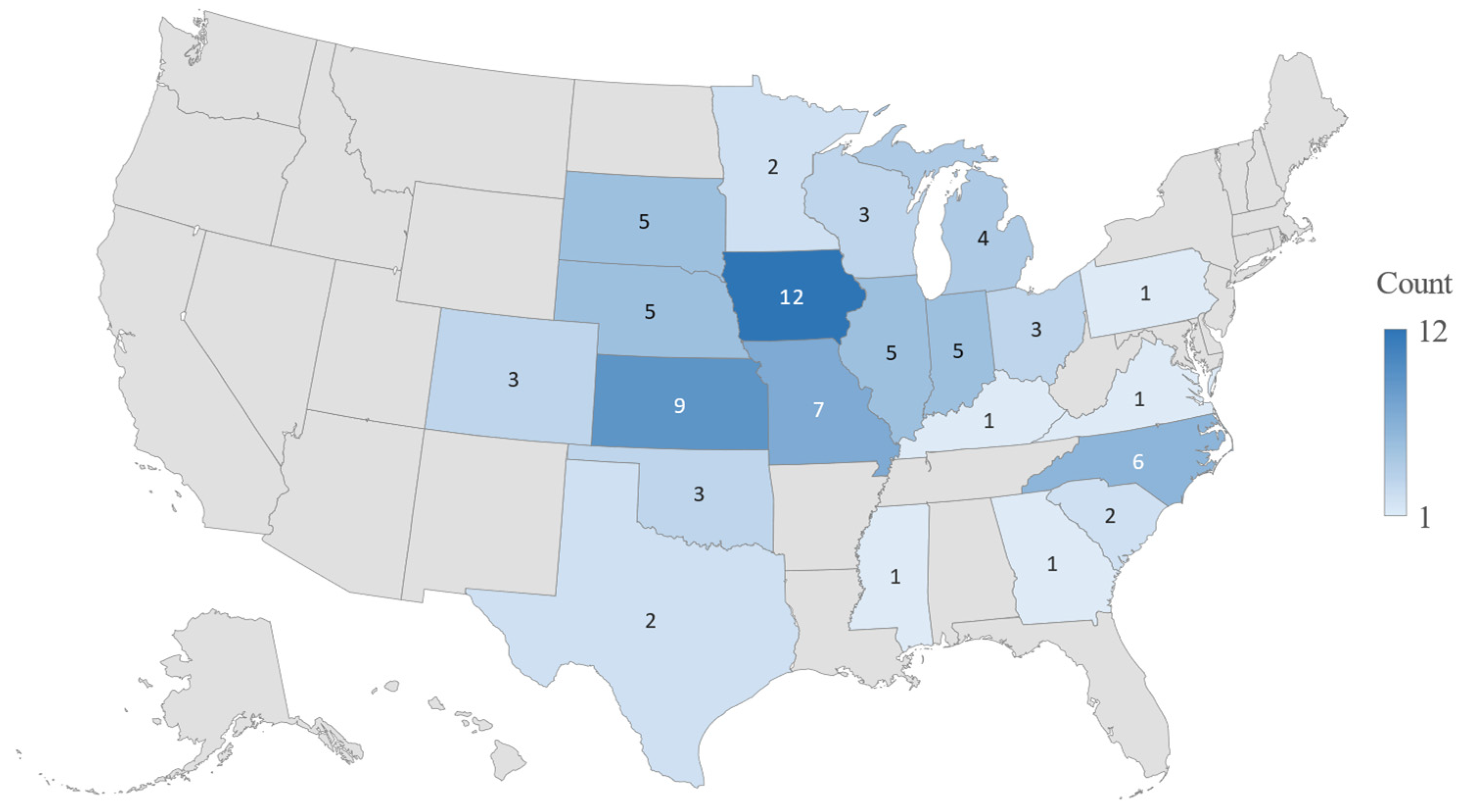
3.1. Demographics
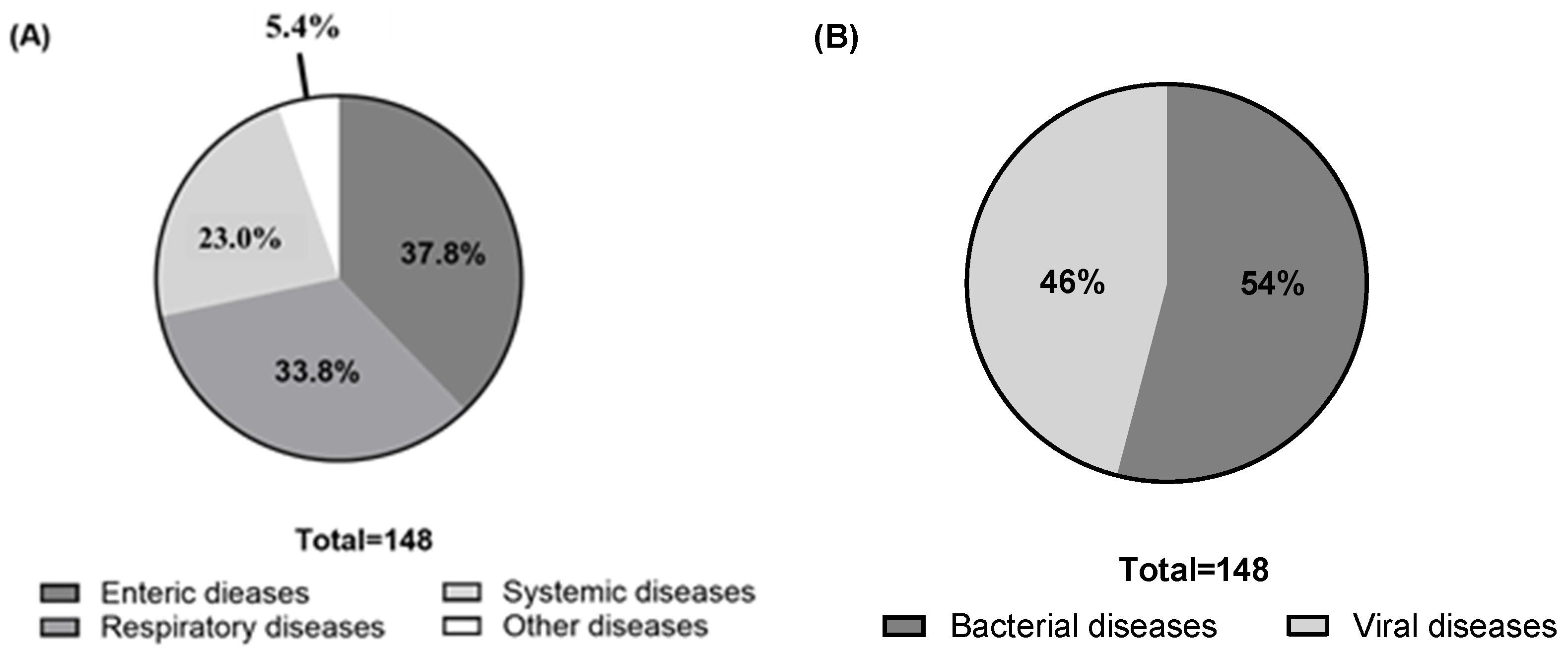
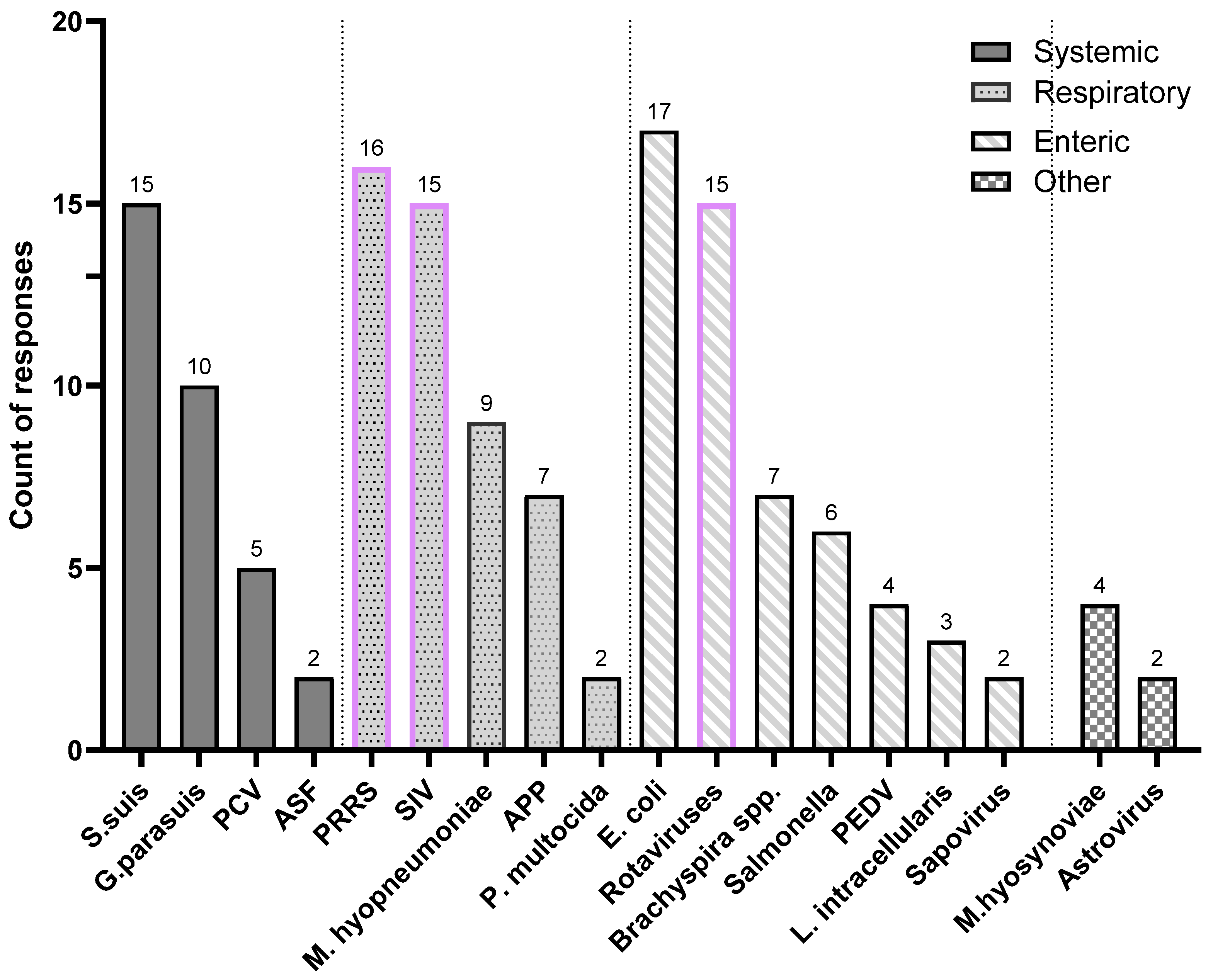
3.2. Prioritization of Swine Diseases in Need of Better Vaccines
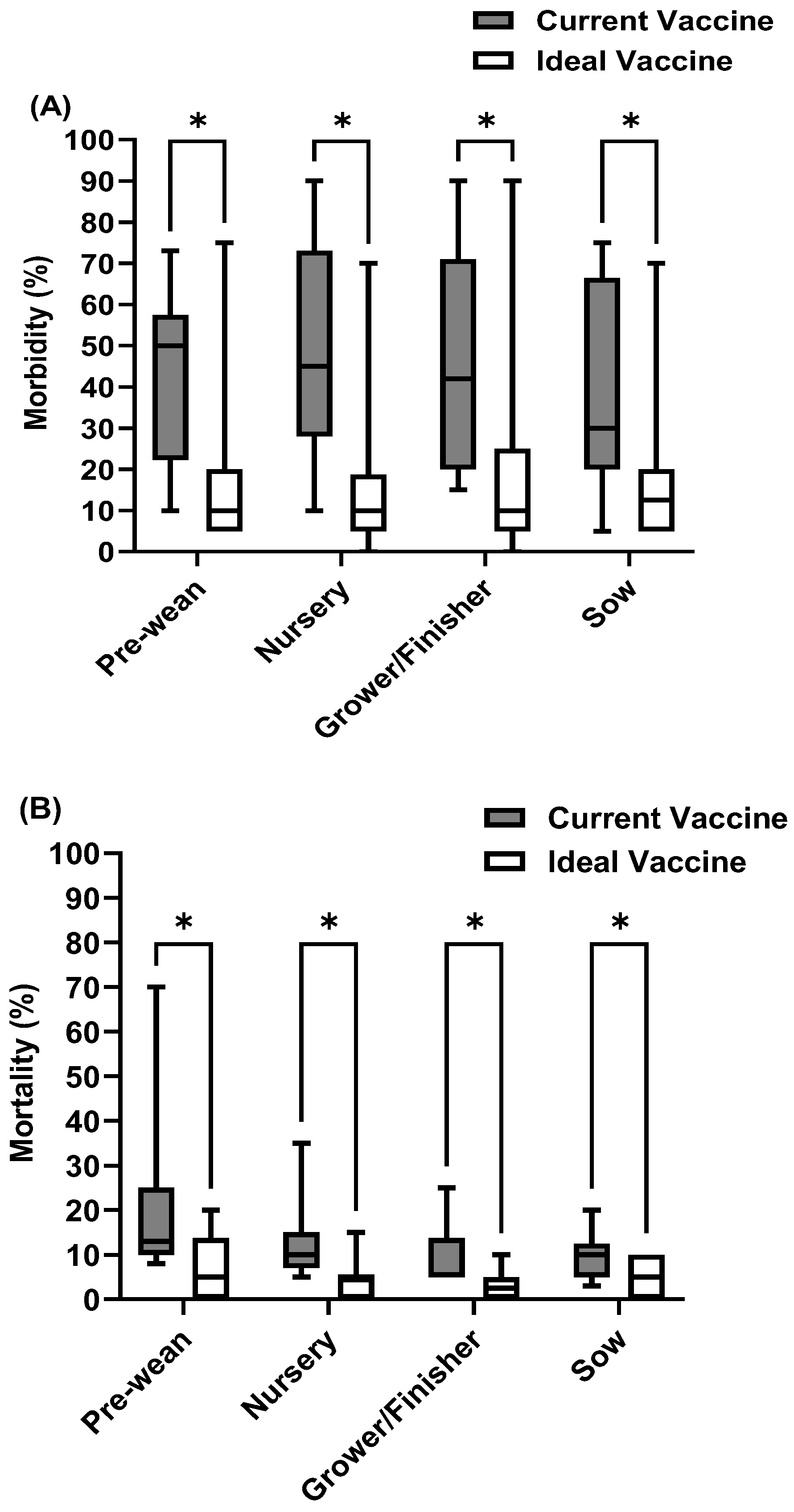
3.3. Current Management Strategies for the Top Swine Viral Diseases
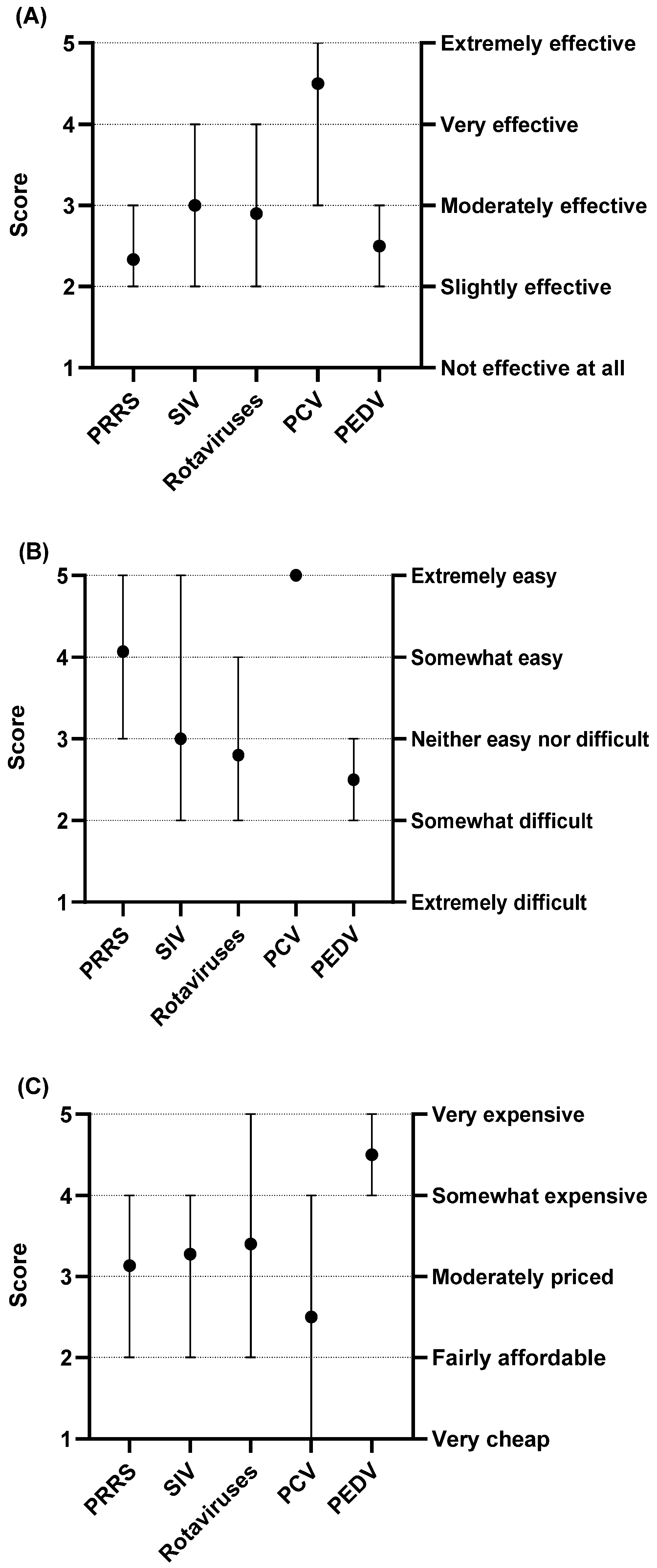
3.4. Challenges of Current Vaccines for the Top Swine Viral Diseases
3.4.1. Specific Limitations That Contribute to Challenges on Effectiveness of Swine Viral Vaccines
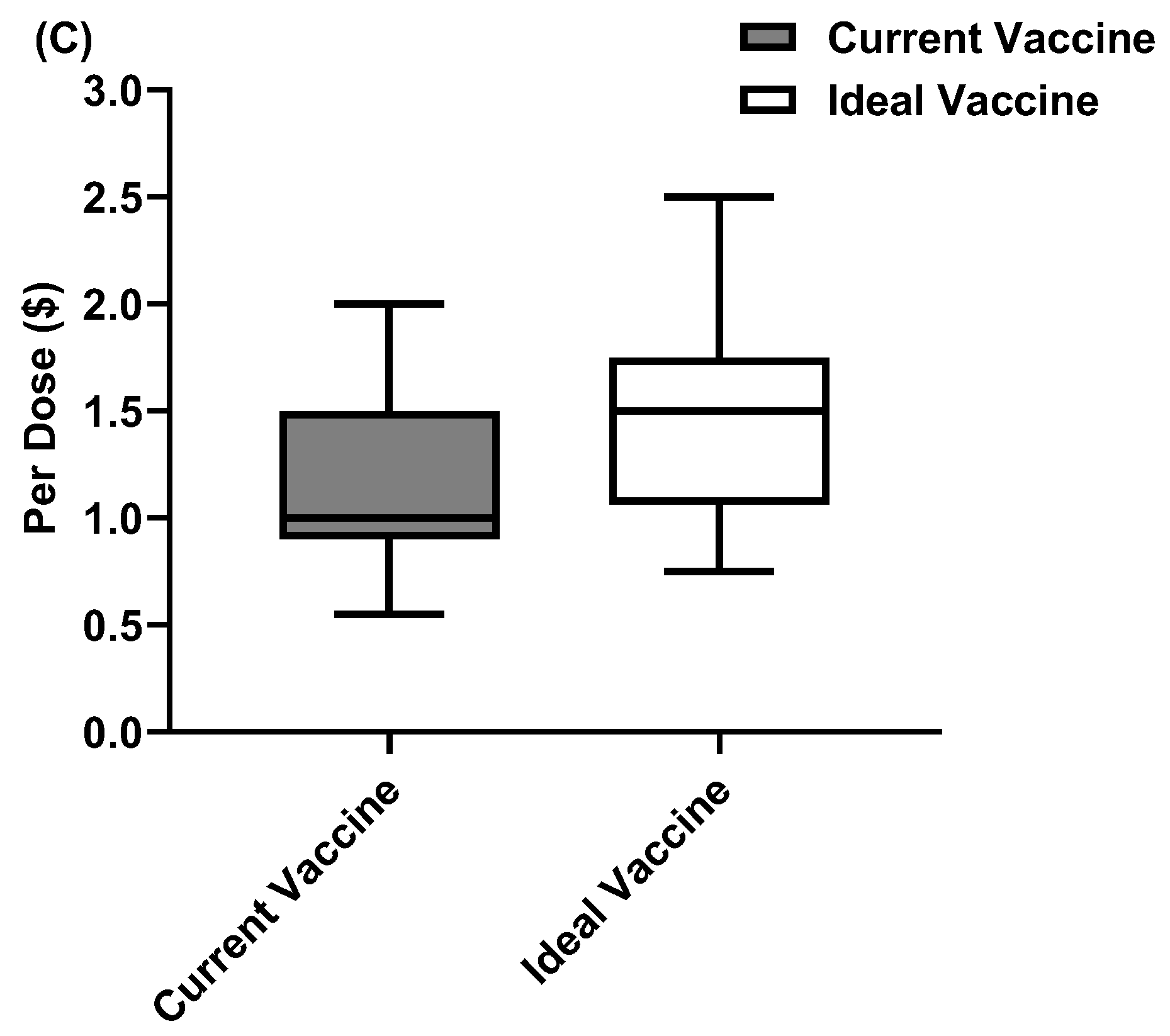
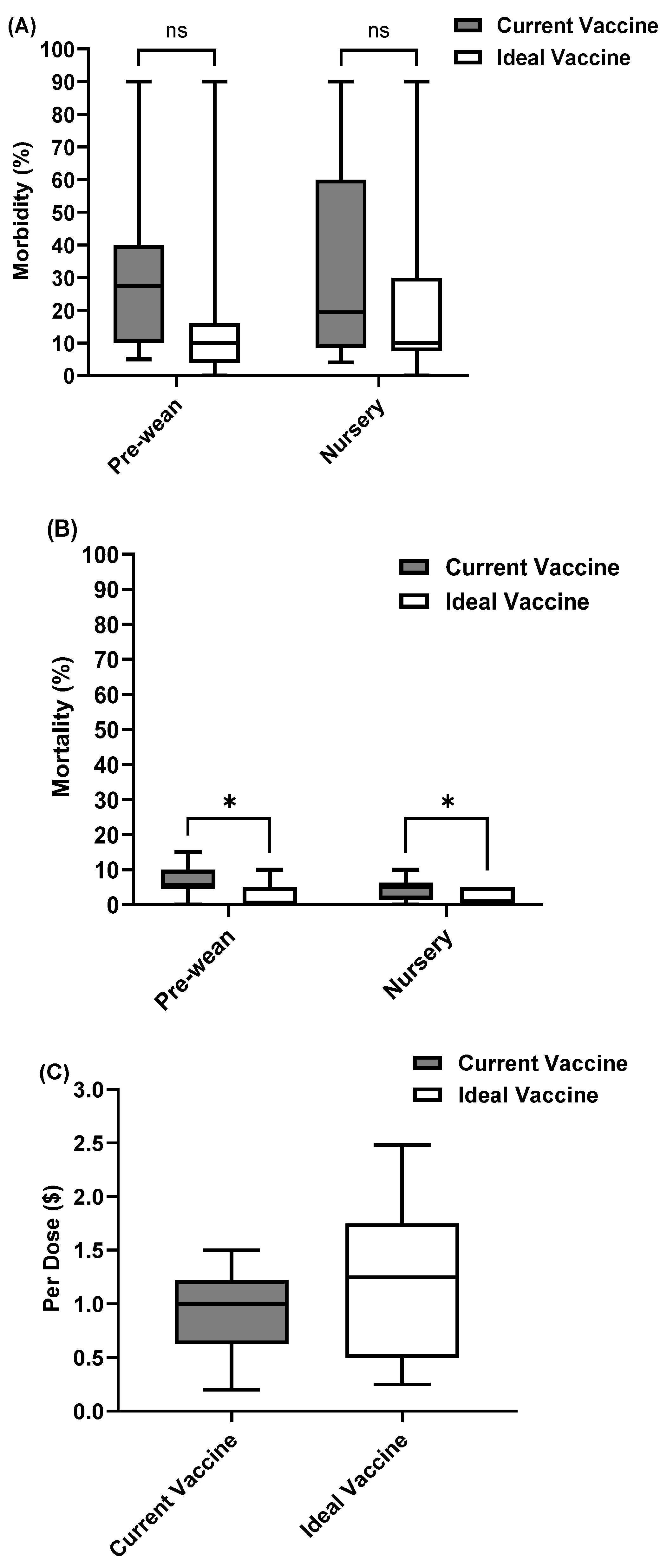
3.4.2. Specific Limitations That Contribute to Challenges on Cost and Availability of Swine Viral Vaccines
3.4.3. Specific Limitations That Contribute to Challenges on Safety of Vaccines
3.5. Future Perspectives and Opportunities for Improved Swine Viral Vaccines
4. Discussion
4.1. Top Swine Viral Diseases and Their Concerns
4.2. Promising Strategies for Vaccine Improvement of the Top Swine Viral Diseases
4.3. Barriers and the Role of Veterinarians
