Proposing Bromo-Epi-Androsterone for Host-Directed Therapy Against Tuberculosis
Abstract
1. Introduction
2. Ageing, Immunosenescence, and TB Susceptibility
2.1. Immune Commitment to Persistent Viral and Environmental Factors
2.1.1. Viral Infections
2.1.2. Environmental and Metabolic Influences
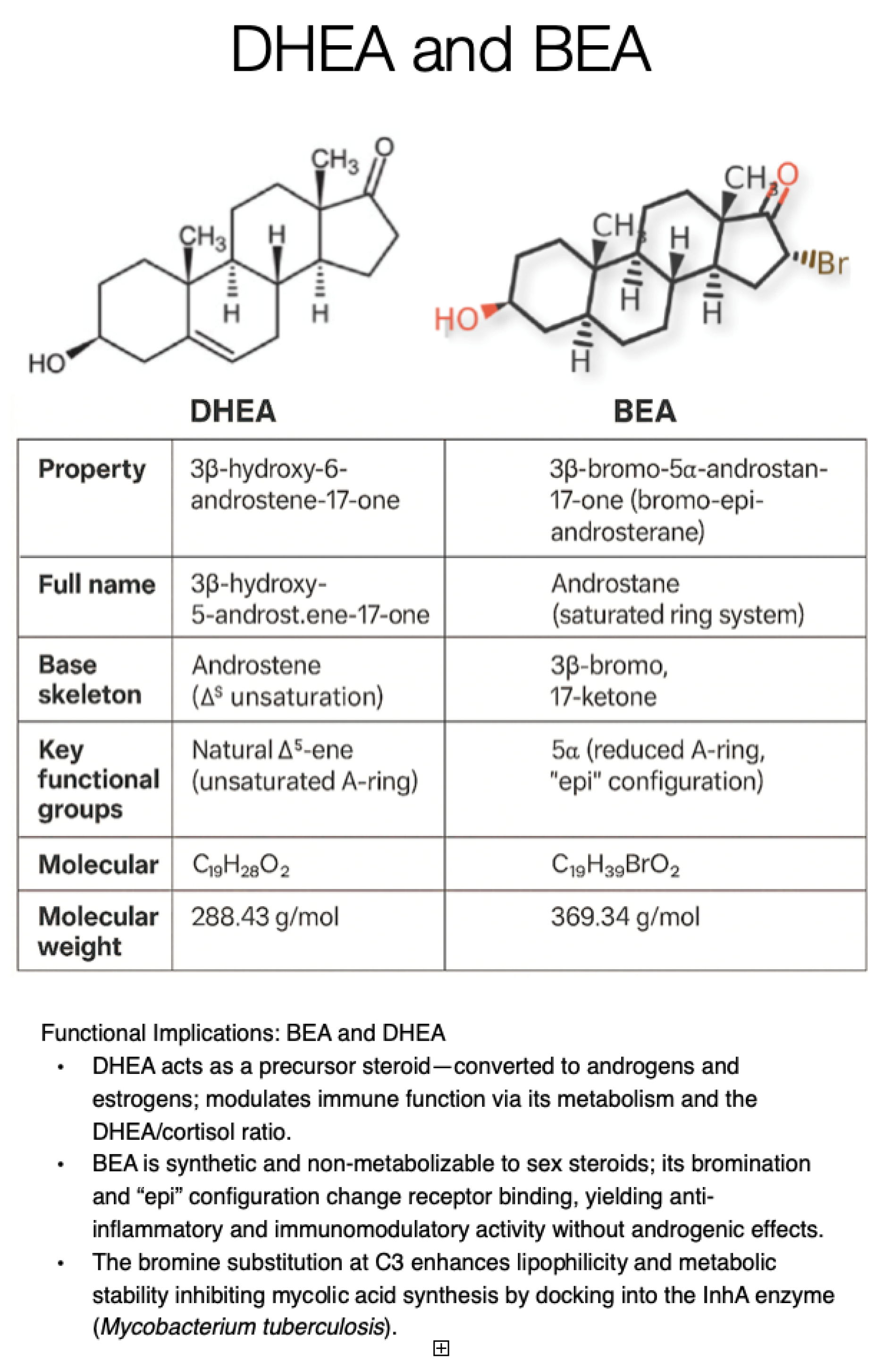
3. Immunosenescence and the Role of DHEA Decline
4. History of Bromoepiandrosterone (BEA, Originally Known as HE2000) as an Anti-Infective Agent
5. Rationale for Bromoepiandrosterone (BEA) for Tuberculosis
6. BEA Activity in Murine Models of Tuberculosis
7. Challenging the Murine Model of MDR-TB
8. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report 2024; World Health Organization: Geneva, Switzerland, 2024; Available online: https://www.who.int/publications/i/item/9789240101531 (accessed on 2 October 2025).