4.4. Limitations of the Study and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kirchhelle, C. Pharming animals: A global history of antibiotics in food production (1935–2017). Palgrave Commun. 2018, 4, 96. [Google Scholar] [CrossRef]
- Mulchandani, R.; Wang, Y.; Gilbert, M.; Van Boeckel, T.P. Global trends in antimicrobial use in food-producing animals: 2020 to 2030. PLoS Glob Public Health 2023, 3, e0001305. [Google Scholar] [CrossRef]
- Rahman, S.; Hollis, A. The effect of antibiotic usage on resistance in humans and food-producing animals: A longitudinal, One Health analysis using European data. Front. Public Health 2023, 11, 1170426. [Google Scholar] [CrossRef]
- Food and Drug Administration. Guidance for Industry #213. Available online: https://www.fda.gov/media/83488/download (accessed on 19 August 2025).
- Food and Drug Administration. 2023 Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals. Available online: https://www.fda.gov/animal-veterinary/antimicrobial-resistance/2023-summary-report-antimicrobials-sold-or-distributed-use-food-producing-animals (accessed on 19 August 2025).
- Machado, I.; Petznick, T.; Poeta Silva, A.P.S.; Wang, C.; Karriker, L.; Linhares, D.C.L.; Silva, G.S. Assessment of changes in antibiotic use in grow-finish pigs after the introduction of PRRSV in a naïve farrow-to-finish system. Prev Vet Med. 2024, 233, 106350. [Google Scholar] [CrossRef] [PubMed]
- Swine Health Information Center. Domestic Swine Disease Monitoring Report. Available online: https://www.swinehealth.org/domestic-disease-surveillance-reports/ (accessed on 19 August 2025).
- Will, K.J.; Magalhaes, E.S.; Moura, C.A.A.; Trevisan, G.; Silva, G.S.; Mellagi, A.P.G.; Ulguim, R.R.; Bortolozzo, F.P.; Linhares, D.C.L. Risk factors associated with piglet pre-weaning mortality in a Midwestern US swine production system from 2020 to 2022. Prev Vet Med. 2024, 232, 106316. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Zhang, H.; Cheng, H.; Liu, M.; Wen, S.; Ren, J. Progress in PRRSV infection and adaptive immune response mechanisms. Viruses 2023, 15, 1442. [Google Scholar] [CrossRef] [PubMed]
- Janke, B.H. Influenza A virus infections in swine: Pathogenesis and diagnosis. Vet. Pathol. 2014, 51, 410–426. [Google Scholar] [CrossRef]
- Charerntantanakul, W. Porcine reproductive and respiratory syndrome virus vaccines: Immunogenicity, efficacy and safety aspects. World J Virol. 2012, 1, 23–30. [Google Scholar] [CrossRef]
- Cui, X.Y.; Xia, D.S.; Luo, L.Z.; An, T.Q. Recombination of porcine reproductive and respiratory syndrome virus: Features, possible mechanisms, and future directions. Viruses 2024, 16, 929. [Google Scholar] [CrossRef]
- Graaf-Rau, A.; Schmies, K.; Breithaupt, A.; Ciminski, K.; Zimmer, G.; Summerfield, A.; Sehl-Ewert, J.; Lillie-Jaschniski, K.; Helmer, C.; Bielenberg, W.; et al. Reassortment incompetent live attenuated and replicon influenza vaccines provide improved protection against influenza in piglets. NPJ Vaccines 2024, 9, 127. [Google Scholar] [CrossRef]
- Salvesen, H.A.; Whitelaw, C.B.A. Current and prospective control strategies of influenza A virus in swine. Porc. Health Manag. 2021, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Petro-Turnquist, E.; Pekarek, M.J.; Weaver, E.A. Swine influenza A virus: Challenges and novel vaccine strategies. Front. Cell. Infect. Microbiol. 2024, 14, 1336013. [Google Scholar] [CrossRef]
- Kumar, D.; Shepherd, F.K.; Springer, N.L.; Mwangi, W.; Marthaler, D.G. Rotavirus infection in swine: Genotypic diversity, immune responses, and role of gut microbiome in rotavirus immunity. Pathogens 2022, 11, 1078. [Google Scholar] [CrossRef]