- World Health Organization. Global Tuberculosis Report 2024—Determinants, Risk Factors, and Treatment Outcomes; World Health Organization: Geneva, Switzerland, 2024; Available online: https://www.who.int/teams/global-programme-on-tuberculosis-and-lung-health/tb-reports/global-tuberculosis-report-2024 (accessed on 2 October 2025).
- Estaji, F.; Kamali, A.; Keikha, M. Strengthening the global Response to Tuberculosis: Insights from the 2024 WHO global TB report. J. Clin. Tuberc. Other Mycobact. Dis. 2025, 39, 100522. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Navasardyan, I.; Miwalian, R.; Petrosyan, A.; Yeganyan, S.; Venketaraman, V. HIV-TB Coinfection: Current Therapeutic Approaches and Drug Interactions. Viruses 2024, 16, 321. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Caraux-Paz, P.; Diamantis, S.; de Wazières, B.; Gallien, S. Tuberculosis in the Elderly. J. Clin. Med. 2021, 10, 5888. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Olmo-Fontánez, A.M.; Turner, J. Tuberculosis in an Aging World. Pathogens 2022, 11, 1101. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Theodorakis, N.; Feretzakis, G.; Hitas, C.; Kreouzi, M.; Kalantzi, S.; Spyridaki, A.; Kollia, Z.; Verykios, V.S.; Nikolaou, M. Immunosenescence: How Aging Increases Susceptibility to Bacterial Infections and Virulence Factors. Microorganisms 2024, 12, 2052. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Müller, J.; Tanner, R.; Matsumiya, M.; Snowden, M.A.; Landry, B.; Satti, I.; Harris, S.A.; O’Shea, M.K.; Stockdale, L.; Marsay, L.; et al. Cytomegalovirus infection is a risk factor for tuberculosis disease in infants. JCI Insight 2019, 4, e130090. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kua, K.P.; Chongmelaxme, B.; Lee, S.W.H. Association Between Cytomegalovirus Infection and Tuberculosis Disease: A Systematic Review and Meta-Analysis of Epidemiological Studies. J. Infect. Dis. 2023, 227, 471–482. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shankar, E.M.; Velu, V.; Kamarulzaman, A.; Larsson, M. Mechanistic insights on immunosenescence and chronic immune activation in HIV-tuberculosis co-infection. World J. Virol. 2015, 4, 17–24. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bizuneh, F.K.; Bizuneh, T.K.; Biwota, G.T.; Abate, B.B.; Ayenew, T.G. Meta-analysis of TB & HIV co-infection mortality rate in sub-Saharan African children, youth, and adolescents. Ital. J. Pediatr. 2025, 51, 210. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Labrie, F.; Luu-The, V.; Labrie, C.; Simard, J. DHEA and its transformation into androgens and estrogens in peripheral target tissues: Intracrinology. Front. Neuroendocrinol. 2001, 22, 185–212. [Google Scholar] [CrossRef] [PubMed]
- Voznesensky, M.; Walsh, S.; Dauser, D.; Brindisi, J.; Kenny, A.M. The association between dehydroepiandosterone and frailty in older men and women. Age Ageing 2009, 38, 401–406. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abrams, D.I.; Shade, S.B.; Couey, P.; McCune, J.M.; Lo, J.; Bacchetti, P.; Chang, B.; Epling, L.; Liegler, T.; Grant, R.M. Dehydroepiandrosterone (DHEA) effects on HIV replication and host immunity: A randomized placebo-controlled study. AIDS Res. Hum. Retroviruses 2007, 23, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Gabay, C. Interleukin-6 and chronic inflammation. Arthritis Res Ther. 2006, 8 (Suppl. S2), S3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Migeon, C.J.; Keller, A.R.; Lawrence, B.; Shepard, T.H., II. Dehydroepiandrosterone and androsterone levels in human plasma: Effect of age and sex; day-to-day and diurnal variations. J. Clin. Endocrinol. Metab. 1957, 17, 1051–1062. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.J.; Nolan, J.J.; Nelson, J.C.; Yen, S.S. Effects of replacement dose of dehydroepiandrosterone in men and women of advancing age. J. Clin. Endocrinol. Metab. 1994, 78, 1360–1367, Erratum in: J. Clin. Endocrinol. Metab. 1995, 80, 2799. [Google Scholar] [CrossRef] [PubMed]
- Buford, T.W.; Willoughby, D.S. Impact of DHEA(S) and cortisol on immune function in aging: A brief review. Appl. Physiol. Nutr. Metab. 2008, 33, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Suzuki, N.; Daynes, R.A.; Engleman, E.G. Dehydroepiandrosterone enhances IL2 production and cytotoxic effector function of human T cells. Clin. Immunol. Immunopathol. 1991, 61 Pt 1, 202–211. [Google Scholar] [CrossRef] [PubMed]