- Chepngeno, J.; Diaz, A.; Paim, F.C.; Saif, L.J.; Vlasova, A.N. Rotavirus C: Prevalence in suckling piglets and development of virus-like particles to assess the influence of maternal immunity on the disease development. Vet Res. 2019, 50, 84. [Google Scholar]
- Guo, Y.; Raev, S.; Kick, M.K.; Raque, M.; Saif, L.J.; Vlasova, A.N. Rotavirus C replication in porcine intestinal enteroids reveals roles for cellular cholesterol and sialic acids. Viruses 2022, 14, 1825. [Google Scholar] [CrossRef] [PubMed]
- Chase, C.; Daniels, C.; Garcia, R.; Milward, F. Needle-free injection technology in swine: Progress toward vaccine efficacy and pork quality. J. Swine Health Prod. 2008, 16, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Madapong, A.; Saeng-Chuto, K.; Tantituvanont, A.; Nilubol, D. Safety of PRRSV-2 MLV vaccines administrated via the intramuscular or intradermal route and evaluation of PRRSV transmission upon needle-free and needle delivery. Sci Rep. 2021, 11, 23107. [Google Scholar]
- Renson, P.; Mahé, S.; Andraud, M.; Le Dimna, M.; Paboeuf, F.; Rose, N.; Bourry, O. Effect of vaccination route (intradermal vs. intramuscular) against porcine reproductive and respiratory syndrome using a modified live vaccine on systemic and mucosal immune response and virus transmission in pigs. BMC Vet Res. 2024, 20, 5. [Google Scholar]
- Aguirre, L.; Li, Y.; Baratelli, M.; Martín-Valls, G.; Cortey, M.; Miranda, J.; Martín, M.; Mateu, E. In the presence of non-neutralising maternally derived antibodies, intradermal and intramuscular vaccination with a modified live vaccine against porcine reproductive and respiratory syndrome virus 1 (PRRSV-1) induce similar levels of neutralising antibodies or interferon-gamma secreting cells. Porc. Health Manag. 2022, 8, 47. [Google Scholar]
- Maragkakis, G.; Athanasiou, L.V.; Korou, L.M.; Chaintoutis, S.C.; Dovas, C.; Perrea, D.N.; Papakonstantinou, G.; Christodoulopoulos, G.; Maes, D.; Papatsiros, V.G. Angiotensin II Blood Serum Levels in Piglets, after Intra-Dermal or Intra-Muscular Vaccination against PRRSV. Vet Sci. 2022, 9, 496. [Google Scholar] [CrossRef] [PubMed]
- Petro-Turnquist, E.; Pekarek, M.; Jeanjaquet, N.; Wooledge, C.; Steffen, D.; Vu, H.; Weaver, E.A. Adenoviral-vectored epigraph vaccine elicits robust, durable, and protective immunity against H3 influenza A virus in swine. Front. Immunol. 2023, 14, 1143451. [Google Scholar] [CrossRef] [PubMed]
- Wesley, R.D.; Lager, K.M. Overcoming maternal antibody interference by vaccination with human adenovirus 5 recombinant viruses expressing the hemagglutinin and the nucleoprotein of swine influenza virus. Vet. Microbiol. 2006, 118, 67–75. [Google Scholar] [CrossRef]
- Anderson, A.V.; Shepherd, F.; Dominguez, F.; Pittman, J.; Marthaler, D.; Karriker, L. Evaluating natural planned exposure protocols on rotavirus shedding patterns in gilts and the impact on their suckling pigs. J. Swine Health Prod. 2023, 31, 10–19. [Google Scholar] [CrossRef]
- Kumar, D.; Anderson, A.V.; Pittman, J.; Springer, N.L.; Marthaler, D.G.; Mwangi, W. Antibody response to Rotavirus C pre-farrow natural planned exposure to gilts and their piglets. Viruses 2022, 14, 2250. [Google Scholar] [CrossRef]
- Kikuti, M.; Geary, E.; Picasso, C.; Medrano, M.; Vilalta, C.; Corzo, C. Review of MSHMP PRRS Chart 2-Prevalence. Morrison Swine Health Monitoring Project. Available online: https://mshmp.umn.edu/reports (accessed on 19 August 2025).
- Valdes-Donoso, P.; Jarvis, L.S. Combining epidemiology and economics to assess control of a viral endemic animal disease: Porcine Reproductive and Respiratory Syndrome (PRRS). PLoS ONE 2022, 17, e0274382. [Google Scholar] [CrossRef]
- Law, J. Swine veterinarians-key players in the pork production chain. Can. Vet. J. 2021, 62, 515–516. [Google Scholar]
- Hockenhull, J.; Turner, A.E.; Reyher, K.K.; Barrett, D.C.; Jones, L.; Hinchliffe, S.; Buller, H.J. Antimicrobial use in food-producing animals: A rapid evidence assessment of stakeholder practices and beliefs. Vet. Rec. 2017, 181, 510. [Google Scholar] [CrossRef]
- Visschers, V.H.; Backhans, A.; Collineau, L.; Iten, D.; Loesken, S.; Postma, M.; Belloc, C.; Dewulf, J.; Emanuelson, U.; Beilage, E.G.; et al. Perceptions of antimicrobial usage, antimicrobial resistance and policy measures to reduce antimicrobial usage in convenient samples of Belgian, French, German, Swedish and Swiss pig farmers. Prev. Vet. Med. 2015, 119, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Coyne, L.A.; Latham, S.M.; Williams, N.J.; Dawson, S.; Donald, I.J.; Pearson, R.B.; Smith, R.F.; Pinchbeck, G.L. Understanding the culture of antimicrobial prescribing in agriculture: A qualitative study of UK pig veterinary surgeons. J. Antimicrob. Chemother. 2016, 71, 3300–3312. [Google Scholar] [CrossRef] [PubMed]

| Disease | Current Management Strategies | Percentage | Score |
|---|---|---|---|
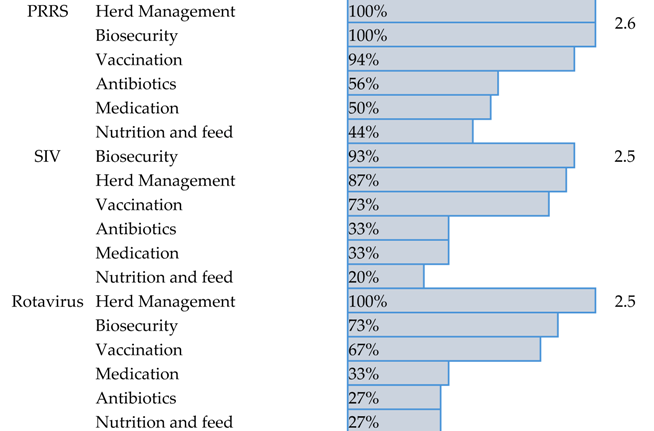 | |||
| Disease | Effective Limitations | Percentage |
|---|---|---|
 | ||
| Group | Commercial Vaccines | Autogenous Vaccines |
|---|---|---|
| PRRS | 100% | 37.5% |
| SIV | 46.7% | 66.7% |
| Rotavirus | 26.7% | 60% |
| Disease | Availability Limitations | Percentage |
|---|---|---|
 | ||
| Disease | Adverse Effects | Percentage |
|---|---|---|
 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Zhang, X.; Apley, M.D.; Gebhardt, J.T.; Karriker, L.; Connor, J.F.; Bromfield, C.; Lubbers, B.; Kittana, H.; Pendell, D.; et al. Viral Vaccines as an Alternative to Antimicrobials: A Perspective from Swine Veterinarians on Challenges, Opportunities, and Future Directions. Pathogens 2025, 14, 1259. https://doi.org/10.3390/pathogens14121259
Li D, Zhang X, Apley MD, Gebhardt JT, Karriker L, Connor JF, Bromfield C, Lubbers B, Kittana H, Pendell D, et al. Viral Vaccines as an Alternative to Antimicrobials: A Perspective from Swine Veterinarians on Challenges, Opportunities, and Future Directions. Pathogens. 2025; 14(12):1259. https://doi.org/10.3390/pathogens14121259
Chicago/Turabian StyleLi, Danqin, Xirui Zhang, Michael D. Apley, Jordan T. Gebhardt, Locke Karriker, Joseph F. Connor, Corinne Bromfield, Brian Lubbers, Hatem Kittana, Dustin Pendell, and et al. 2025. "Viral Vaccines as an Alternative to Antimicrobials: A Perspective from Swine Veterinarians on Challenges, Opportunities, and Future Directions" Pathogens 14, no. 12: 1259. https://doi.org/10.3390/pathogens14121259
APA StyleLi, D., Zhang, X., Apley, M. D., Gebhardt, J. T., Karriker, L., Connor, J. F., Bromfield, C., Lubbers, B., Kittana, H., Pendell, D., Madera, R., Muro, N., Craig, A., Shenkenberg, B., Li, Y., Wang, L., & Shi, J. (2025). Viral Vaccines as an Alternative to Antimicrobials: A Perspective from Swine Veterinarians on Challenges, Opportunities, and Future Directions. Pathogens, 14(12), 1259. https://doi.org/10.3390/pathogens14121259