- van Weerden, W.M.; Bierings, H.G.; van Steenbrugge, G.J.; de Jong, F.H.; Schröder, F.H. Adrenal glands of mouse and rat do not synthesize androgens. Life Sci. 1992, 50, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Henderson, E.; Schwartz, A.; Pashko, L.; Abou-Gharbia, M.; Swern, D. Dehydroepiandrosterone and 16 alpha-bromo-epiandrosterone: Inhibitors of Epstein-Barr virus-induced transformation of human lymphocytes. Carcinogenesis 1981, 2, 683–686. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Qassem, M.; Kyriacou, P.A. Measuring stress: A review of the current cortisol and dehydroepiandrosterone (DHEA) measurement techniques and considerations for the future of mental health monitoring. Stress 2023, 26, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Frincke, J.M.; Stickney, D.R.; Onizuka-Handa, N.; Garsd, A.; Reading, C.; Krudsood, S.; Wilairatana, P.; Looareesuwan, S. Reduction of parasite levels in patients with uncomplicated malaria by treatment with HE2000. Am. J. Trop. Med. Hyg. 2007, 76, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, A.T.; Thiemann, O.H. 16-bromoepiandrosterone, an activator of the mammalian immune system, inhibits glucose 6-phosphate dehydrogenase from Trypanosoma cruzi and is toxic to these parasites grown in culture. Bioorg. Med. Chem. 2010, 18, 4762–4768. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hernández-Pando, R.; Aguilar-Leon, D.; Orozco, H.; Serrano, A.; Ahlem, C.; Trauger, R.; Schramm, B.; Reading, C.; Frincke, J.; Rook, G.A. 16alpha-Bromoepiandrosterone restores T helper cell type 1 activity and accelerates chemotherapy-induced bacterial clearance in a model of progressive pulmonary tuberculosis. J. Infect. Dis. 2005, 191, 299–306. [Google Scholar] [CrossRef] [PubMed]
- López-Torres, M.O.; Marquina-Castillo, B.; Ramos-Espinosa, O.; Mata-Espinosa, D.; Barrios-Payan, J.A.; Baay-Guzman, G.; Yepez, S.H.; Bini, E.; Torre-Villalvazo, I.; Torres, N.; et al. 16α-Bromoepiandrosterone as a new candidate for experimental diabetes-tuberculosis co-morbidity treatment. Clin. Exp. Immunol. 2021, 205, 232–245. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nicoletti, F.; Conrad, D.; Wang, A.; Pieters, R.; Mangano, K.; van Heeckeren, A.; White, S.K.; Frincke, J.; Reading, C.L.; Auci, D.L.; et al. 16alpha-Bromoepiandrosterone (HE2000) limits non-productive inflammation and stimulates immunity in lungs. Clin. Exp. Immunol. 2009, 158, 308–316. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reading, C.; Dowding, C.; Schramm, B.; Garsd, A.; Onizuka-Handa, N.; Stickney, D.; Frincke, J. Improvement in immune parameters and human immunodeficiency virus-1 viral response in individuals treated with 16alpha-bromoepiandrosterone (HE2000). Clin. Microbiol. Infect. 2006, 12, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Stickney, D.R.; Noveljic, Z.; Garsd, A.; Destiche, D.A.; Frincke, J.M. Safety and activity of the immune modulator HE2000 on the incidence of tuberculosis and other opportunistic infections in AIDS patients. Antimicrob. Agents Chemother. 2007, 51, 2639–2641. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dow, C.T.; Obaid, L. Proposing Bromo-Epi-Androsterone (BEA) for Post-Traumatic Stress Disorder (PTSD). Cells 2025, 14, 1120. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dow, C.T.; Obaid, L. Proposing Bromo-Epi-Androsterone for Host-Directed Therapy Against Tuberculosis. Pathogens 2025, 14, 1179. https://doi.org/10.3390/pathogens14111179
Dow CT, Obaid L. Proposing Bromo-Epi-Androsterone for Host-Directed Therapy Against Tuberculosis. Pathogens. 2025; 14(11):1179. https://doi.org/10.3390/pathogens14111179
Chicago/Turabian StyleDow, Coad Thomas, and Liam Obaid. 2025. "Proposing Bromo-Epi-Androsterone for Host-Directed Therapy Against Tuberculosis" Pathogens 14, no. 11: 1179. https://doi.org/10.3390/pathogens14111179
APA StyleDow, C. T., & Obaid, L. (2025). Proposing Bromo-Epi-Androsterone for Host-Directed Therapy Against Tuberculosis. Pathogens, 14(11), 1179. https://doi.org/10.3390/pathogens14111179




